

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Bos taurus) | BDBM222435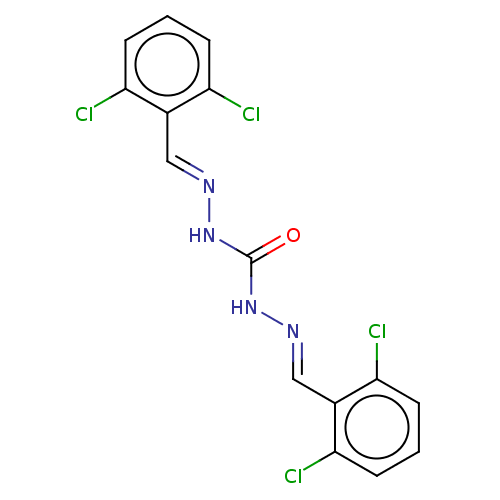 (N'',N'''-Bis[(E)-(2,6-dichlorophenyl)methylide...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Karachi; Government College University Faisalabad | Assay Description Kinetic studies were performed by using different concentration of inhibitors over different concentrations of substrate (4-NPA) such as 0.175, 0.35,... | Bioorg Chem 72: 89-101 (2017) Article DOI: 10.1016/j.bioorg.2017.03.014 BindingDB Entry DOI: 10.7270/Q2125RHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM222429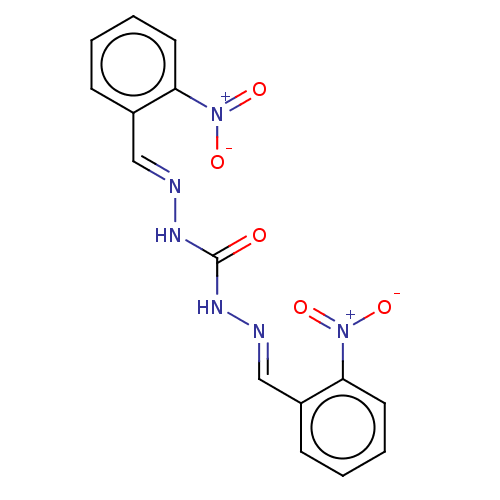 (N'',N'''-Bis[(E)-(2-nitrophenyl)methylidene]ca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Karachi; Government College University Faisalabad | Assay Description Kinetic studies were performed by using different concentration of inhibitors over different concentrations of substrate (4-NPA) such as 0.175, 0.35,... | Bioorg Chem 72: 89-101 (2017) Article DOI: 10.1016/j.bioorg.2017.03.014 BindingDB Entry DOI: 10.7270/Q2125RHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM222428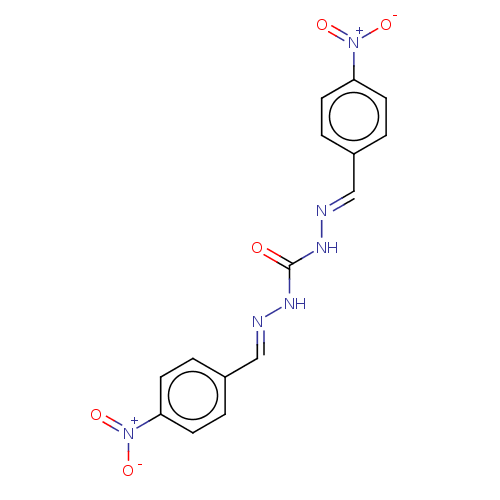 (N'',N'''-Bis[(E)-(4-nitrophenyl)methylidene]ca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Karachi; Government College University Faisalabad | Assay Description Kinetic studies were performed by using different concentration of inhibitors over different concentrations of substrate (4-NPA) such as 0.175, 0.35,... | Bioorg Chem 72: 89-101 (2017) Article DOI: 10.1016/j.bioorg.2017.03.014 BindingDB Entry DOI: 10.7270/Q2125RHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM222431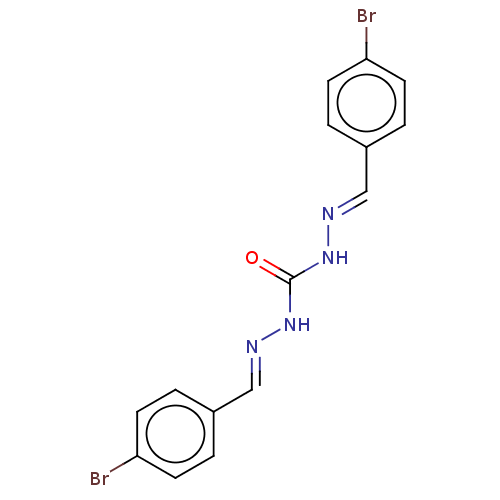 (N'',N'''-Bis[(E)-(4-bromophenyl)methylidene]ca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Karachi; Government College University Faisalabad | Assay Description Kinetic studies were performed by using different concentration of inhibitors over different concentrations of substrate (4-NPA) such as 0.175, 0.35,... | Bioorg Chem 72: 89-101 (2017) Article DOI: 10.1016/j.bioorg.2017.03.014 BindingDB Entry DOI: 10.7270/Q2125RHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM222139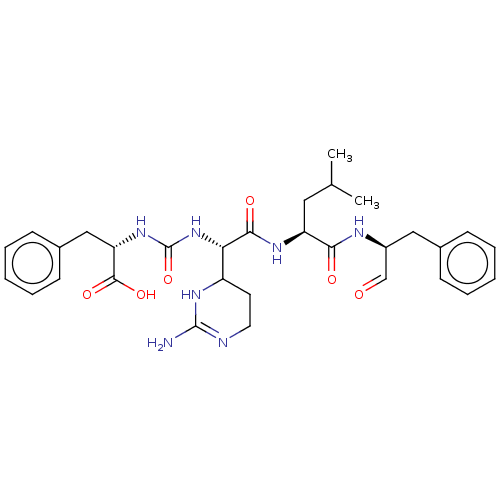 (Chymostatin | US11859014, Compound chymostatin) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi | Assay Description The change in optical density per minute (OD/min) was obtained by incorporating various concentrations of compounds over a range of substrate (SPpNA)... | Bioorg Chem 70: 210-221 (2017) Article DOI: 10.1016/j.bioorg.2017.01.001 BindingDB Entry DOI: 10.7270/Q2QR4W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM222430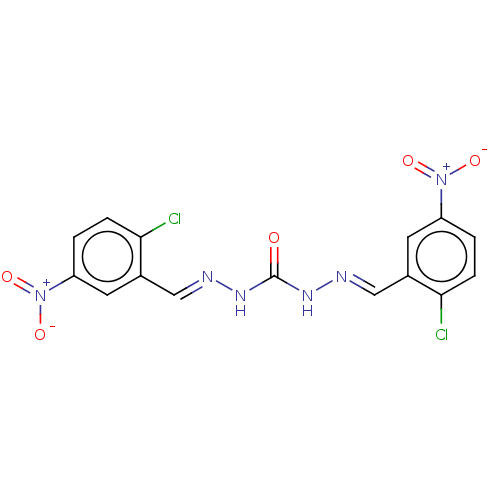 (N'',N'''-Bis[(E)-(2-chloro-5-nitrophenyl)methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Karachi; Government College University Faisalabad | Assay Description Kinetic studies were performed by using different concentration of inhibitors over different concentrations of substrate (4-NPA) such as 0.175, 0.35,... | Bioorg Chem 72: 89-101 (2017) Article DOI: 10.1016/j.bioorg.2017.03.014 BindingDB Entry DOI: 10.7270/Q2125RHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Bos taurus) | BDBM222433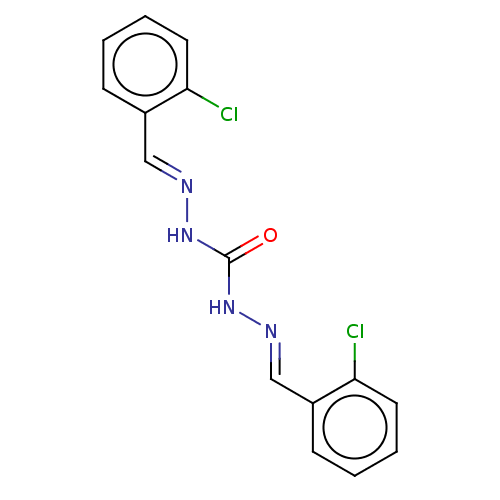 (N'',N'''-Bis[(E)-(2-chlorophenyl)methylidene]c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 4.