 Found 133 hits with Last Name = 'perron' and Initial = 'dc'
Found 133 hits with Last Name = 'perron' and Initial = 'dc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50305006

(5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C23H19N7S/c24-5-6-30-12-17(22-18(30)11-26-23(25)28-22)14-7-15-10-27-29-21(15)16(8-14)20-9-13-3-1-2-4-19(13)31-20/h1-4,7-12H,5-6,24H2,(H,27,29)(H2,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50305013

(3-(2-amino-7-(7-(benzo[b]thiophen-2-yl)-1H-indazol...)Show SMILES Nc1ncc2n(CCC(O)=O)cc(-c3cc(-c4cc5ccccc5s4)c4[nH]ncc4c3)c2n1 Show InChI InChI=1S/C24H18N6O2S/c25-24-26-11-18-23(28-24)17(12-30(18)6-5-21(31)32)14-7-15-10-27-29-22(15)16(8-14)20-9-13-3-1-2-4-19(13)33-20/h1-4,7-12H,5-6H2,(H,27,29)(H,31,32)(H2,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit alpha
(Homo sapiens (Human)) | BDBM50305006

(5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C23H19N7S/c24-5-6-30-12-17(22-18(30)11-26-23(25)28-22)14-7-15-10-27-29-21(15)16(8-14)20-9-13-3-1-2-4-19(13)31-20/h1-4,7-12H,5-6,24H2,(H,27,29)(H2,25,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of IKK1 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50305007
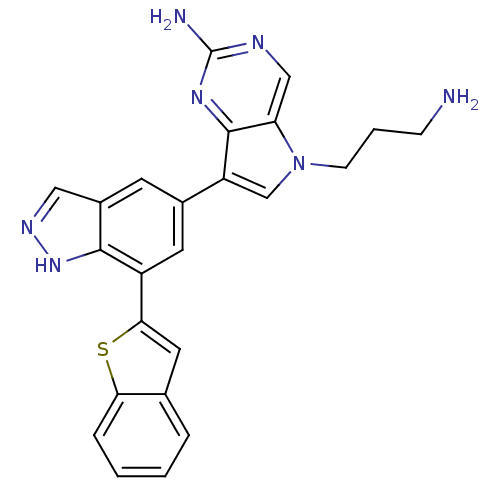
(5-(3-aminopropyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-...)Show SMILES NCCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C24H21N7S/c25-6-3-7-31-13-18(23-19(31)12-27-24(26)29-23)15-8-16-11-28-30-22(16)17(9-15)21-10-14-4-1-2-5-20(14)32-21/h1-2,4-5,8-13H,3,6-7,25H2,(H,28,30)(H2,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50305010

(3-(2-amino-7-(7-(benzo[b]thiophen-2-yl)-1H-indazol...)Show SMILES Nc1ncc2n(CCCO)cc(-c3cc(-c4cc5ccccc5s4)c4[nH]ncc4c3)c2n1 Show InChI InChI=1S/C24H20N6OS/c25-24-26-12-19-23(28-24)18(13-30(19)6-3-7-31)15-8-16-11-27-29-22(16)17(9-15)21-10-14-4-1-2-5-20(14)32-21/h1-2,4-5,8-13,31H,3,6-7H2,(H,27,29)(H2,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50305014

(3-(2-amino-7-(7-(benzo[b]thiophen-2-yl)-1H-indazol...)Show SMILES NC(=O)CCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C24H19N7OS/c25-21(32)5-6-31-12-17(23-18(31)11-27-24(26)29-23)14-7-15-10-28-30-22(15)16(8-14)20-9-13-3-1-2-4-19(13)33-20/h1-4,7-12H,5-6H2,(H2,25,32)(H,28,30)(H2,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50305006

(5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C23H19N7S/c24-5-6-30-12-17(22-18(30)11-26-23(25)28-22)14-7-15-10-27-29-21(15)16(8-14)20-9-13-3-1-2-4-19(13)31-20/h1-4,7-12H,5-6,24H2,(H,27,29)(H2,25,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CHK2 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50305006

