

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM19461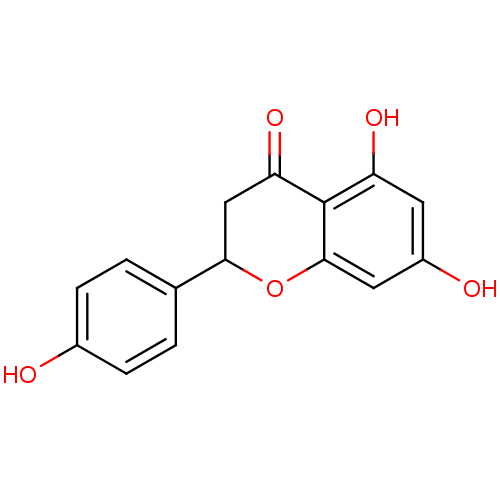 (α-CA inhibitor, 5 | 5,7-dihydroxy-2-(4-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 113 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM239159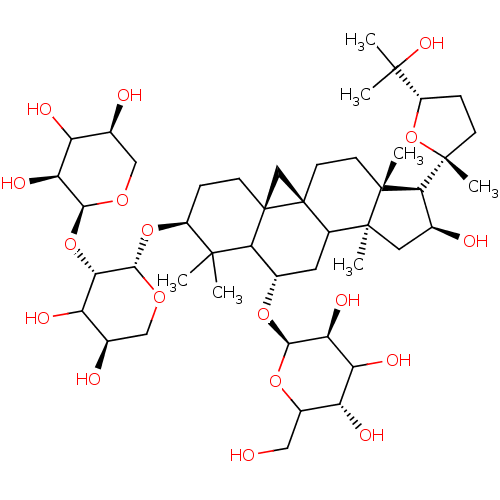 (α-CA inhibitor, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 578 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM239157 (α-CA inhibitor, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 830 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM239159 (α-CA inhibitor, 7) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 940 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM19461 (α-CA inhibitor, 5 | 5,7-dihydroxy-2-(4-hydrox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 970 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM239160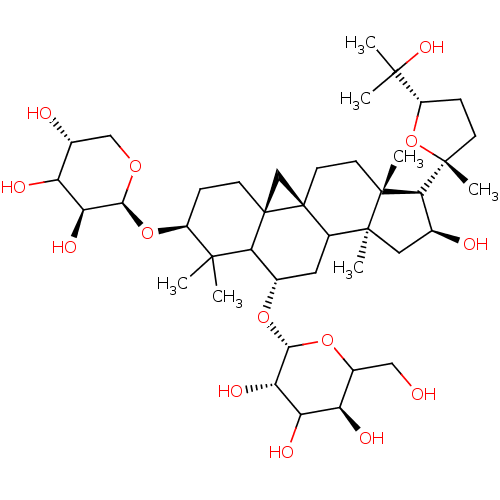 (α-CA inhibitor, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 970 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM19461 (α-CA inhibitor, 5 | 5,7-dihydroxy-2-(4-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM239161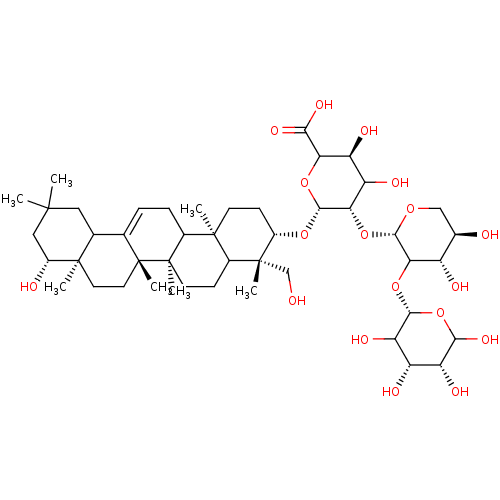 (α-CA inhibitor, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM23417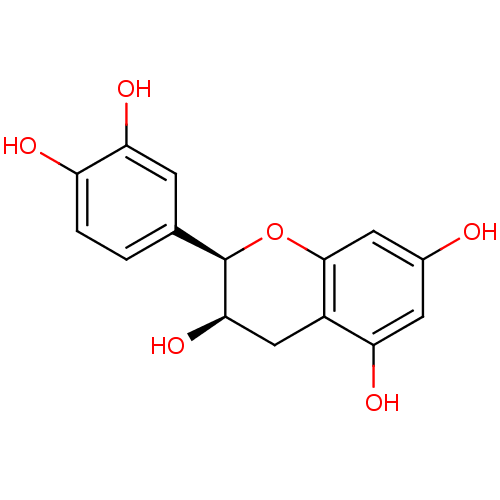 (α-CA inhibitor, 4 | (-)-Epicatechin | (2R,3R)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM239162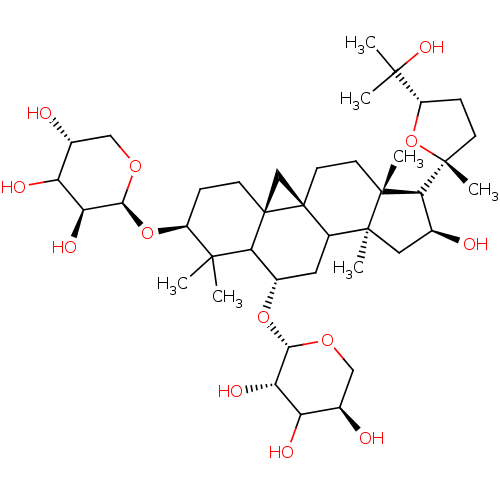 (α-CA inhibitor, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 3 (Bos taurus (Cattle)) | BDBM239159 (α-CA inhibitor, 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM239160 (α-CA inhibitor, 8) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (Homo sapiens (Human)) | BDBM19461 (α-CA inhibitor, 5 | 5,7-dihydroxy-2-(4-hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM239157 (α-CA inhibitor, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 3 (Bos taurus (Cattle)) | BDBM239160 (α-CA inhibitor, 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM239161 (α-CA inhibitor, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 3 (Bos taurus (Cattle)) | BDBM239161 (α-CA inhibitor, 9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM239162 (α-CA