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
University of Karachi; Government College University Faisalabad | Assay Description Kinetic studies were performed by using different concentration of inhibitors over different concentrations of substrate (4-NPA) such as 0.175, 0.35,... | Bioorg Chem 72: 89-101 (2017) Article DOI: 10.1016/j.bioorg.2017.03.014 BindingDB Entry DOI: 10.7270/Q2125RHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50289012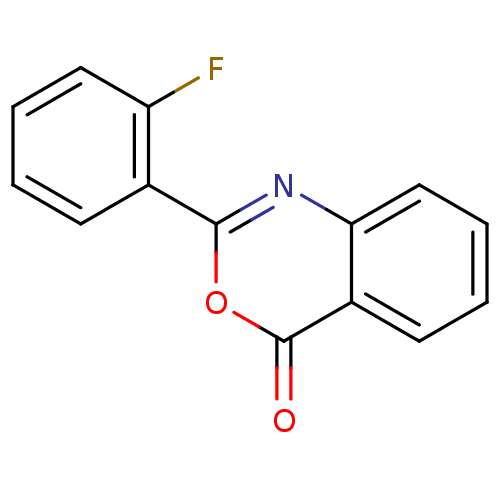 (2-(2-Fluoro-phenyl)-benzo[d][1,3]oxazin-4-one | 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi | Assay Description The change in optical density per minute (OD/min) was obtained by incorporating various concentrations of compounds over a range of substrate (SPpNA)... | Bioorg Chem 70: 210-221 (2017) Article DOI: 10.1016/j.bioorg.2017.01.001 BindingDB Entry DOI: 10.7270/Q2QR4W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50448175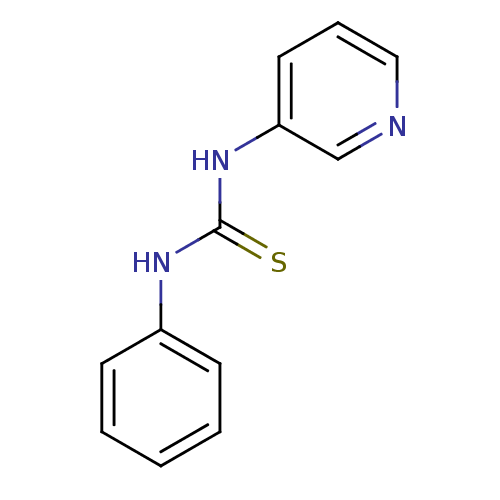 (CHEMBL3120552) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Karachi Curated by ChEMBL | Assay Description Competitive inhibition of jack bean urease using urea as substrate assessed as ammonia production after 15 mins by Lineweaver-Burk/Dixon plot analysi... | Eur J Med Chem 74: 314-23 (2014) Article DOI: 10.1016/j.ejmech.2014.01.001 BindingDB Entry DOI: 10.7270/Q2VH5QBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50077011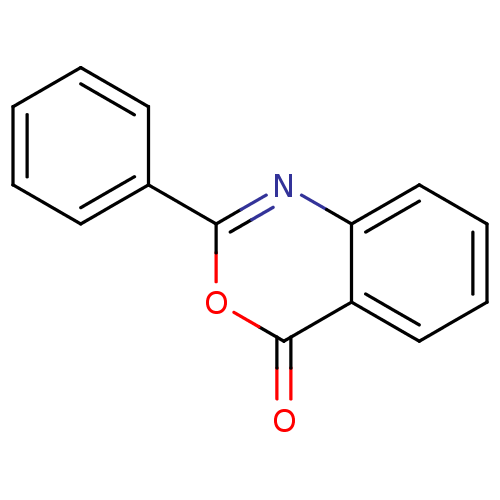 (2-Phenyl-4H-3,1-benzoxazin-4-one (3) | 2-Phenyl-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi | Assay Description The change in optical density per minute (OD/min) was obtained by incorporating various concentrations of compounds over a range of substrate (SPpNA)... | Bioorg Chem 70: 210-221 (2017) Article DOI: 10.1016/j.bioorg.2017.01.001 BindingDB Entry DOI: 10.7270/Q2QR4W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50289006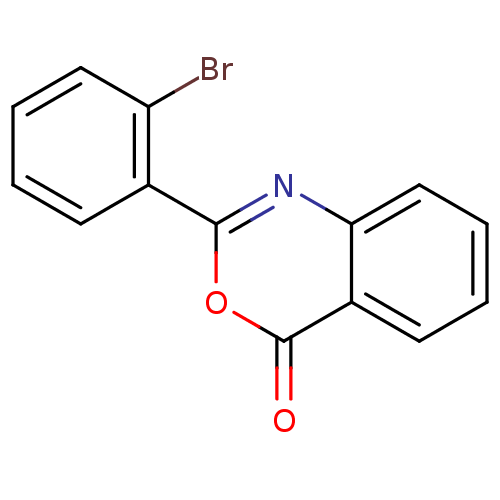 (2-(2-Bromo-phenyl)-benzo[d][1,3]oxazin-4-one | 2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi | Assay Description The change in optical density per minute (OD/min) was obtained by incorporating various concentrations of compounds over a range of substrate (SPpNA)... | Bioorg Chem 70: 210-221 (2017) Article DOI: 10.1016/j.bioorg.2017.01.001 BindingDB Entry DOI: 10.7270/Q2QR4W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM222117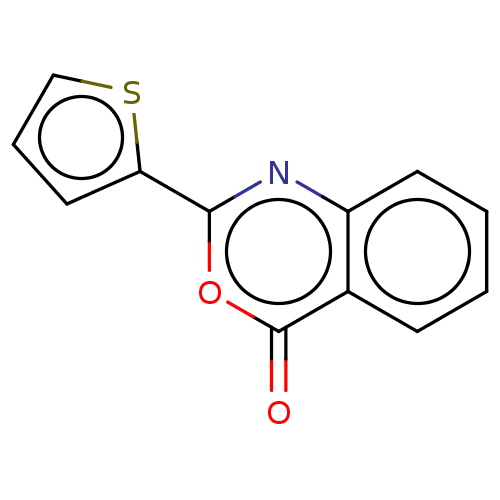 (2-(2-Thienyl)-4H-3,1-benzoxazin-4-one (4) | US1158...