(5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C23H19N7S/c24-5-6-30-12-17(22-18(30)11-26-23(25)28-22)14-7-15-10-27-29-21(15)16(8-14)20-9-13-3-1-2-4-19(13)31-20/h1-4,7-12H,5-6,24H2,(H,27,29)(H2,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of pdk1 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50305003

(7-(7-(benzo[b]thiophen-2-yl)-1H-indazol-5-yl)-5H-p...)Show SMILES Nc1ncc2[nH]cc(-c3cc(-c4cc5ccccc5s4)c4n[nH]cc4c3)c2n1 Show InChI InChI=1S/C21H14N6S/c22-21-24-10-16-20(26-21)15(9-23-16)12-5-13-8-25-27-19(13)14(6-12)18-7-11-3-1-2-4-17(11)28-18/h1-10,23H,(H,25,27)(H2,22,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50305006

(5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C23H19N7S/c24-5-6-30-12-17(22-18(30)11-26-23(25)28-22)14-7-15-10-27-29-21(15)16(8-14)20-9-13-3-1-2-4-19(13)31-20/h1-4,7-12H,5-6,24H2,(H,27,29)(H2,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PKCeta |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Sphingosine-1-phosphate lyase 1
(Homo sapiens (Human)) | BDBM50500988

(CHEMBL3798814)Show SMILES CCCCOc1cc(C)c(CNCC(NC(=O)c2cc(C)on2)c2ccccc2)cc1C Show InChI InChI=1S/C26H33N3O3/c1-5-6-12-31-25-14-18(2)22(13-19(25)3)16-27-17-24(21-10-8-7-9-11-21)28-26(30)23-15-20(4)32-29-23/h7-11,13-15,24,27H,5-6,12,16-17H2,1-4H3,(H,28,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human sphingosine 1-phosphate lyase after 22 hrs by umbelliferone fluorescence analysis |
Bioorg Med Chem Lett 26: 2297-302 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.043
BindingDB Entry DOI: 10.7270/Q2ZS30K0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50305006

(5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C23H19N7S/c24-5-6-30-12-17(22-18(30)11-26-23(25)28-22)14-7-15-10-27-29-21(15)16(8-14)20-9-13-3-1-2-4-19(13)31-20/h1-4,7-12H,5-6,24H2,(H,27,29)(H2,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Sphingosine-1-phosphate lyase 1
(Homo sapiens (Human)) | BDBM50500982

