 Found 555 hits with Last Name = 'pero' and Initial = 'j'
Found 555 hits with Last Name = 'pero' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM149478
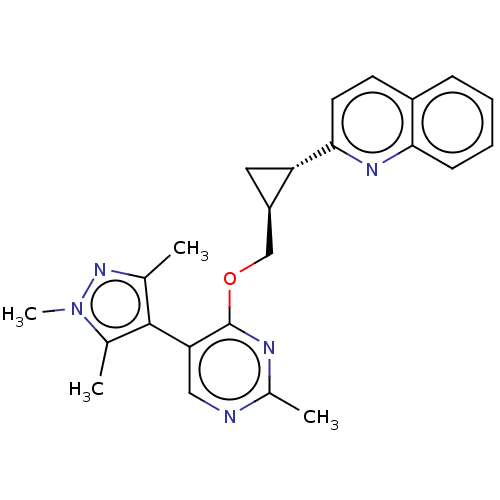
(US8975261, I-46)Show SMILES Cc1nn(C)c(C)c1-c1cnc(C)nc1OC[C@H]1C[C@@H]1c1ccc2ccccc2n1 |r,wU:17.18,wD:19.22,(-.31,2.36,;1.02,3.13,;2.49,2.65,;3.39,3.9,;4.73,4.67,;2.49,5.14,;3.26,6.48,;1.02,4.67,;-.31,5.44,;-.31,6.98,;-1.65,7.75,;-2.98,6.98,;-4.31,7.75,;-2.98,5.44,;-1.65,4.67,;-1.65,3.13,;-2.42,1.79,;-1.65,.46,;-.31,-.31,;-1.65,-1.08,;-2.42,-2.41,;-3.96,-2.41,;-4.73,-3.75,;-3.96,-5.08,;-4.73,-6.41,;-3.96,-7.75,;-2.42,-7.75,;-1.65,-6.41,;-2.42,-5.08,;-1.65,-3.75,)| Show InChI InChI=1S/C24H25N5O/c1-14-23(15(2)29(4)28-14)20-12-25-16(3)26-24(20)30-13-18-11-19(18)22-10-9-17-7-5-6-8-21(17)27-22/h5-10,12,18-19H,11,13H2,1-4H3/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... |
US Patent US8975261 (2015)
BindingDB Entry DOI: 10.7270/Q2JS9P5F |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM149477
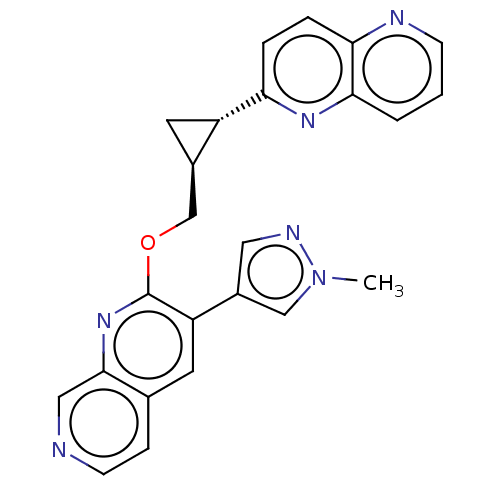
(US8975261, I-42)Show SMILES Cn1cc(cn1)-c1cc2ccncc2nc1OC[C@H]1C[C@@H]1c1ccc2ncccc2n1 |r| Show InChI InChI=1S/C24H20N6O/c1-30-13-17(11-27-30)19-9-15-6-8-25-12-23(15)29-24(19)31-14-16-10-18(16)20-4-5-21-22(28-20)3-2-7-26-21/h2-9,11-13,16,18H,10,14H2,1H3/t16-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... |
US Patent US8975261 (2015)
BindingDB Entry DOI: 10.7270/Q2JS9P5F |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM149487