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi | Assay Description The change in optical density per minute (OD/min) was obtained by incorporating various concentrations of compounds over a range of substrate (SPpNA)... | Bioorg Chem 70: 210-221 (2017) Article DOI: 10.1016/j.bioorg.2017.01.001 BindingDB Entry DOI: 10.7270/Q2QR4W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM222124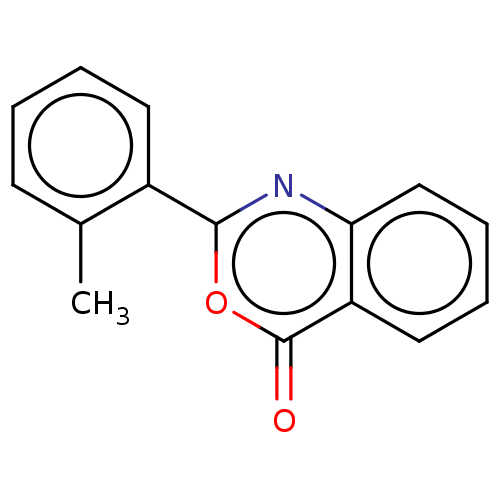 (2-(2-Methylphenyl)-4H-3,1-benzoxazin-4-one (13)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi | Assay Description The change in optical density per minute (OD/min) was obtained by incorporating various concentrations of compounds over a range of substrate (SPpNA)... | Bioorg Chem 70: 210-221 (2017) Article DOI: 10.1016/j.bioorg.2017.01.001 BindingDB Entry DOI: 10.7270/Q2QR4W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50448176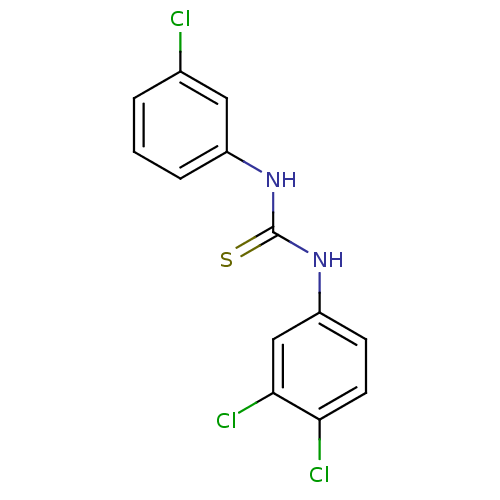 (CHEMBL3120570) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Karachi Curated by ChEMBL | Assay Description Competitive inhibition of jack bean urease using urea as substrate assessed as ammonia production after 15 mins by Lineweaver-Burk/Dixon plot analysi... | Eur J Med Chem 74: 314-23 (2014) Article DOI: 10.1016/j.ejmech.2014.01.001 BindingDB Entry DOI: 10.7270/Q2VH5QBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50448174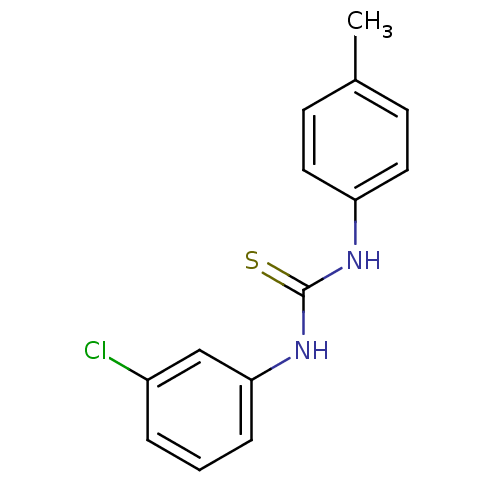 (CHEMBL3120548) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Karachi Curated by ChEMBL | Assay Description Non-competitive inhibition of jack bean urease using urea as substrate assessed as ammonia production after 15 mins by Lineweaver-Burk/Dixon plot ana... | Eur J Med Chem 74: 314-23 (2014) Article DOI: 10.1016/j.ejmech.2014.01.001 BindingDB Entry DOI: 10.7270/Q2VH5QBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50289000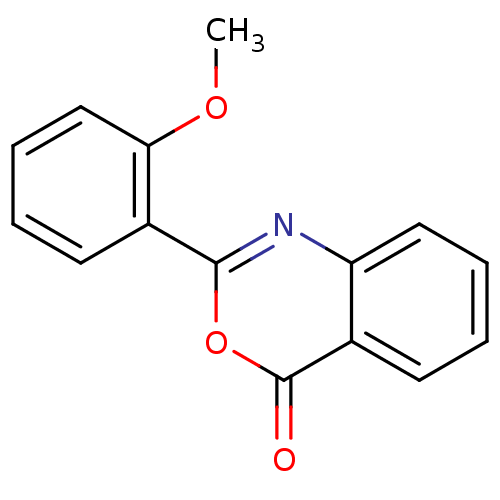 (2-(2-Methoxy-phenyl)-benzo[d][1,3]oxazin-4-one | 2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi | Assay Description The change in optical density per minute (OD/min) was obtained by incorporating various concentrations of compounds over a range of substrate (SPpNA)... | Bioorg Chem 70: 210-221 (2017) Article DOI: 10.1016/j.bioorg.2017.01.001 BindingDB Entry DOI: 10.7270/Q2QR4W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM222118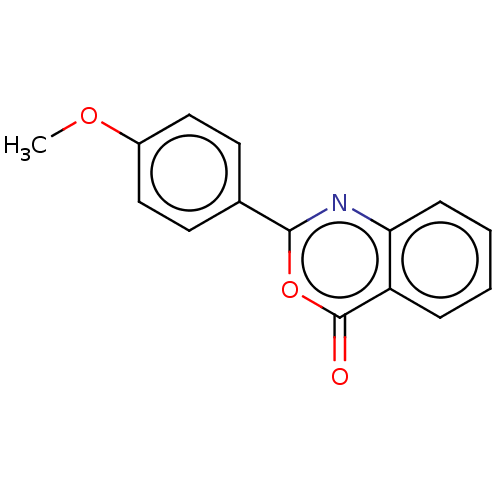 (2-(4-Methoxyphenyl)-4H-3,1-benzoxazin-4-one (5)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi | Assay Description The change in optical density per minute (OD/min) was obtained by incorporating various concentrations of compounds over a range of substrate (SPpNA)... | Bioorg Chem 70: 210-221 (2017) Article DOI: 10.1016/j.bioorg.2017.01.001 BindingDB Entry DOI: 10.7270/Q2QR4W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50448177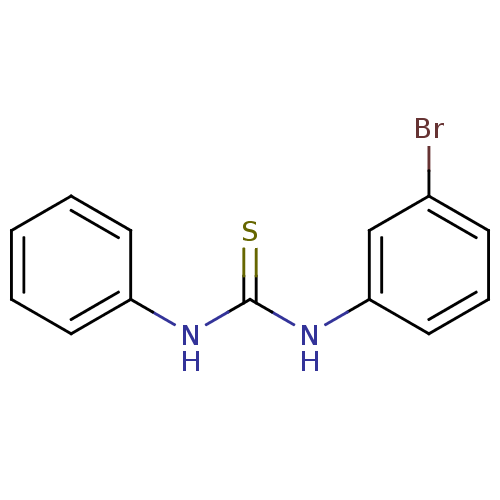 (CHEMBL1576403) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Karachi Curated by ChEMBL | Assay Description Mixed-type inhibition of jack bean urease using urea as substrate assessed as ammonia production