(CHEMBL3798147)Show SMILES COc1cc(C)c(CNCC(NC(=O)c2cc(C)on2)c2ccc(cc2)C#CCO)cc1C Show InChI InChI=1S/C26H29N3O4/c1-17-13-25(32-4)18(2)12-22(17)15-27-16-24(28-26(31)23-14-19(3)33-29-23)21-9-7-20(8-10-21)6-5-11-30/h7-10,12-14,24,27,30H,11,15-16H2,1-4H3,(H,28,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human sphingosine 1-phosphate lyase after 22 hrs by umbelliferone fluorescence analysis |
Bioorg Med Chem Lett 26: 2297-302 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.043
BindingDB Entry DOI: 10.7270/Q2ZS30K0 |
More data for this
Ligand-Target Pair | |
Sphingosine-1-phosphate lyase 1
(Homo sapiens (Human)) | BDBM50500974
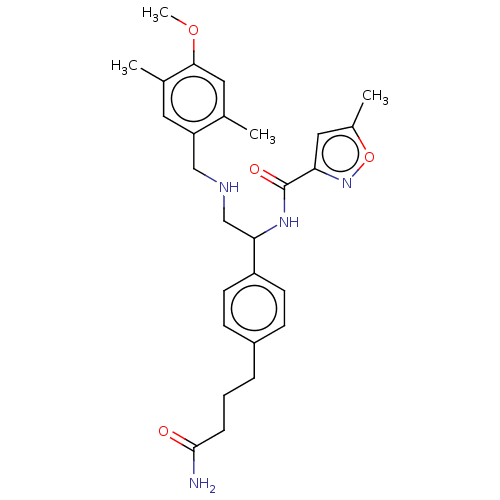
(CHEMBL3799061)Show SMILES COc1cc(C)c(CNCC(NC(=O)c2cc(C)on2)c2ccc(CCCC(N)=O)cc2)cc1C Show InChI InChI=1S/C27H34N4O4/c1-17-13-25(34-4)18(2)12-22(17)15-29-16-24(30-27(33)23-14-19(3)35-31-23)21-10-8-20(9-11-21)6-5-7-26(28)32/h8-14,24,29H,5-7,15-16H2,1-4H3,(H2,28,32)(H,30,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human sphingosine 1-phosphate lyase after 22 hrs by umbelliferone fluorescence analysis |
Bioorg Med Chem Lett 26: 2297-302 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.043
BindingDB Entry DOI: 10.7270/Q2ZS30K0 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50305001
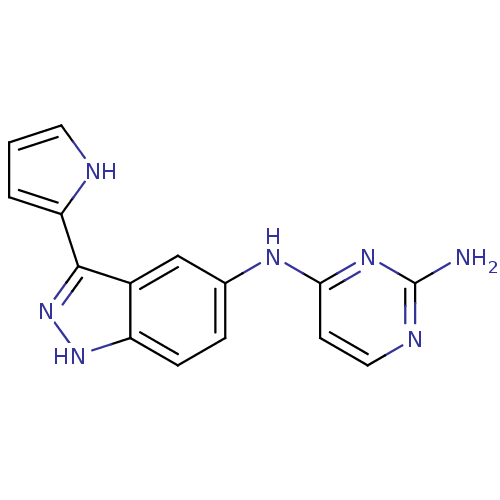
(CHEMBL592288 | N4-(3-(1H-pyrrol-2-yl)-1H-indazol-5...)Show InChI InChI=1S/C15H13N7/c16-15-18-7-5-13(20-15)19-9-3-4-11-10(8-9)14(22-21-11)12-2-1-6-17-12/h1-8,17H,(H,21,22)(H3,16,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50305000

(CHEMBL600081 | N4-(3-(benzo[b]thiophen-2-yl)-1H-in...)Show SMILES Nc1nccc(Nc2ccc3[nH]nc(-c4cc5ccccc5s4)c3c2)n1 Show InChI InChI=1S/C19H14N6S/c20-19-21-8-7-17(23-19)22-12-5-6-14-13(10-12)18(25-24-14)16-9-11-3-1-2-4-15(11)26-16/h1-10H,(H,24,25)(H3,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50305006

(5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C23H19N7S/c24-5-6-30-12-17(22-18(30)11-26-23(25)28-22)14-7-15-10-27-29-21(15)16(8-14)20-9-13-3-1-2-4-19(13)31-20/h1-4,7-12H,5-6,24H2,(H,27,29)(H2,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of GSK3alpha |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
MAP/microtubule affinity-regulating kinase 3
(Homo sapiens (Human)) | BDBM50305006

(5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C23H19N7S/c24-5-6-30-12-17(22-18(30)11-26-23(25)28-22)14-7-15-10-27-29-21(15)16(8-14)20-9-13-3-1-2-4-19(13)31-20/h1-4,7-12H,5-6,24H2,(H,27,29)(H2,25,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CTAK1 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Sphingosine-1-phosphate lyase 1
(Homo sapiens (Human)) | BDBM50500988

(CHEMBL3798814)Show SMILES CCCCOc1cc(C)c(CNCC(NC(=O)c2cc(C)on2)c2ccccc2)cc1C Show InChI InChI=1S/C26H33N3O3/c1-5-6-12-31-25-14-18(2)22(13-19(25)3)16-27-17-24(21-10-8-7-9-11-21)28-26(30)23-15-20(4)32-29-23/h7-11,13-15,24,27H,5-6,12,16-17H2,1-4H3,(H,28,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type full-length human sphingosine 1-phosphate lyase transfected in HEK293-H cells preincubated for 30 mins followed by addition o... |
Bioorg Med Chem Lett 26: 2297-302 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.043
BindingDB Entry DOI: 10.7270/Q2ZS30K0 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50305006