(US8975261, I-31)Show SMILES COc1ccc(nc1)[C@H]1C[C@@H]1COc1nc(C)ncc1C1=CCC(O)CC1 |r,t:23| Show InChI InChI=1S/C21H25N3O3/c1-13-22-11-19(14-3-5-16(25)6-4-14)21(24-13)27-12-15-9-18(15)20-8-7-17(26-2)10-23-20/h3,7-8,10-11,15-16,18,25H,4-6,9,12H2,1-2H3/t15-,16?,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... |
US Patent US8975261 (2015)
BindingDB Entry DOI: 10.7270/Q2JS9P5F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50054764
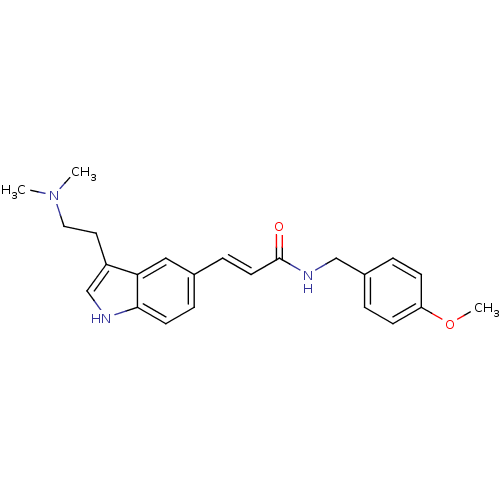
((E)-3-[3-(2-Dimethylamino-ethyl)-1H-indol-5-yl]-N-...)Show SMILES COc1ccc(CNC(=O)\C=C\c2ccc3[nH]cc(CCN(C)C)c3c2)cc1 Show InChI InChI=1S/C23H27N3O2/c1-26(2)13-12-19-16-24-22-10-6-17(14-21(19)22)7-11-23(27)25-15-18-4-8-20(28-3)9-5-18/h4-11,14,16,24H,12-13,15H2,1-3H3,(H,25,27)/b11-7+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1D receptor beta expressed in CHO-K1 cells. |
J Med Chem 39: 4717-26 (1997)
Article DOI: 10.1021/jm9604890
BindingDB Entry DOI: 10.7270/Q2R210GS |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM149485
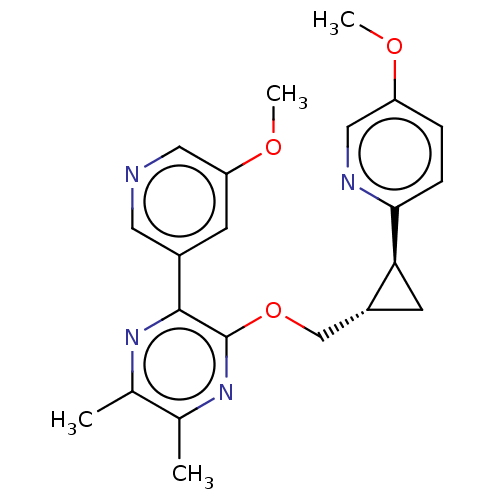
(US8975261, I-36)Show SMILES COc1ccc(nc1)[C@H]1C[C@@H]1COc1nc(C)c(C)nc1-c1cncc(OC)c1 |r| Show InChI InChI=1S/C22H24N4O3/c1-13-14(2)26-22(21(25-13)15-7-18(28-4)10-23-9-15)29-12-16-8-19(16)20-6-5-17(27-3)11-24-20/h5-7,9-11,16,19H,8,12H2,1-4H3/t16-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... |
US Patent US8975261 (2015)
BindingDB Entry DOI: 10.7270/Q2JS9P5F |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM149475
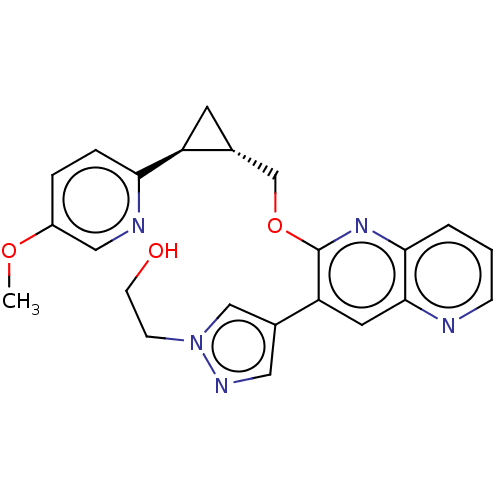
(US8975261, LL3)Show SMILES COc1ccc(nc1)[C@H]1C[C@@H]1COc1nc2cccnc2cc1-c1cnn(CCO)c1 |r| Show InChI InChI=1S/C23H23N5O3/c1-30-17-4-5-20(25-12-17)18-9-15(18)14-31-23-19(16-11-26-28(13-16)7-8-29)10-22-21(27-23)3-2-6-24-22/h2-6,10-13,15,18,29H,7-9,14H2,1H3/t15-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... |
US Patent US8975261 (2015)
BindingDB Entry DOI: 10.7270/Q2JS9P5F |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM149476
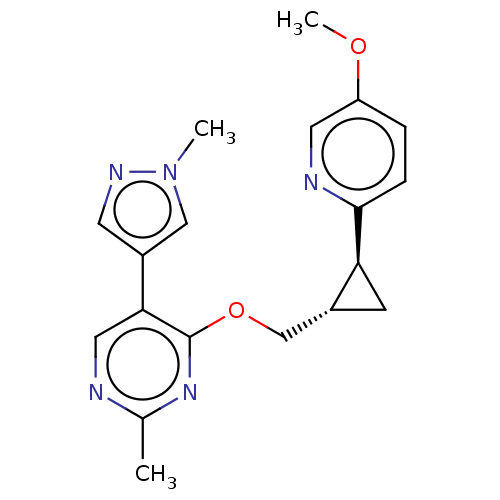
(US8975261, MM4)Show SMILES COc1ccc(nc1)[C@H]1C[C@@H]1COc1nc(C)ncc1-c1cnn(C)c1 |r| Show InChI InChI=1S/C19H21N5O2/c1-12-20-9-17(14-7-22-24(2)10-14)19(23-12)26-11-13-6-16(13)18-5-4-15(25-3)8-21-18/h4-5,7-10,13,16H,6,11H2,1-3H3/t13-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... |
US Patent US8975261 (2015)
BindingDB Entry DOI: 10.7270/Q2JS9P5F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50054764