after 15 mins by Lineweaver-Burk/Dixon plot analysis | Eur J Med Chem 74: 314-23 (2014) Article DOI: 10.1016/j.ejmech.2014.01.001 BindingDB Entry DOI: 10.7270/Q2VH5QBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50448178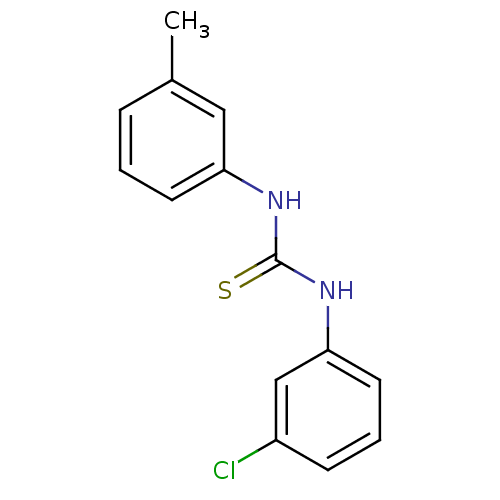 (CHEMBL3120574) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Karachi Curated by ChEMBL | Assay Description Mixed-type inhibition of jack bean urease using urea as substrate assessed as ammonia production after 15 mins by Lineweaver-Burk/Dixon plot analysis | Eur J Med Chem 74: 314-23 (2014) Article DOI: 10.1016/j.ejmech.2014.01.001 BindingDB Entry DOI: 10.7270/Q2VH5QBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM222121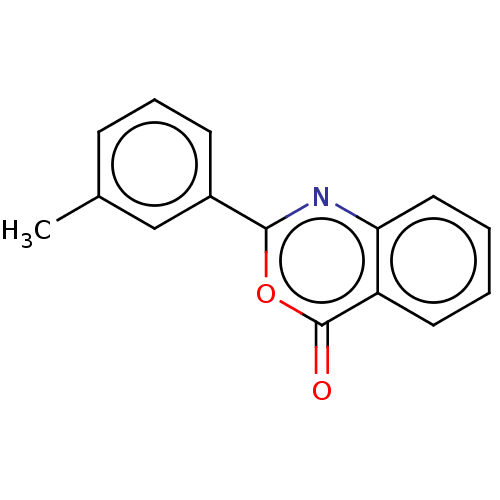 (2-(3-Methylphenyl)-4H-3,1-benzoxazin-4-one (10)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi | Assay Description The change in optical density per minute (OD/min) was obtained by incorporating various concentrations of compounds over a range of substrate (SPpNA)... | Bioorg Chem 70: 210-221 (2017) Article DOI: 10.1016/j.bioorg.2017.01.001 BindingDB Entry DOI: 10.7270/Q2QR4W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM222119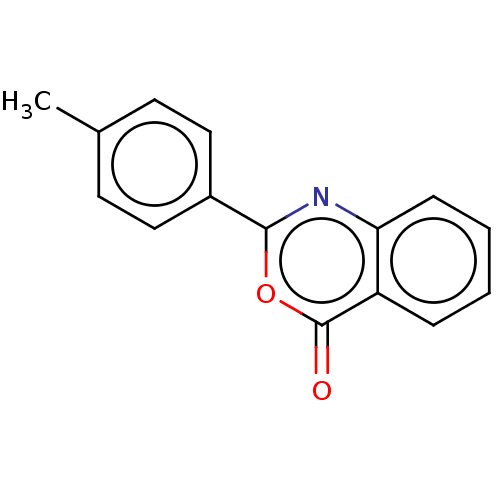 (2-(4-Methylphenyl)-4H-3,1-benzoxazin-4-one (7)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi | Assay Description The change in optical density per minute (OD/min) was obtained by incorporating various concentrations of compounds over a range of substrate (SPpNA)... | Bioorg Chem 70: 210-221 (2017) Article DOI: 10.1016/j.bioorg.2017.01.001 BindingDB Entry DOI: 10.7270/Q2QR4W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50448179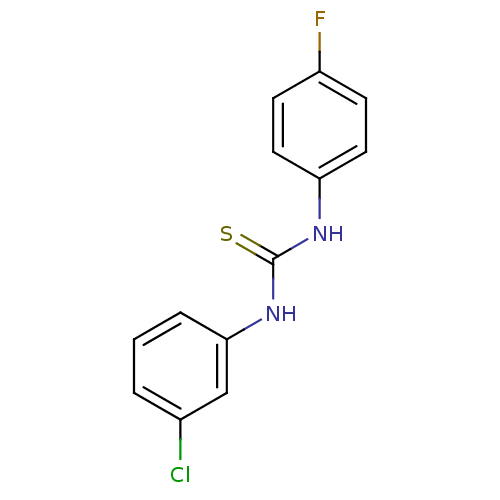 (CHEMBL1544729) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Karachi Curated by ChEMBL | Assay Description Mixed-type inhibition of jack bean urease using urea as substrate assessed as ammonia production after 15 mins by Lineweaver-Burk/Dixon plot analysis | Eur J Med Chem 74: 314-23 (2014) Article DOI: 10.1016/j.ejmech.2014.01.001 BindingDB Entry DOI: 10.7270/Q2VH5QBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM222123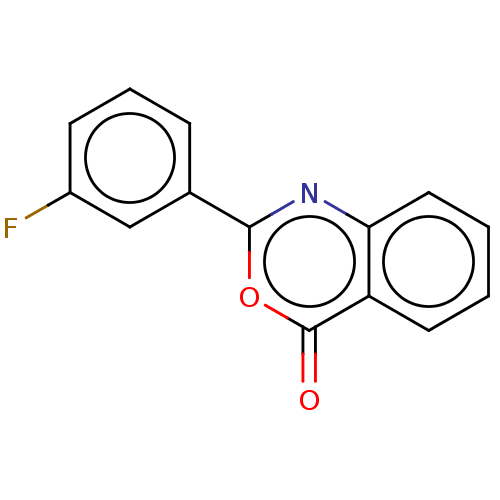 (2-(3-Fluorophenyl)-4H-3,1-benzoxazin-4-one (12)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi | Assay Description The change in optical density per minute (OD/min) was obtained by incorporating various concentrations of compounds over a range of substrate (SPpNA)... | Bioorg Chem 70: 210-221 (2017) Article DOI: 10.1016/j.bioorg.2017.01.001 BindingDB Entry DOI: 10.7270/Q2QR4W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50448184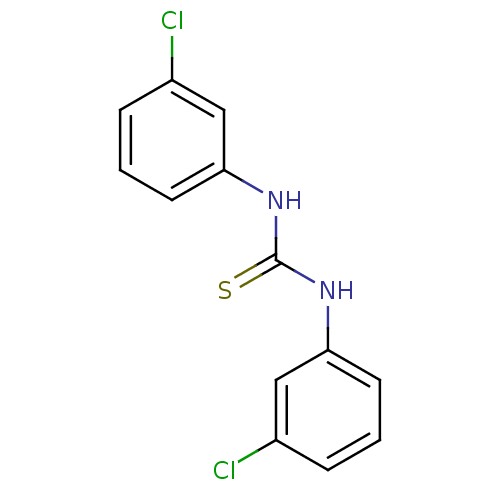 (CHEMBL3120558) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Karachi Curated by ChEMBL | Assay Description Mixed-type inhibition of jack bean urease using urea as substrate assessed as ammonia production after 15 mins by Lineweaver-Burk/Dixon plot analysis | Eur J Med Chem 74: 314-23 (2014) Article