(5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C23H19N7S/c24-5-6-30-12-17(22-18(30)11-26-23(25)28-22)14-7-15-10-27-29-21(15)16(8-14)20-9-13-3-1-2-4-19(13)31-20/h1-4,7-12H,5-6,24H2,(H,27,29)(H2,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/P25 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Sphingosine-1-phosphate lyase 1
(Homo sapiens (Human)) | BDBM50500983

(CHEMBL3798874)Show SMILES Cc1cc(no1)C(=O)NC(CNCc1cc(C)c(OCC(F)(F)F)cc1C)c1ccccc1 Show InChI InChI=1S/C24H26F3N3O3/c1-15-10-22(32-14-24(25,26)27)16(2)9-19(15)12-28-13-21(18-7-5-4-6-8-18)29-23(31)20-11-17(3)33-30-20/h4-11,21,28H,12-14H2,1-3H3,(H,29,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type full-length human sphingosine 1-phosphate lyase transfected in HEK293-H cells preincubated for 30 mins followed by addition o... |
Bioorg Med Chem Lett 26: 2297-302 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.043
BindingDB Entry DOI: 10.7270/Q2ZS30K0 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50305008

(5-(4-aminobutyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C25H23N7S/c26-7-3-4-8-32-14-19(24-20(32)13-28-25(27)30-24)16-9-17-12-29-31-23(17)18(10-16)22-11-15-5-1-2-6-21(15)33-22/h1-2,5-6,9-14H,3-4,7-8,26H2,(H,29,31)(H2,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Sphingosine-1-phosphate lyase 1
(Homo sapiens (Human)) | BDBM50500975

(CHEMBL3799085)Show SMILES CCOc1cc(C)c(CNCC(NC(=O)c2cc(C)on2)c2ccccc2)cc1C Show InChI InChI=1S/C24H29N3O3/c1-5-29-23-12-16(2)20(11-17(23)3)14-25-15-22(19-9-7-6-8-10-19)26-24(28)21-13-18(4)30-27-21/h6-13,22,25H,5,14-15H2,1-4H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human sphingosine 1-phosphate lyase after 22 hrs by umbelliferone fluorescence analysis |
Bioorg Med Chem Lett 26: 2297-302 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.043
BindingDB Entry DOI: 10.7270/Q2ZS30K0 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50304999
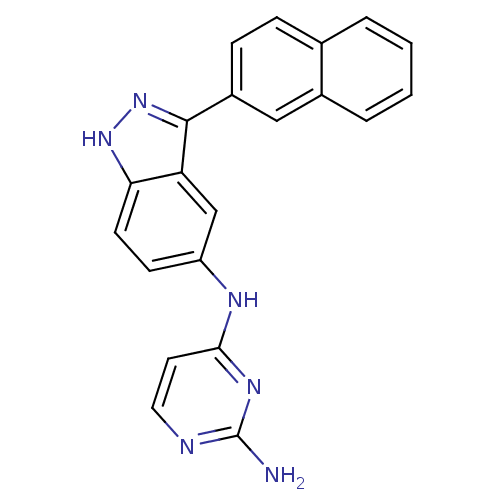
(CHEMBL590834 | N4-(3-(naphthalen-2-yl)-1H-indazol-...)Show SMILES Nc1nccc(Nc2ccc3[nH]nc(-c4ccc5ccccc5c4)c3c2)n1 Show InChI InChI=1S/C21H16N6/c22-21-23-10-9-19(25-21)24-16-7-8-18-17(12-16)20(27-26-18)15-6-5-13-3-1-2-4-14(13)11-15/h1-12H,(H,26,27)(H3,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50305006