((E)-3-[3-(2-Dimethylamino-ethyl)-1H-indol-5-yl]-N-...)Show SMILES COc1ccc(CNC(=O)\C=C\c2ccc3[nH]cc(CCN(C)C)c3c2)cc1 Show InChI InChI=1S/C23H27N3O2/c1-26(2)13-12-19-16-24-22-10-6-17(14-21(19)22)7-11-23(27)25-15-18-4-8-20(28-3)9-5-18/h4-11,14,16,24H,12-13,15H2,1-3H3,(H,25,27)/b11-7+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1D receptor alpha expressed in CHO-K1 cells. |
J Med Chem 39: 4717-26 (1997)
Article DOI: 10.1021/jm9604890
BindingDB Entry DOI: 10.7270/Q2R210GS |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM149486
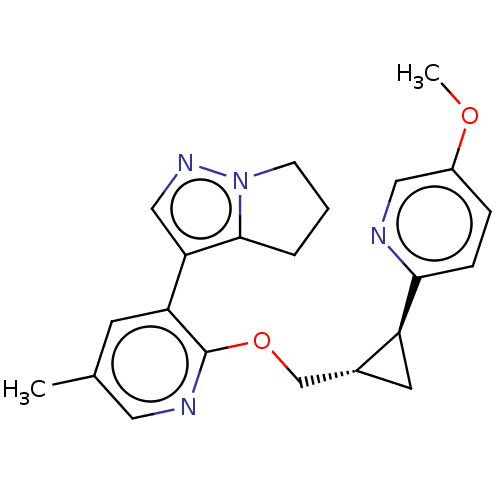
(US8975261, I-39)Show SMILES COc1ccc(nc1)[C@H]1C[C@@H]1COc1ncc(C)cc1-c1cnn2CCCc12 |r| Show InChI InChI=1S/C22H24N4O2/c1-14-8-18(19-12-25-26-7-3-4-21(19)26)22(24-10-14)28-13-15-9-17(15)20-6-5-16(27-2)11-23-20/h5-6,8,10-12,15,17H,3-4,7,9,13H2,1-2H3/t15-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... |
US Patent US8975261 (2015)
BindingDB Entry DOI: 10.7270/Q2JS9P5F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM81967

(CAS_3035990 | DOB,R(-) | NSC_3035990)Show SMILES COC(CO)C(=O)OC(C)c1cccc2nc3c(cccc3nc12)C(O)=O Show InChI InChI=1S/C19H18N2O6/c1-10(27-19(25)15(9-22)26-2)11-5-3-7-13-16(11)20-14-8-4-6-12(18(23)24)17(14)21-13/h3-8,10,15,22H,9H2,1-2H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM28582
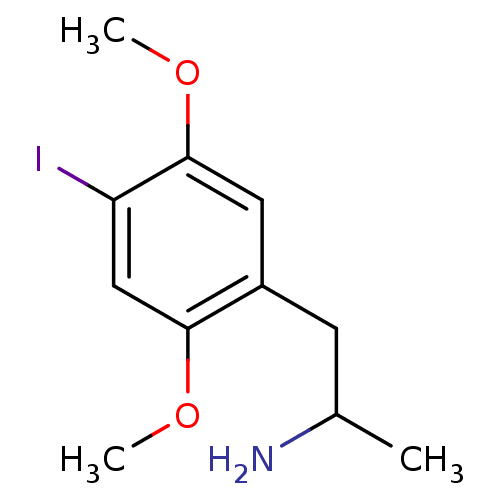
(1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine | CHE...)Show InChI InChI=1S/C11H16INO2/c1-7(13)4-8-5-11(15-3)9(12)6-10(8)14-2/h5-7H,4,13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM81965