DOI: 10.1016/j.ejmech.2014.01.001 BindingDB Entry DOI: 10.7270/Q2VH5QBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50448182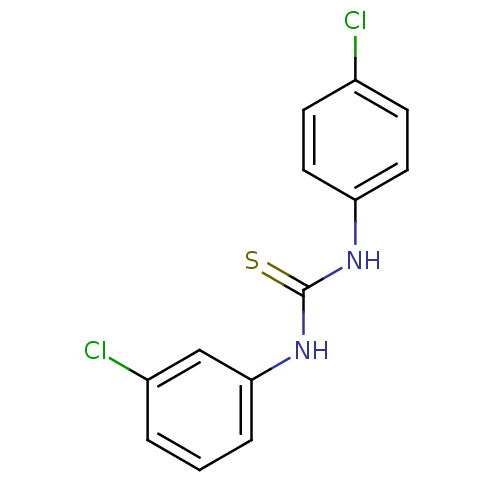 (CHEMBL3120560) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Karachi Curated by ChEMBL | Assay Description Mixed-type inhibition of jack bean urease using urea as substrate assessed as ammonia production after 15 mins by Lineweaver-Burk/Dixon plot analysis | Eur J Med Chem 74: 314-23 (2014) Article DOI: 10.1016/j.ejmech.2014.01.001 BindingDB Entry DOI: 10.7270/Q2VH5QBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50448180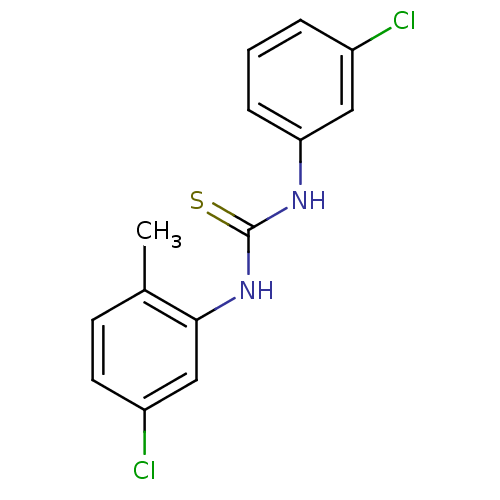 (CHEMBL3120571) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Karachi Curated by ChEMBL | Assay Description Mixed-type inhibition of jack bean urease using urea as substrate assessed as ammonia production after 15 mins by Lineweaver-Burk/Dixon plot analysis | Eur J Med Chem 74: 314-23 (2014) Article DOI: 10.1016/j.ejmech.2014.01.001 BindingDB Entry DOI: 10.7270/Q2VH5QBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50448185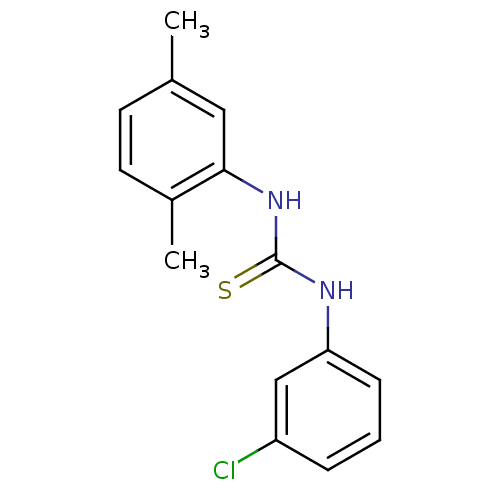 (CHEMBL3120566) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Karachi Curated by ChEMBL | Assay Description Mixed-type inhibition of jack bean urease using urea as substrate assessed as ammonia production after 15 mins by Lineweaver-Burk/Dixon plot analysis | Eur J Med Chem 74: 314-23 (2014) Article DOI: 10.1016/j.ejmech.2014.01.001 BindingDB Entry DOI: 10.7270/Q2VH5QBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50229993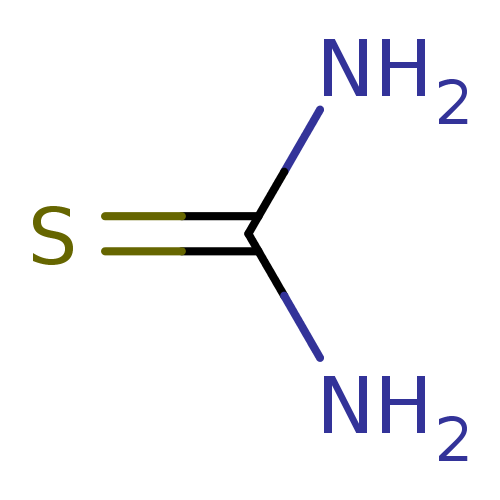 (2-thiourea | CHEMBL260876 | Thiocarbamid | Thiohar...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Karachi Curated by ChEMBL | Assay Description Competitive inhibition of jack bean urease using urea as substrate assessed as ammonia production after 15 mins by Lineweaver-Burk/Dixon plot analysi... | Eur J Med Chem 74: 314-23 (2014) Article DOI: 10.1016/j.ejmech.2014.01.001 BindingDB Entry DOI: 10.7270/Q2VH5QBM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM33703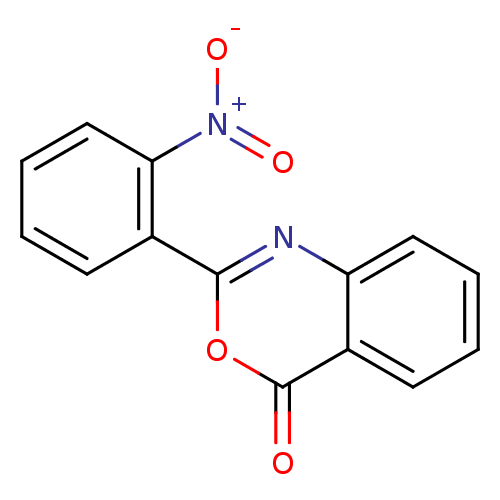 (2-(2-Nitro-phenyl)-benzo[d][1,3]oxazin-4-one | 2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi | Assay Description The change in optical density per minute (OD/min) was obtained by incorporating various concentrations of compounds over a range of substrate (SPpNA)... | Bioorg Chem 70: 210-221 (2017) Article DOI: 10.1016/j.bioorg.2017.01.001 BindingDB Entry DOI: 10.7270/Q2QR4W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM222125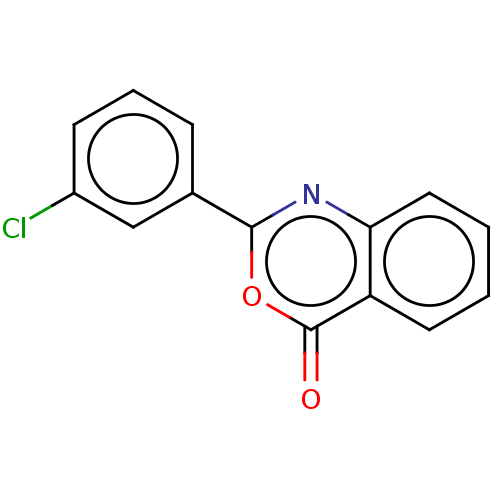 (2-(3-Chlorophenyl)-4H-3,1-benzoxazin-4-one (14)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi | Assay Description The change in optical density per minute (OD/min) was obtained by incorporating various concentrations of compounds over a range of substrate (SPpNA)... | Bioorg Chem 70: 210-221 (2017) Article DOI: 10.1016/j.bioorg.2017.01.001 BindingDB Entry DOI: 