inhibitor, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 3 (Bos taurus (Cattle)) | BDBM19461 (α-CA inhibitor, 5 | 5,7-dihydroxy-2-(4-hydrox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM23417 (α-CA inhibitor, 4 | (-)-Epicatechin | (2R,3R)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM239159 (α-CA inhibitor, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 3 (Bos taurus (Cattle)) | BDBM239162 (α-CA inhibitor, 10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM239160 (α-CA inhibitor, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 3 (Bos taurus (Cattle)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM239157 (α-CA inhibitor, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM239161 (α-CA inhibitor, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM23417 (α-CA inhibitor, 4 | (-)-Epicatechin | (2R,3R)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM239162 (α-CA inhibitor, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (Homo sapiens (Human)) | BDBM23417 (α-CA inhibitor, 4 | (-)-Epicatechin | (2R,3R)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 3 (Bos taurus (Cattle)) | BDBM26187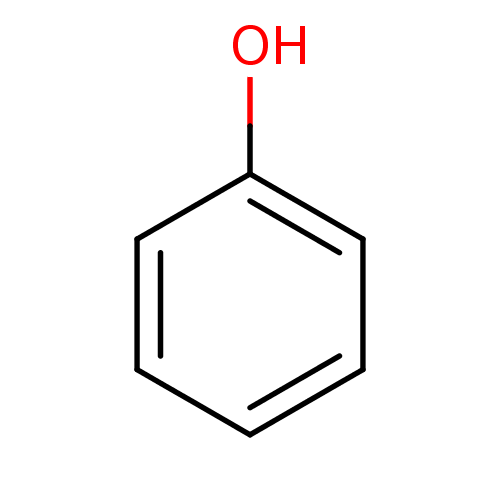 (α-CA inhibitor, 11 | CHEMBL14060 | US9688816,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (Homo sapiens (Human)) | BDBM239159 (α-CA inhibitor, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 3 (Bos taurus (Cattle)) | BDBM239157 (α-CA inhibitor, 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 3 (Bos taurus (Cattle)) | BDBM239158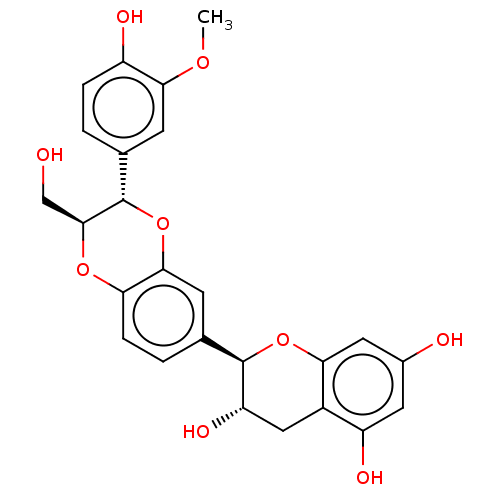 (α-CA inhibitor, 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 3 (Bos taurus (Cattle)) | BDBM26188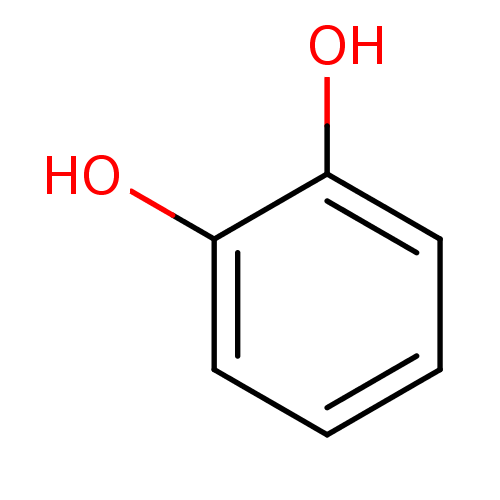 (α-CA inhibitor, 12 | 1,2-Dihydroxybenzene, XI...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (Homo sapiens (Human)) | BDBM239160 (α-CA inhibitor, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 6.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (Homo sapiens (Human)) | BDBM239161 (α-CA inhibitor, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (Homo sapiens (Human)) | BDBM239157 (α-CA inhibitor, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 3 (Bos taurus (Cattle)) | BDBM23417 (α-CA inhibitor, 4 | (-)-Epicatechin | (2R,3R)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 3 (Bos taurus (Cattle)) | BDBM23416 (α-CA inhibitor, 3 | (+)-Catechin | (2R,3S)-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 9.