(1-(4-ethyl-2,5-dimethoxyphenyl)propan-2-amine | CA...)Show InChI InChI=1S/C13H21NO2/c1-5-10-7-13(16-4)11(6-9(2)14)8-12(10)15-3/h7-9H,5-6,14H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM149479
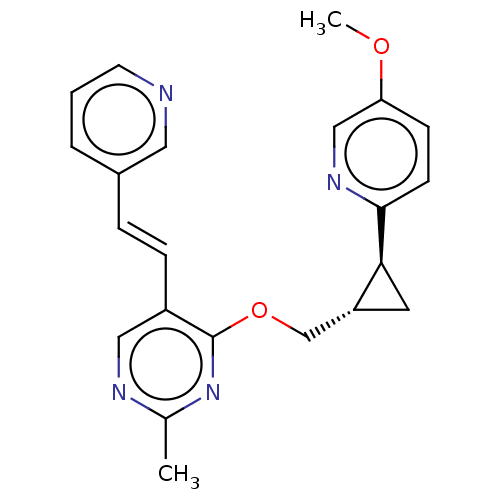
(US8975261, TT1)Show SMILES COc1ccc(nc1)[C@H]1C[C@@H]1COc1nc(C)ncc1\C=C\c1cccnc1 |r| Show InChI InChI=1S/C22H22N4O2/c1-15-24-12-17(6-5-16-4-3-9-23-11-16)22(26-15)28-14-18-10-20(18)21-8-7-19(27-2)13-25-21/h3-9,11-13,18,20H,10,14H2,1-2H3/b6-5+/t18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... |
US Patent US8975261 (2015)
BindingDB Entry DOI: 10.7270/Q2JS9P5F |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM149488
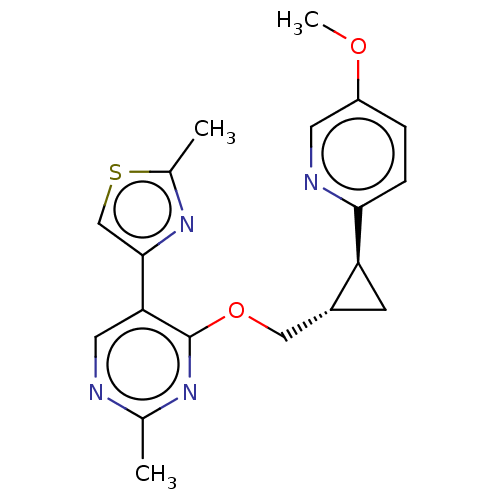
(US8975261, I-57)Show SMILES COc1ccc(nc1)[C@H]1C[C@@H]1COc1nc(C)ncc1-c1csc(C)n1 |r| Show InChI InChI=1S/C19H20N4O2S/c1-11-20-8-16(18-10-26-12(2)23-18)19(22-11)25-9-13-6-15(13)17-5-4-14(24-3)7-21-17/h4-5,7-8,10,13,15H,6,9H2,1-3H3/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... |
US Patent US8975261 (2015)
BindingDB Entry DOI: 10.7270/Q2JS9P5F |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM149483
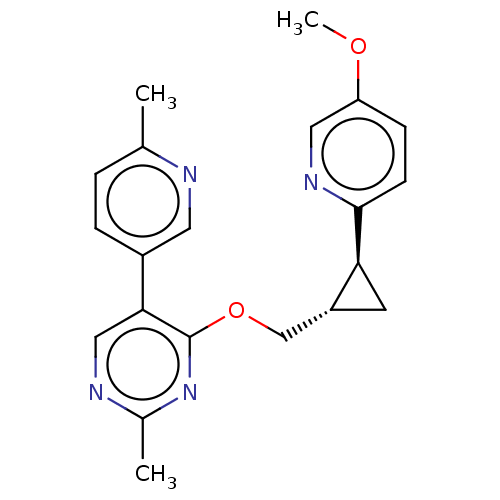
(US8975261, I-73)Show SMILES COc1ccc(nc1)[C@H]1C[C@@H]1COc1nc(C)ncc1-c1ccc(C)nc1 |r| Show InChI InChI=1S/C21H22N4O2/c1-13-4-5-15(9-22-13)19-11-23-14(2)25-21(19)27-12-16-8-18(16)20-7-6-17(26-3)10-24-20/h4-7,9-11,16,18H,8,12H2,1-3H3/t16-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... |
US Patent US8975261 (2015)
BindingDB Entry DOI: 10.7270/Q2JS9P5F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(BOVINE) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM28582

(1-(4-iodo-2,5-dimethoxyphenyl)propan-2-amine | CHE...)Show InChI InChI=1S/C11H16INO2/c1-7(13)4-8-5-11(15-3)9(12)6-10(8)14-2/h5-7H,4,13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM81966

(DOB,S(+))Show SMILES COC(CO)C(=O)O[C@@H](C)c1cccc2nc3c(cccc3nc12)C(O)=O |r| Show InChI InChI=1S/C19H18N2O6/c1-10(27-19(25)15(9-22)26-2)11-5-3-7-13-16(11)20-14-8-4-6-12(18(23)24)17(14)21-13/h3-8,10,15,22H,9H2,1-2H3,(H,23,24)/t10-,15?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM21395