10.7270/Q2QR4W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50018905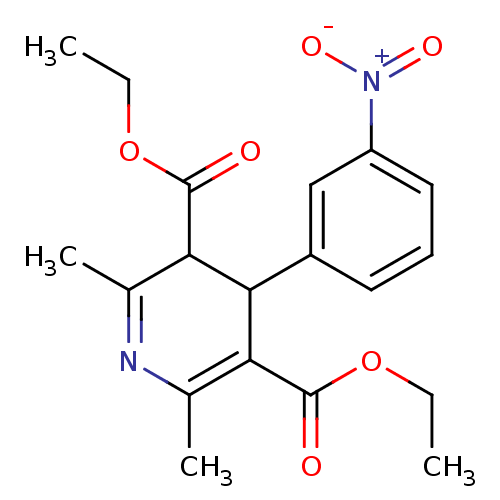 (2,6-Dimethyl-4-(3-nitro-phenyl)-1,4-dihydro-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Non-competitive inhibition of Saccharomyces cerevisiae alpha-glucosidase using p-nitrophenyl alpha-D-glucopyranoside as substrate preincubated for 15... | Eur J Med Chem 95: 199-209 (2015) Article DOI: 10.1016/j.ejmech.2015.03.018 BindingDB Entry DOI: 10.7270/Q2K075Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM222122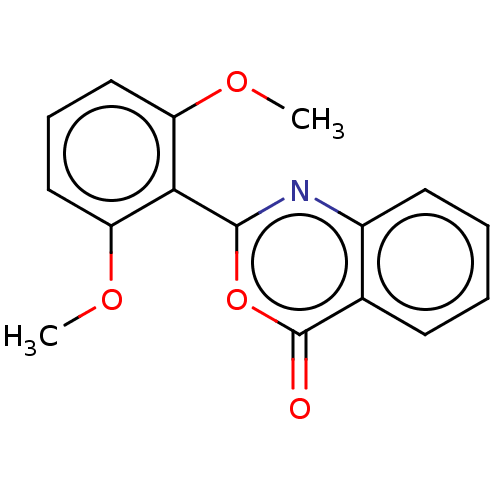 (2-(2,6-Dimethoxyphenyl)-4H-3,1-benzoxazin-4-one (1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi | Assay Description The change in optical density per minute (OD/min) was obtained by incorporating various concentrations of compounds over a range of substrate (SPpNA)... | Bioorg Chem 70: 210-221 (2017) Article DOI: 10.1016/j.bioorg.2017.01.001 BindingDB Entry DOI: 10.7270/Q2QR4W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM222120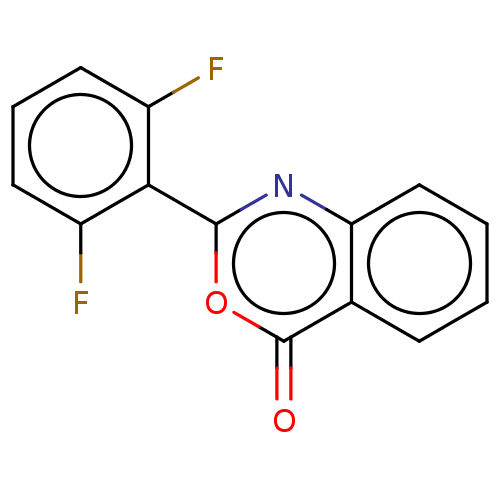 (2-(2,6-Difluorophenyl)-4H-3,1-benzoxazin-4-one (8)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi | Assay Description The change in optical density per minute (OD/min) was obtained by incorporating various concentrations of compounds over a range of substrate (SPpNA)... | Bioorg Chem 70: 210-221 (2017) Article DOI: 10.1016/j.bioorg.2017.01.001 BindingDB Entry DOI: 10.7270/Q2QR4W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oligo-1,6-glucosidase IMA1 (Saccharomyces cerevisiae S288c (Baker's yeast)) | BDBM50074020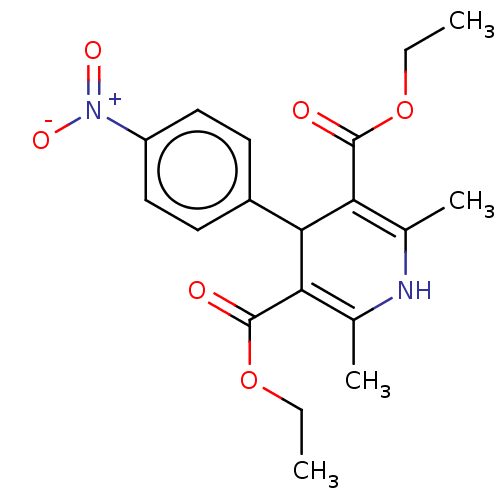 (CHEMBL1099206) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Competitive inhibition of Saccharomyces cerevisiae alpha-glucosidase using p-nitrophenyl alpha-D-glucopyranoside as substrate preincubated for 15 min... | Eur J Med Chem 95: 199-209 (2015) Article DOI: 10.1016/j.ejmech.2015.03.018 BindingDB Entry DOI: 10.7270/Q2K075Z5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM222127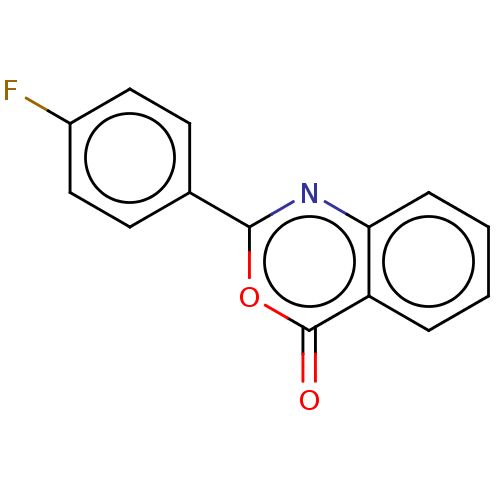 (2-(4-Fluorophenyl)-4H-3,1-benzoxazin-4-one (16)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 9.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi | Assay Description The change in optical density per minute (OD/min) was obtained by incorporating various concentrations of compounds over a range of substrate (SPpNA)... | Bioorg Chem 70: 210-221 (2017) Article DOI: 10.1016/j.bioorg.2017.01.001 BindingDB Entry DOI: 10.7270/Q2QR4W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50304608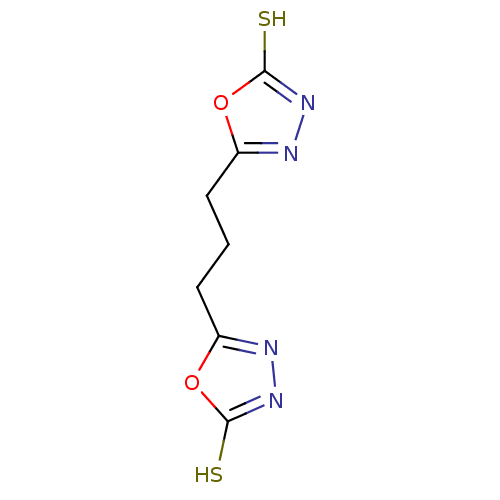 (5,5'-(propane-1,3-diyl)bis(1,3,4-oxadiazole-2(3H)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Noncompetitive inhibition of human recombinant NPP1 by Dixon plot analysis | Bioorg Med Chem 17: 7816-22 (2009) Article DOI: 10.1016/j.bmc.2009.09.011 BindingDB Entry DOI: 10.7270/Q2ZC82X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM222126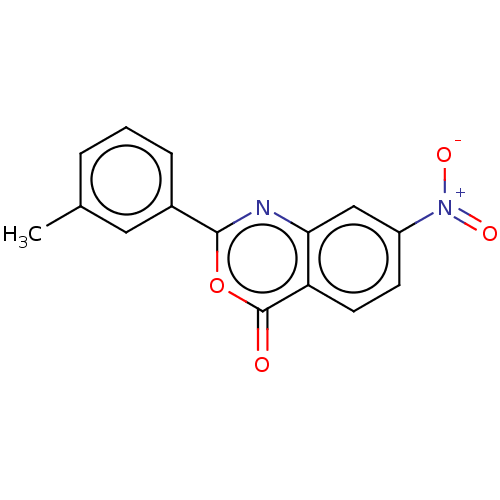 (2-(3-Methylphenyl)-7-nitro-4H-3,1-benzoxazin-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.62E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi | Assay Description The change in optical density per minute (OD/min) was obtained by incorporating various concentrations of compounds over a range of substrate (SPpNA)... | Bioorg Chem 70: 210-221 (2017) Article DOI: 10.1016/j.bioorg.2017.01.001 BindingDB Entry DOI: 10.7270/Q2QR4W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM222128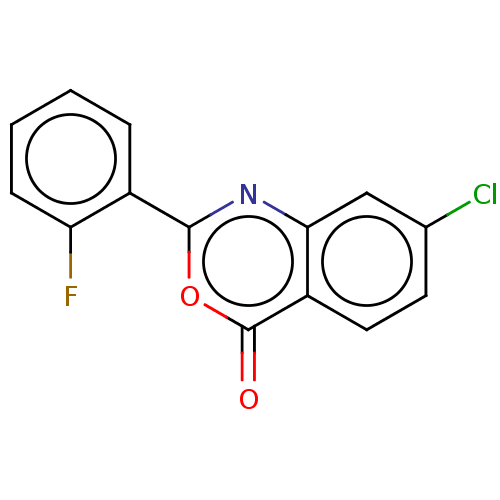 (7-Chloro-2-(2-fluorophenyl)-4H-3,1-benzoxazin-4-on...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 3.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi | Assay Description The change in optical density per minute (OD/min) was obtained by incorporating various concentrations of compounds over a range of substrate (SPpNA)... | Bioorg Chem 70: 210-221 (2017) Article DOI: 10.1016/j.bioorg.2017.01.001 BindingDB Entry DOI: 10.7270/Q2QR4W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50449429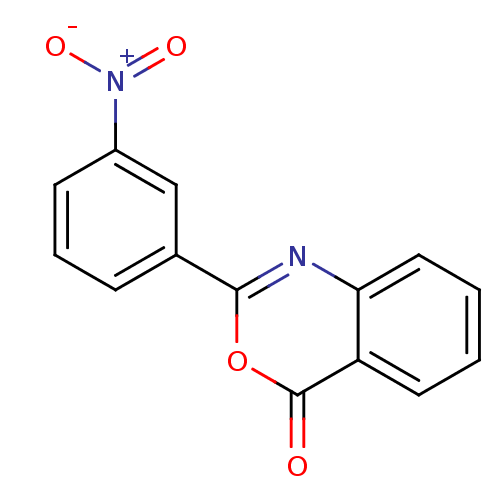 (2-(3-Nitrophenyl)-4H-3,1-benzoxazin-4-one (18) | C...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.41E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi | Assay Description The change in optical density per minute (OD/min) was obtained by incorporating various concentrations of compounds over a range of substrate (SPpNA)... | Bioorg Chem 70: 210-221 (2017) Article DOI: 10.1016/j.bioorg.2017.01.001 BindingDB Entry DOI: 10.7270/Q2QR4W00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50304607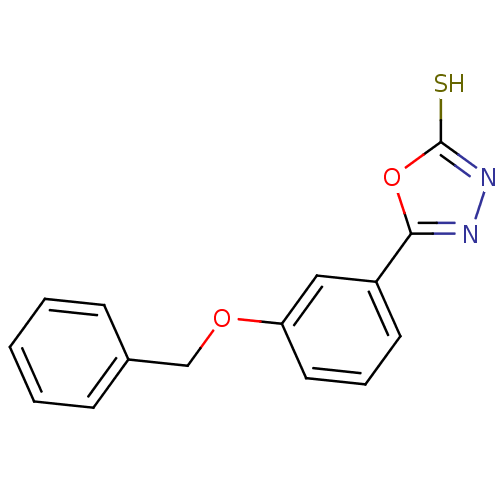 (5-(3-(benzyloxy)phenyl)-1,3,4-oxadiazole-2(3H)-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Noncompetitive inhibition of human recombinant NPP1 by Dixon plot analysis | Bioorg Med Chem 17: 7816-22 (2009) Article DOI: 10.1016/j.bmc.2009.09.011 BindingDB Entry DOI: 10.7270/Q2ZC82X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50304609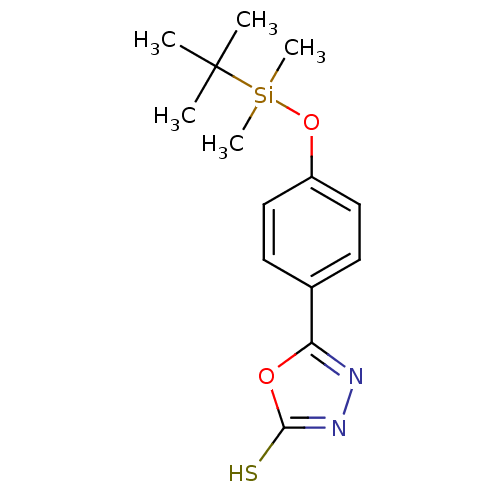 (5-[4-t-Butyldimethylsilyloxy phenyl]-1,3,4-oxadiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Noncompetitive inhibition of human recombinant NPP1 by Dixon plot analysis | Bioorg Med Chem 17: 7816-22 (2009) Article DOI: 10.1016/j.bmc.2009.09.011 BindingDB Entry DOI: 10.7270/Q2ZC82X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50304606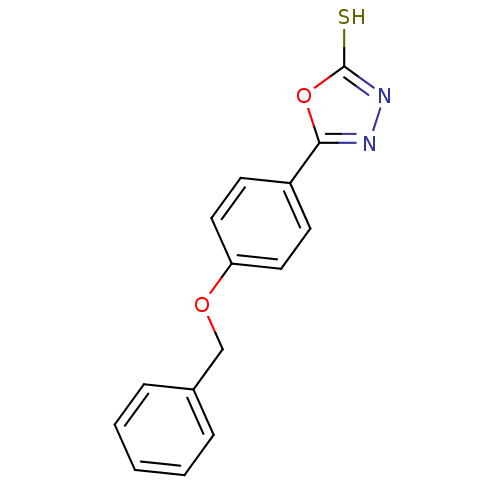 (5-(4-(benzyloxy)phenyl)-1,3,4-oxadiazole-2(3H)-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Noncompetitive inhibition of human recombinant NPP1 by Dixon plot analysis | Bioorg Med Chem 17: 7816-22 (2009) Article DOI: 10.1016/j.bmc.2009.09.011 BindingDB Entry DOI: 10.7270/Q2ZC82X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 1 (Homo sapiens (Human)) | BDBM50304605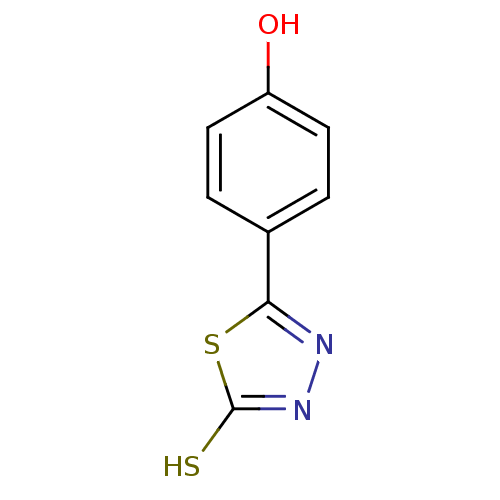 (5-(4-hydroxyphenyl)-1,3,4-thiadiazole-2(3H)-thione...