(5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C23H19N7S/c24-5-6-30-12-17(22-18(30)11-26-23(25)28-22)14-7-15-10-27-29-21(15)16(8-14)20-9-13-3-1-2-4-19(13)31-20/h1-4,7-12H,5-6,24H2,(H,27,29)(H2,25,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CDC2 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK4
(Homo sapiens (Human)) | BDBM50305006

(5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C23H19N7S/c24-5-6-30-12-17(22-18(30)11-26-23(25)28-22)14-7-15-10-27-29-21(15)16(8-14)20-9-13-3-1-2-4-19(13)31-20/h1-4,7-12H,5-6,24H2,(H,27,29)(H2,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PLK4 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM50305006

(5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C23H19N7S/c24-5-6-30-12-17(22-18(30)11-26-23(25)28-22)14-7-15-10-27-29-21(15)16(8-14)20-9-13-3-1-2-4-19(13)31-20/h1-4,7-12H,5-6,24H2,(H,27,29)(H2,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ROCK1 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50305006

(5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C23H19N7S/c24-5-6-30-12-17(22-18(30)11-26-23(25)28-22)14-7-15-10-27-29-21(15)16(8-14)20-9-13-3-1-2-4-19(13)31-20/h1-4,7-12H,5-6,24H2,(H,27,29)(H2,25,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50305006

(5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C23H19N7S/c24-5-6-30-12-17(22-18(30)11-26-23(25)28-22)14-7-15-10-27-29-21(15)16(8-14)20-9-13-3-1-2-4-19(13)31-20/h1-4,7-12H,5-6,24H2,(H,27,29)(H2,25,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DYRK1A |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Sphingosine-1-phosphate lyase 1
(Homo sapiens (Human)) | BDBM50500979

(CHEMBL3798254)Show SMILES COc1cc(C)c(CNC[C@@H](NC(=O)c2cc(C)on2)c2ccccc2)cc1C |r| Show InChI InChI=1S/C23H27N3O3/c1-15-11-22(28-4)16(2)10-19(15)13-24-14-21(18-8-6-5-7-9-18)25-23(27)20-12-17(3)29-26-20/h5-12,21,24H,13-14H2,1-4H3,(H,25,27)/t21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 485 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human sphingosine 1-phosphate lyase after 22 hrs by umbelliferone fluorescence analysis |
Bioorg Med Chem Lett 26: 2297-302 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.043
BindingDB Entry DOI: 10.7270/Q2ZS30K0 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50305016

(7-(7-(benzo[b]thiophen-2-yl)-1H-indazol-5-yl)-5-is...)Show SMILES CC(C)n1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C24H20N6S/c1-13(2)30-12-18(23-19(30)11-26-24(25)28-23)15-7-16-10-27-29-22(16)17(8-15)21-9-14-5-3-4-6-20(14)31-21/h3-13H,1-2H3,(H,27,29)(H2,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50305006

(5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C23H19N7S/c24-5-6-30-12-17(22-18(30)11-26-23(25)28-22)14-7-15-10-27-29-21(15)16(8-14)20-9-13-3-1-2-4-19(13)31-20/h1-4,7-12H,5-6,24H2,(H,27,29)(H2,25,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PAK1 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform delta
(Homo sapiens (Human)) | BDBM50305006

(5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C23H19N7S/c24-5-6-30-12-17(22-18(30)11-26-23(25)28-22)14-7-15-10-27-29-21(15)16(8-14)20-9-13-3-1-2-4-19(13)31-20/h1-4,7-12H,5-6,24H2,(H,27,29)(H2,25,26,28) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Ck1delta |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50305006

(5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C23H19N7S/c24-5-6-30-12-17(22-18(30)11-26-23(25)28-22)14-7-15-10-27-29-21(15)16(8-14)20-9-13-3-1-2-4-19(13)31-20/h1-4,7-12H,5-6,24H2,(H,27,29)(H2,25,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MST2 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Sphingosine-1-phosphate lyase 1
(Homo sapiens (Human)) | BDBM50500983