(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50054764

((E)-3-[3-(2-Dimethylamino-ethyl)-1H-indol-5-yl]-N-...)Show SMILES COc1ccc(CNC(=O)\C=C\c2ccc3[nH]cc(CCN(C)C)c3c2)cc1 Show InChI InChI=1S/C23H27N3O2/c1-26(2)13-12-19-16-24-22-10-6-17(14-21(19)22)7-11-23(27)25-15-18-4-8-20(28-3)9-5-18/h4-11,14,16,24H,12-13,15H2,1-3H3,(H,25,27)/b11-7+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1A receptor expressed in CHO-K1 cells. |
J Med Chem 39: 4717-26 (1997)
Article DOI: 10.1021/jm9604890
BindingDB Entry DOI: 10.7270/Q2R210GS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50014407
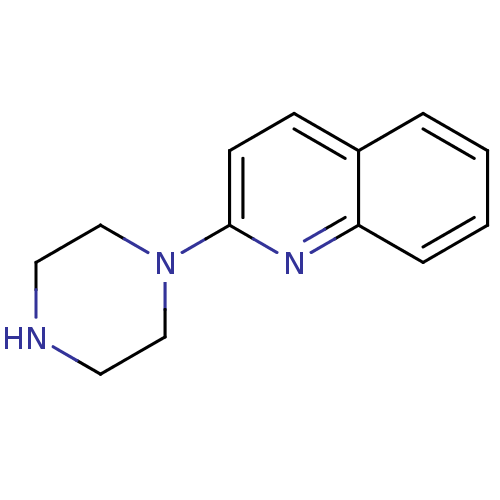
(2-(piperazin-1-yl)quinoline | 2-Piperazin-1-yl-qui...)Show InChI InChI=1S/C13H15N3/c1-2-4-12-11(3-1)5-6-13(15-12)16-9-7-14-8-10-16/h1-6,14H,7-10H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by PDSP Ki Database
| |
Eur J Pharmacol 168: 387-92 (1989)
Article DOI: 10.1016/0014-2999(89)90802-9
BindingDB Entry DOI: 10.7270/Q23R0RC5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(BOVINE) | BDBM30704
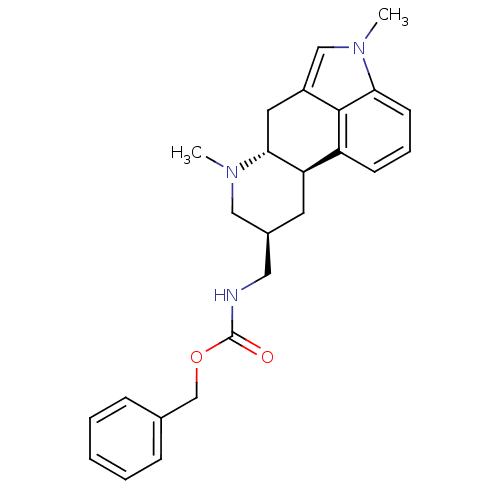
((phenylmethyl) N-[[(6aR,9S,10aR)-4,7-dimethyl-6,6a...)Show SMILES CN1C[C@H](CNC(=O)OCc2ccccc2)C[C@H]2[C@H]1Cc1cn(C)c3cccc2c13 Show InChI InChI=1S/C25H29N3O2/c1-27-14-18(13-26-25(29)30-16-17-7-4-3-5-8-17)11-21-20-9-6-10-22-24(20)19(12-23(21)27)15-28(22)2/h3-10,15,18,21,23H,11-14,16H2,1-2H3,(H,26,29)/t18-,21+,23+/m0/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(BOVINE) | BDBM21395

(3-(2-(4-(4-Fluorobenzoyl)piperidinol)ethyl)-2,4(1H...)Show SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=O)[nH]c3ccccc3c2=O)CC1 Show InChI InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM149480

(US8975261, RR4)Show SMILES COc1ccc(nc1)[C@H]1C[C@@H]1COc1nc2cccnc2cc1C1CCCN(C1)C(C)=O |r| Show InChI InChI=1S/C25H28N4O3/c1-16(30)29-10-4-5-17(14-29)21-12-24-23(6-3-9-26-24)28-25(21)32-15-18-11-20(18)22-8-7-19(31-2)13-27-22/h3,6-9,12-13,17-18,20H,4-5,10-11,14-15H2,1-2H3/t17?,18-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... |
US Patent US8975261 (2015)
BindingDB Entry DOI: 10.7270/Q2JS9P5F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50054762
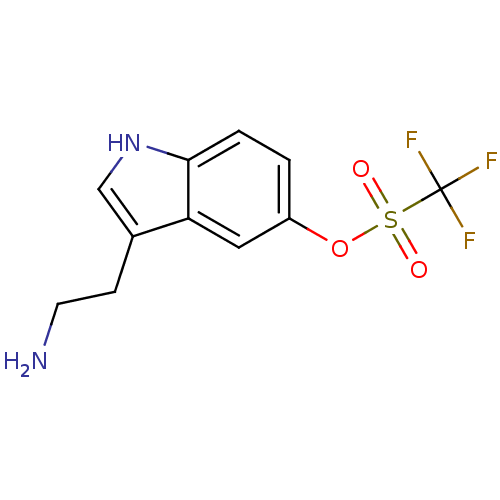
(CHEMBL143512 | Trifluoro-methanesulfonic acid 3-(2...)Show InChI InChI=1S/C11H11F3N2O3S/c12-11(13,14)20(17,18)19-8-1-2-10-9(5-8)7(3-4-15)6-16-10/h1-2,5-6,16H,3-4,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1D receptor alpha expressed in CHO-K1 cells. |
J Med Chem 39: 4717-26 (1997)
Article DOI: 10.1021/jm9604890
BindingDB Entry DOI: 10.7270/Q2R210GS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50024204

(1H-imidazo[4,5-c]pyridine derivative | 2N-[4,7-dim...)Show SMILES CN(C)S(=O)(=O)N[C@H]1C[C@H]2[C@@H](Cc3cn(C)c4cccc2c34)N(C)C1 Show InChI InChI=1S/C18H26N4O2S/c1-20(2)25(23,24)19-13-9-15-14-6-5-7-16-18(14)12(10-21(16)3)8-17(15)22(4)11-13/h5-7,10,13,15,17,19H,8-9,11H2,1-4H3/t13-,15+,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM149482

(US8975261, I-54)Show SMILES Cc1ncc(-c2cnn(C)c2)c(OC[C@H]2C[C@@H]2c2ccn(C)n2)n1 |r| Show InChI InChI=1S/C17H20N6O/c1-11-18-8-15(13-7-19-23(3)9-13)17(20-11)24-10-12-6-14(12)16-4-5-22(2)21-16/h4-5,7-9,12,14H,6,10H2,1-3H3/t12-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... |
US Patent US8975261 (2015)
BindingDB Entry DOI: 10.7270/Q2JS9P5F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50054761