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 6 (Homo sapiens (Human)) | BDBM239162 (α-CA inhibitor, 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 3.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 3 (Bos taurus (Cattle)) | BDBM26189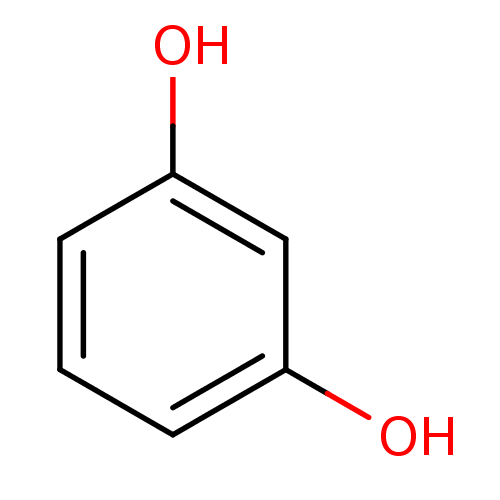 (α-CA inhibitor, 13 | 1,3-Dihydroxybenzene, XI...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.96E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 3 (Bos taurus (Cattle)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.63E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50376851 (CHEMBL259127) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human HDAC1 in U937 cells by immunoprecipitation assay | Bioorg Med Chem Lett 18: 2530-5 (2008) Article DOI: 10.1016/j.bmcl.2008.03.055 BindingDB Entry DOI: 10.7270/Q2930V17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19410 (CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Sapienza Universit£ di Roma Curated by ChEMBL | Assay Description Inhibition of human HDAC1 in U937 cells by immunoprecipitation assay | Bioorg Med Chem Lett 18: 2530-5 (2008) Article DOI: 10.1016/j.bmcl.2008.03.055 BindingDB Entry DOI: 10.7270/Q2930V17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RmtA (Emericella nidulans) | BDBM50376145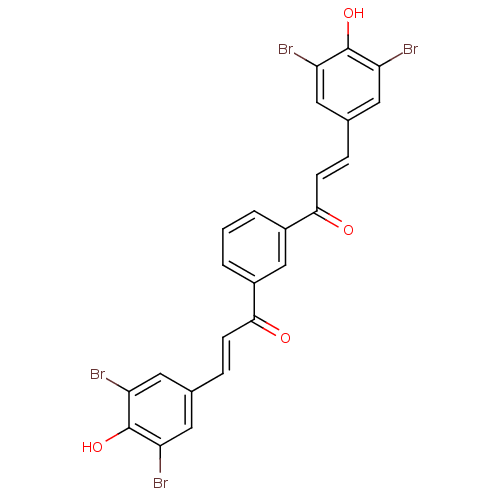 (CHEMBL410106) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Inhibition of Aspergillus nidulans RmtA | J Med Chem 51: 2279-90 (2008) Article DOI: 10.1021/jm701595q BindingDB Entry DOI: 10.7270/Q28C9X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RmtA (Emericella nidulans) | BDBM50376155 (CHEMBL265209) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Roma La Sapienza Curated by ChEMBL | Assay Description Inhibition of Aspergillus nidulans RmtA | J Med Chem 51: 2279-90 (2008) Article DOI: 10.1021/jm701595q BindingDB Entry DOI: 10.7270/Q28C9X48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 94 total ) | Next | Last >> |