(CHEMBL3798874)Show SMILES Cc1cc(no1)C(=O)NC(CNCc1cc(C)c(OCC(F)(F)F)cc1C)c1ccccc1 Show InChI InChI=1S/C24H26F3N3O3/c1-15-10-22(32-14-24(25,26)27)16(2)9-19(15)12-28-13-21(18-7-5-4-6-8-18)29-23(31)20-11-17(3)33-30-20/h4-11,21,28H,12-14H2,1-3H3,(H,29,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human sphingosine 1-phosphate lyase after 22 hrs by umbelliferone fluorescence analysis |
Bioorg Med Chem Lett 26: 2297-302 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.043
BindingDB Entry DOI: 10.7270/Q2ZS30K0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Sgk1
(Homo sapiens (Human)) | BDBM50305006

(5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C23H19N7S/c24-5-6-30-12-17(22-18(30)11-26-23(25)28-22)14-7-15-10-27-29-21(15)16(8-14)20-9-13-3-1-2-4-19(13)31-20/h1-4,7-12H,5-6,24H2,(H,27,29)(H2,25,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of SGK |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Sphingosine-1-phosphate lyase 1
(Homo sapiens (Human)) | BDBM50500986

(CHEMBL3797779)Show SMILES COc1cc(C)c(CNCC(NC(=O)c2cc(C)on2)c2ccc(F)cc2)cc1C Show InChI InChI=1S/C23H26FN3O3/c1-14-10-22(29-4)15(2)9-18(14)12-25-13-21(17-5-7-19(24)8-6-17)26-23(28)20-11-16(3)30-27-20/h5-11,21,25H,12-13H2,1-4H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human sphingosine 1-phosphate lyase after 22 hrs by umbelliferone fluorescence analysis |
Bioorg Med Chem Lett 26: 2297-302 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.043
BindingDB Entry DOI: 10.7270/Q2ZS30K0 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50304994

(CHEMBL589650 | N4-(7-(naphthalen-2-yl)-1H-indazol-...)Show SMILES Nc1nccc(Nc2cc(-c3ccc4ccccc4c3)c3[nH]ncc3c2)n1 Show InChI InChI=1S/C21H16N6/c22-21-23-8-7-19(26-21)25-17-10-16-12-24-27-20(16)18(11-17)15-6-5-13-3-1-2-4-14(13)9-15/h1-12H,(H,24,27)(H3,22,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Sphingosine-1-phosphate lyase 1
(Homo sapiens (Human)) | BDBM50500980