(CHEMBL143767 | Trifluoro-methanesulfonic acid 3-(2...)Show SMILES CN(C)CCc1c[nH]c2ccc(OS(=O)(=O)C(F)(F)F)cc12 Show InChI InChI=1S/C13H15F3N2O3S/c1-18(2)6-5-9-8-17-12-4-3-10(7-11(9)12)21-22(19,20)13(14,15)16/h3-4,7-8,17H,5-6H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1D receptor alpha expressed in CHO-K1 cells. |
J Med Chem 39: 4717-26 (1997)
Article DOI: 10.1021/jm9604890
BindingDB Entry DOI: 10.7270/Q2R210GS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM21342
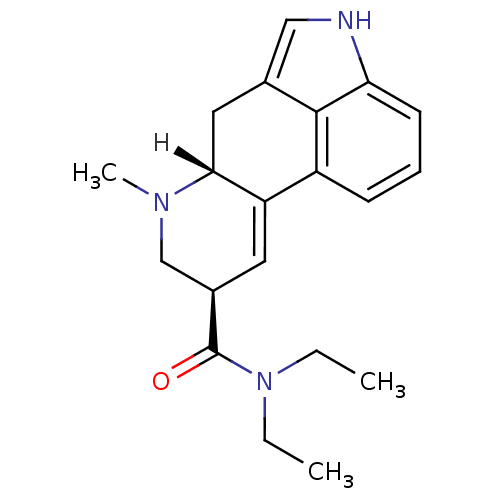
((4R,7R)-N,N-diethyl-6-methyl-6,11-diazatetracyclo[...)Show SMILES [H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](CN2C)C(=O)N(CC)CC)c34 |c:12| Show InChI InChI=1S/C20H25N3O/c1-4-23(5-2)20(24)14-9-16-15-7-6-8-17-19(15)13(11-21-17)10-18(16)22(3)12-14/h6-9,11,14,18,21H,4-5,10,12H2,1-3H3/t14-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM149484

(US8975261, I-55)Show SMILES COc1ccc(nc1)[C@H]1C[C@@H]1COc1nc(C)ncc1-c1nnc(s1)C1CC1 |r| Show InChI InChI=1S/C20H21N5O2S/c1-11-21-9-16(20-25-24-19(28-20)12-3-4-12)18(23-11)27-10-13-7-15(13)17-6-5-14(26-2)8-22-17/h5-6,8-9,12-13,15H,3-4,7,10H2,1-2H3/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... |
US Patent US8975261 (2015)
BindingDB Entry DOI: 10.7270/Q2JS9P5F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM81957
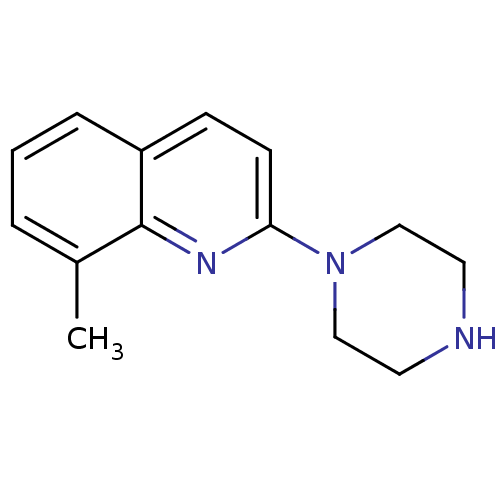
(QUIPAZINE,8-Me)Show InChI InChI=1S/C14H17N3/c1-11-3-2-4-12-5-6-13(16-14(11)12)17-9-7-15-8-10-17/h2-6,15H,7-10H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by PDSP Ki Database
| |
Eur J Pharmacol 168: 387-92 (1989)
Article DOI: 10.1016/0014-2999(89)90802-9
BindingDB Entry DOI: 10.7270/Q23R0RC5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50005265
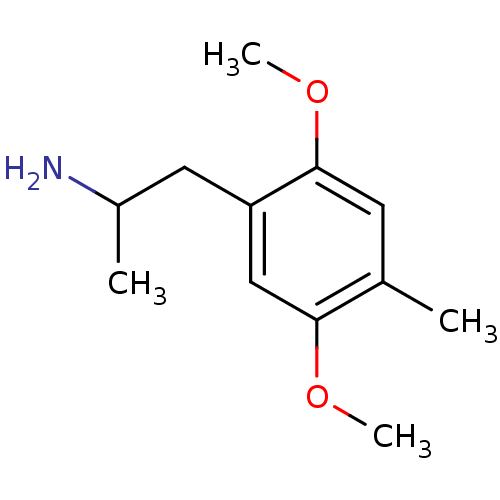
((+/-)2-(2,5-Dimethoxy-4-methyl-phenyl)-1-methyl-et...)Show InChI InChI=1S/C12H19NO2/c1-8-5-12(15-4)10(6-9(2)13)7-11(8)14-3/h5,7,9H,6,13H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50014407