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Noncompetitive inhibition of human recombinant NPP1 by Dixon plot analysis | Bioorg Med Chem 17: 7816-22 (2009) Article DOI: 10.1016/j.bmc.2009.09.011 BindingDB Entry DOI: 10.7270/Q2ZC82X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50532145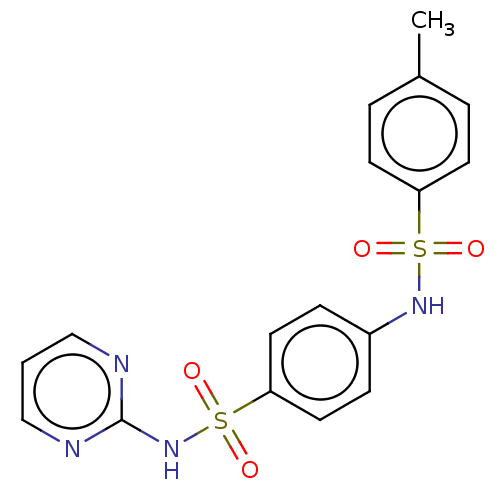 (CHEMBL1549559) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as reduction in ammonia production using urea as substrate after 15 mins by indophenol method | Bioorg Med Chem 27: 1009-1022 (2019) Article DOI: 10.1016/j.bmc.2019.01.043 BindingDB Entry DOI: 10.7270/Q2FN19P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Escherichia coli) | BDBM20079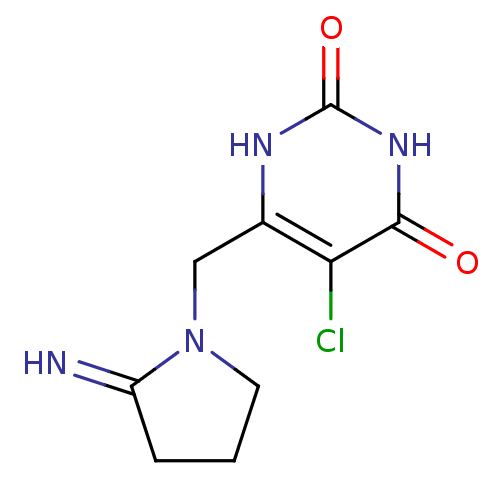 (5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of Escherichia coli thymidine phosphorylase | Bioorg Med Chem 17: 2983-8 (2009) Article DOI: 10.1016/j.bmc.2009.03.020 BindingDB Entry DOI: 10.7270/Q2GX4FC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM17638 (2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi Curated by ChEMBL | Assay Description Inhibition of COX1 | Eur J Med Chem 45: 4058-64 (2010) Article DOI: 10.1016/j.ejmech.2010.05.065 BindingDB Entry DOI: 10.7270/Q2GQ6ZQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucuronidase (Homo sapiens (Human)) | BDBM50284071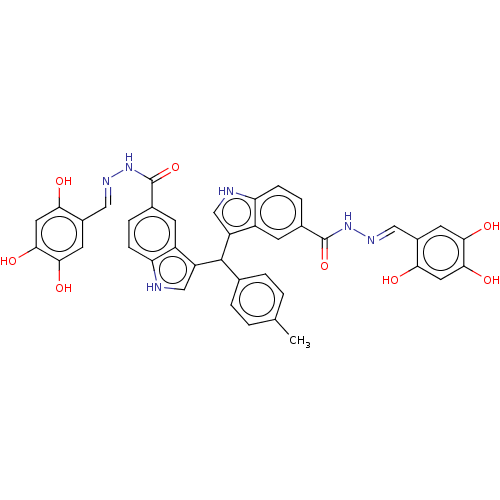 (CHEMBL4165927) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Imam Abdulrahman Bin Faisal University Curated by ChEMBL | Assay Description Inhibition of human beta-glucuronidase | Eur J Med Chem 143: 1757-1767 (2018) Article DOI: 10.1016/j.ejmech.2017.10.071 BindingDB Entry DOI: 10.7270/Q23N25X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucuronidase (Homo sapiens (Human)) | BDBM50284029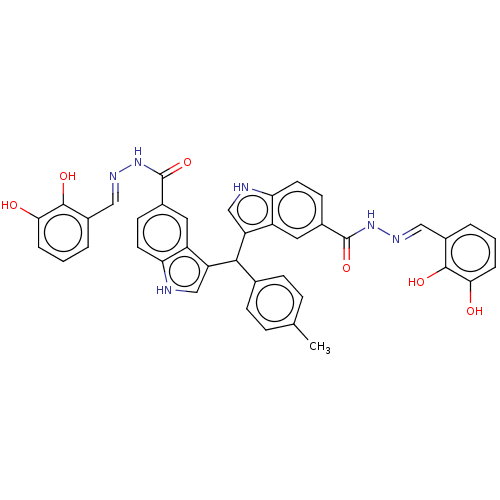 (CHEMBL4168635) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Imam Abdulrahman Bin Faisal University Curated by ChEMBL | Assay Description Inhibition of human beta-glucuronidase | Eur J Med Chem 143: 1757-1767 (2018) Article DOI: 10.1016/j.ejmech.2017.10.071 BindingDB Entry DOI: 10.7270/Q23N25X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucuronidase (Homo sapiens (Human)) | BDBM50284055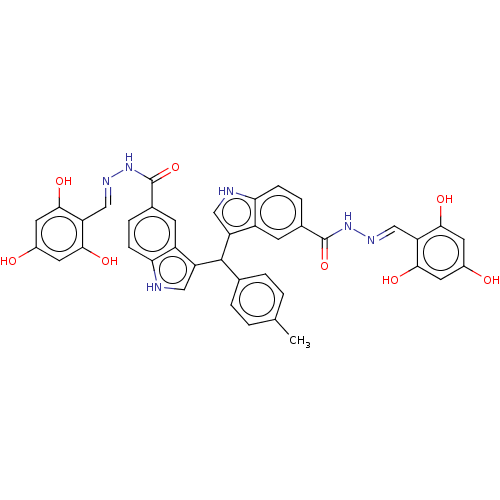 (CHEMBL4177204) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Imam Abdulrahman Bin Faisal University Curated by ChEMBL | Assay Description Inhibition of human beta-glucuronidase | Eur J Med Chem 143: 1757-1767 (2018) Article DOI: 10.1016/j.ejmech.2017.10.071 BindingDB Entry DOI: 10.7270/Q23N25X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-glucuronidase (Homo sapiens (Human)) | BDBM50284072 (CHEMBL4173852) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Imam Abdulrahman Bin Faisal University Curated by ChEMBL | Assay Description Inhibition of human beta-glucuronidase | Eur J Med Chem 143: 1757-1767 (2018) Article DOI: 10.1016/j.ejmech.2017.10.071 BindingDB Entry DOI: 10.7270/Q23N25X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 521 total ) | Next | Last >> |