(CHEMBL3798062)Show SMILES COc1cc(C)c(CNCC(NC(=O)c2cc(on2)C2CC2)c2ccccc2)cc1C Show InChI InChI=1S/C25H29N3O3/c1-16-12-23(30-3)17(2)11-20(16)14-26-15-22(18-7-5-4-6-8-18)27-25(29)21-13-24(31-28-21)19-9-10-19/h4-8,11-13,19,22,26H,9-10,14-15H2,1-3H3,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human sphingosine 1-phosphate lyase after 22 hrs by umbelliferone fluorescence analysis |
Bioorg Med Chem Lett 26: 2297-302 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.043
BindingDB Entry DOI: 10.7270/Q2ZS30K0 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50305011
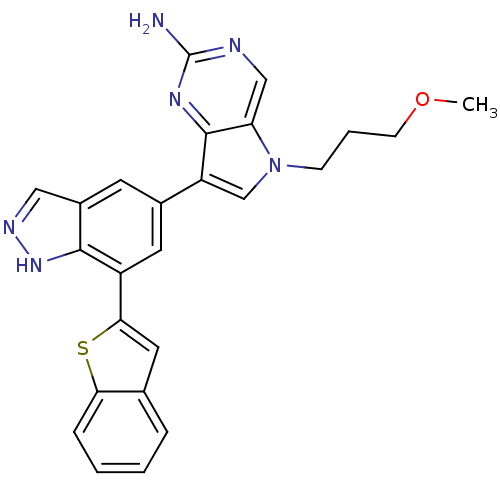
(7-(7-(benzo[b]thiophen-2-yl)-1H-indazol-5-yl)-5-(3...)Show SMILES COCCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C25H22N6OS/c1-32-8-4-7-31-14-19(24-20(31)13-27-25(26)29-24)16-9-17-12-28-30-23(17)18(10-16)22-11-15-5-2-3-6-21(15)33-22/h2-3,5-6,9-14H,4,7-8H2,1H3,(H,28,30)(H2,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Sphingosine-1-phosphate lyase 1
(Homo sapiens (Human)) | BDBM50500981
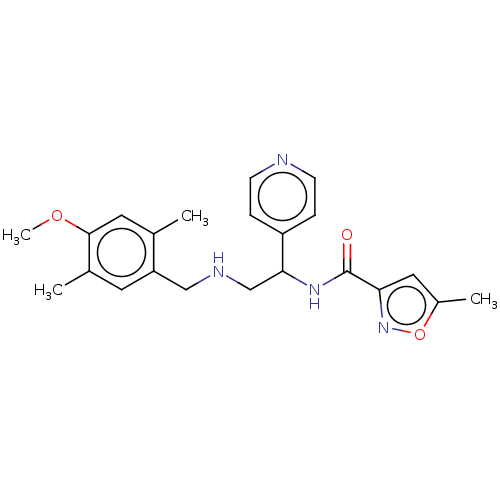
(CHEMBL3799254)Show SMILES COc1cc(C)c(CNCC(NC(=O)c2cc(C)on2)c2ccncc2)cc1C Show InChI InChI=1S/C22H26N4O3/c1-14-10-21(28-4)15(2)9-18(14)12-24-13-20(17-5-7-23-8-6-17)25-22(27)19-11-16(3)29-26-19/h5-11,20,24H,12-13H2,1-4H3,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human sphingosine 1-phosphate lyase after 22 hrs by umbelliferone fluorescence analysis |
Bioorg Med Chem Lett 26: 2297-302 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.043
BindingDB Entry DOI: 10.7270/Q2ZS30K0 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50305006

(5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C23H19N7S/c24-5-6-30-12-17(22-18(30)11-26-23(25)28-22)14-7-15-10-27-29-21(15)16(8-14)20-9-13-3-1-2-4-19(13)31-20/h1-4,7-12H,5-6,24H2,(H,27,29)(H2,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PKCdelta |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Sphingosine-1-phosphate lyase 1
(Homo sapiens (Human)) | BDBM50500975

(CHEMBL3799085)Show SMILES CCOc1cc(C)c(CNCC(NC(=O)c2cc(C)on2)c2ccccc2)cc1C Show InChI InChI=1S/C24H29N3O3/c1-5-29-23-12-16(2)20(11-17(23)3)14-25-15-22(19-9-7-6-8-10-19)26-24(28)21-13-18(4)30-27-21/h6-13,22,25H,5,14-15H2,1-4H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type full-length human sphingosine 1-phosphate lyase transfected in HEK293-H cells preincubated for 30 mins followed by addition o... |
Bioorg Med Chem Lett 26: 2297-302 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.043
BindingDB Entry DOI: 10.7270/Q2ZS30K0 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50305006

(5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C23H19N7S/c24-5-6-30-12-17(22-18(30)11-26-23(25)28-22)14-7-15-10-27-29-21(15)16(8-14)20-9-13-3-1-2-4-19(13)31-20/h1-4,7-12H,5-6,24H2,(H,27,29)(H2,25,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D2
(Homo sapiens (Human)) | BDBM50305006

(5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C23H19N7S/c24-5-6-30-12-17(22-18(30)11-26-23(25)28-22)14-7-15-10-27-29-21(15)16(8-14)20-9-13-3-1-2-4-19(13)31-20/h1-4,7-12H,5-6,24H2,(H,27,29)(H2,25,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PKD2 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50305009