(2-(piperazin-1-yl)quinoline | 2-Piperazin-1-yl-qui...)Show InChI InChI=1S/C13H15N3/c1-2-4-12-11(3-1)5-6-13(15-12)16-9-7-14-8-10-16/h1-6,14H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(BOVINE) | BDBM50016900
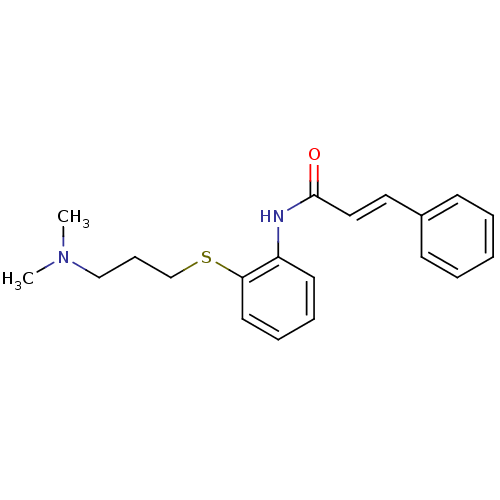
((E)-N-[2-(3-Dimethylamino-propylsulfanyl)-phenyl]-...)Show InChI InChI=1S/C20H24N2OS/c1-22(2)15-8-16-24-19-12-7-6-11-18(19)21-20(23)14-13-17-9-4-3-5-10-17/h3-7,9-14H,8,15-16H2,1-2H3,(H,21,23)/b14-13+ | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM30704

((phenylmethyl) N-[[(6aR,9S,10aR)-4,7-dimethyl-6,6a...)Show SMILES CN1C[C@H](CNC(=O)OCc2ccccc2)C[C@H]2[C@H]1Cc1cn(C)c3cccc2c13 Show InChI InChI=1S/C25H29N3O2/c1-27-14-18(13-26-25(29)30-16-17-7-4-3-5-8-17)11-21-20-9-6-10-22-24(20)19(12-23(21)27)15-28(22)2/h3-10,15,18,21,23H,11-14,16H2,1-2H3,(H,26,29)/t18-,21+,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50005835

((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...)Show InChI InChI=1S/C14H21N3O2S/c1-15-20(18,19)10-11-4-5-14-13(8-11)12(9-16-14)6-7-17(2)3/h4-5,8-9,15-16H,6-7,10H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| DrugBank
Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1D receptor alpha expressed in CHO-K1 cells. |
J Med Chem 39: 4717-26 (1997)
Article DOI: 10.1021/jm9604890
BindingDB Entry DOI: 10.7270/Q2R210GS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(BOVINE) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50016900

((E)-N-[2-(3-Dimethylamino-propylsulfanyl)-phenyl]-...)Show InChI InChI=1S/C20H24N2OS/c1-22(2)15-8-16-24-19-12-7-6-11-18(19)21-20(23)14-13-17-9-4-3-5-10-17/h3-7,9-14H,8,15-16H2,1-2H3,(H,21,23)/b14-13+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(BOVINE) | BDBM50024204

(1H-imidazo[4,5-c]pyridine derivative | 2N-[4,7-dim...)Show SMILES CN(C)S(=O)(=O)N[C@H]1C[C@H]2[C@@H](Cc3cn(C)c4cccc2c34)N(C)C1 Show InChI InChI=1S/C18H26N4O2S/c1-20(2)25(23,24)19-13-9-15-14-6-5-7-16-18(14)12(10-21(16)3)8-17(15)22(4)11-13/h5-7,10,13,15,17,19H,8-9,11H2,1-4H3/t13-,15+,17+/m0/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(BOVINE) | BDBM21342

((4R,7R)-N,N-diethyl-6-methyl-6,11-diazatetracyclo[...)Show SMILES [H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](CN2C)C(=O)N(CC)CC)c34 |c:12| Show InChI InChI=1S/C20H25N3O/c1-4-23(5-2)20(24)14-9-16-15-7-6-8-17-19(15)13(11-21-17)10-18(16)22(3)12-14/h6-9,11,14,18,21H,4-5,10,12H2,1-3H3/t14-,18-/m1/s1 | PDB
Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001888

((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...)Show InChI InChI=1S/C17H19ClN2S/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20/h3-4,6-9,12H,5,10-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50024210
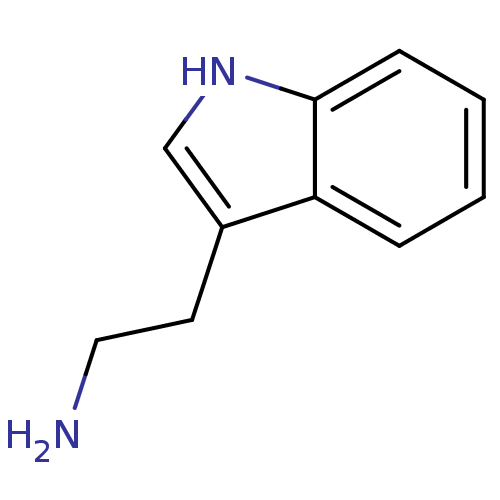
(1H-indole-3-ethanamine | 2-(1H-indol-3-yl)ethanami...)Show InChI InChI=1S/C10H12N2/c11-6-5-8-7-12-10-4-2-1-3-9(8)10/h1-4,7,12H,5-6,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50081701

(3-[2-(dimethylamino)ethyl]-1H-indol-4-ol | 4-hydro...)Show InChI InChI=1S/C12H16N2O/c1-14(2)7-6-9-8-13-10-4-3-5-11(15)12(9)10/h3-5,8,13,15H,6-7H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50054757
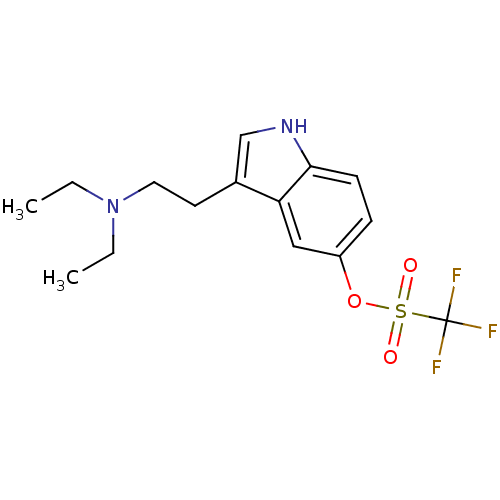
(CHEMBL143510 | Trifluoro-methanesulfonic acid 3-(2...)Show SMILES CCN(CC)CCc1c[nH]c2ccc(OS(=O)(=O)C(F)(F)F)cc12 Show InChI InChI=1S/C15H19F3N2O3S/c1-3-20(4-2)8-7-11-10-19-14-6-5-12(9-13(11)14)23-24(21,22)15(16,17)18/h5-6,9-10,19H,3-4,7-8H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1D receptor alpha expressed in CHO-K1 cells. |
J Med Chem 39: 4717-26 (1997)
Article DOI: 10.1021/jm9604890
BindingDB Entry DOI: 10.7270/Q2R210GS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50171269
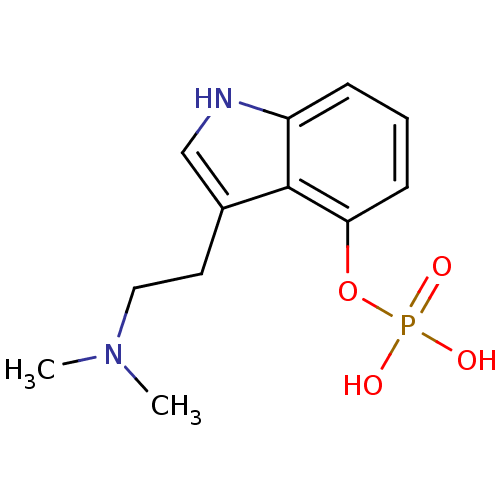
(3-[2-(dimethylamino)ethyl]-1H-indol-4-yl dihydroge...)Show InChI InChI=1S/C12H17N2O4P/c1-14(2)7-6-9-8-13-10-4-3-5-11(12(9)10)18-19(15,16)17/h3-5,8,13H,6-7H2,1-2H3,(H2,15,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50054762

(CHEMBL143512 | Trifluoro-methanesulfonic acid 3-(2...)Show InChI InChI=1S/C11H11F3N2O3S/c12-11(13,14)20(17,18)19-8-1-2-10-9(5-8)7(3-4-15)6-16-10/h1-2,5-6,16H,3-4,15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1D receptor beta expressed in CHO-K1 cells. |
J Med Chem 39: 4717-26 (1997)
Article DOI: 10.1021/jm9604890
BindingDB Entry DOI: 10.7270/Q2R210GS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM30707
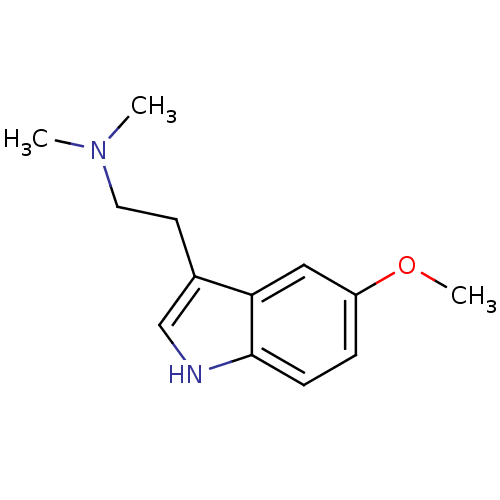
(2-(5-methoxy-1H-indol-3-yl)-N,N-dimethyl-ethanamin...)Show InChI InChI=1S/C13H18N2O/c1-15(2)7-6-10-9-14-13-5-4-11(16-3)8-12(10)13/h4-5,8-9,14H,6-7H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM81958

(N-Propylquipazine)Show InChI InChI=1S/C16H21N3/c1-2-9-18-10-12-19(13-11-18)16-8-7-14-5-3-4-6-15(14)17-16/h3-8H,2,9-13H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by PDSP Ki Database
| |
Eur J Pharmacol 168: 387-92 (1989)
Article DOI: 10.1016/0014-2999(89)90802-9
BindingDB Entry DOI: 10.7270/Q23R0RC5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data