(7-(7-(benzo[b]thiophen-2-yl)-1H-indazol-5-yl)-5-(2...)Show SMILES Nc1ncc2n(CCC3CCNCC3)cc(-c3cc(-c4cc5ccccc5s4)c4[nH]ncc4c3)c2n1 Show InChI InChI=1S/C28H27N7S/c29-28-31-15-23-27(33-28)22(16-35(23)10-7-17-5-8-30-9-6-17)19-11-20-14-32-34-26(20)21(12-19)25-13-18-3-1-2-4-24(18)36-25/h1-4,11-17,30H,5-10H2,(H,32,34)(H2,29,31,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
Sphingosine-1-phosphate lyase 1
(Homo sapiens (Human)) | BDBM50500984

(CHEMBL3799908)Show SMILES COc1cc(C)c(CNCC(NC(=O)c2cc(C)on2)c2ccccc2)cc1C Show InChI InChI=1S/C23H27N3O3/c1-15-11-22(28-4)16(2)10-19(15)13-24-14-21(18-8-6-5-7-9-18)25-23(27)20-12-17(3)29-26-20/h5-12,21,24H,13-14H2,1-4H3,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human sphingosine 1-phosphate lyase after 22 hrs by umbelliferone fluorescence analysis |
Bioorg Med Chem Lett 26: 2297-302 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.043
BindingDB Entry DOI: 10.7270/Q2ZS30K0 |
More data for this
Ligand-Target Pair | |
Sphingosine-1-phosphate lyase 1
(Homo sapiens (Human)) | BDBM50500976
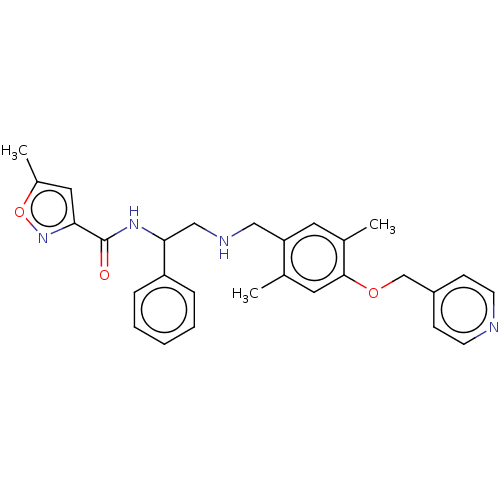
(CHEMBL3798106)Show SMILES Cc1cc(no1)C(=O)NC(CNCc1cc(C)c(OCc2ccncc2)cc1C)c1ccccc1 Show InChI InChI=1S/C28H30N4O3/c1-19-14-27(34-18-22-9-11-29-12-10-22)20(2)13-24(19)16-30-17-26(23-7-5-4-6-8-23)31-28(33)25-15-21(3)35-32-25/h4-15,26,30H,16-18H2,1-3H3,(H,31,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human sphingosine 1-phosphate lyase after 22 hrs by umbelliferone fluorescence analysis |
Bioorg Med Chem Lett 26: 2297-302 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.043
BindingDB Entry DOI: 10.7270/Q2ZS30K0 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50305006

(5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C23H19N7S/c24-5-6-30-12-17(22-18(30)11-26-23(25)28-22)14-7-15-10-27-29-21(15)16(8-14)20-9-13-3-1-2-4-19(13)31-20/h1-4,7-12H,5-6,24H2,(H,27,29)(H2,25,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50305006

(5-(2-aminoethyl)-7-(7-(benzo[b]thiophen-2-yl)-1H-i...)Show SMILES NCCn1cc(-c2cc(-c3cc4ccccc4s3)c3[nH]ncc3c2)c2nc(N)ncc12 Show InChI InChI=1S/C23H19N7S/c24-5-6-30-12-17(22-18(30)11-26-23(25)28-22)14-7-15-10-27-29-21(15)16(8-14)20-9-13-3-1-2-4-19(13)31-20/h1-4,7-12H,5-6,24H2,(H,27,29)(H2,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 |
Bioorg Med Chem Lett 20: 334-7 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.103
BindingDB Entry DOI: 10.7270/Q2VD6ZJJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data