

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50345693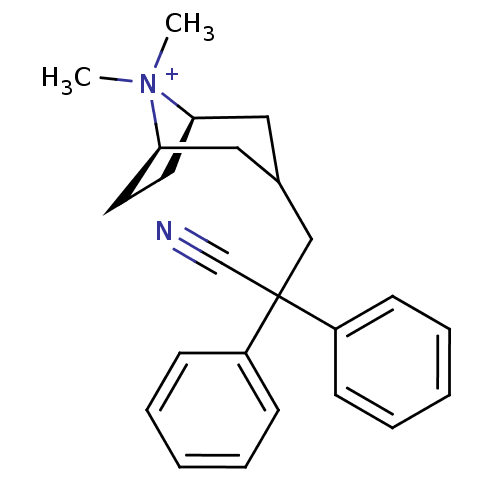 ((3-endo)-3-(2-Cyano-2,2-diphenylethyl)-8,8-dimethy...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human muscarinic M1 receptor expressed in CHO cells by scintillation proximity assay | J Med Chem 52: 5241-52 (2010) Article DOI: 10.1021/jm900736e BindingDB Entry DOI: 10.7270/Q2PK0H54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50345693 ((3-endo)-3-(2-Cyano-2,2-diphenylethyl)-8,8-dimethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay | J Med Chem 52: 5241-52 (2010) Article DOI: 10.1021/jm900736e BindingDB Entry DOI: 10.7270/Q2PK0H54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50381654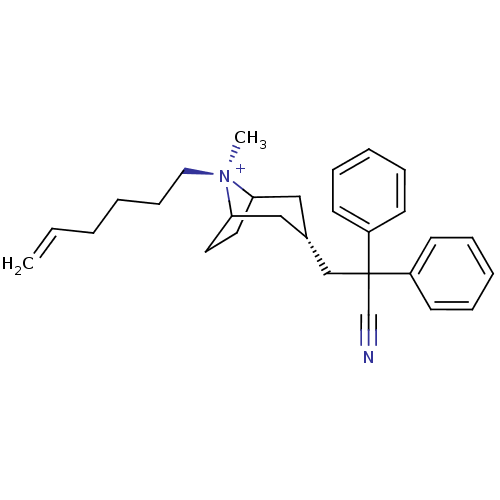 (CHEMBL2023764) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl scopolamine from muscarinic acetylcholine M2 receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 22: 3366-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.015 BindingDB Entry DOI: 10.7270/Q2C82B9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50381654 (CHEMBL2023764) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl scopolamine from muscarinic acetylcholine M1 receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 22: 3366-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.015 BindingDB Entry DOI: 10.7270/Q2C82B9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50381654 (CHEMBL2023764) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl scopolamine from muscarinic acetylcholine M3 receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 22: 3366-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.015 BindingDB Entry DOI: 10.7270/Q2C82B9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50345693 ((3-endo)-3-(2-Cyano-2,2-diphenylethyl)-8,8-dimethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human muscarinic M2 receptor expressed in CHO cells by scintillation proximity assay | J Med Chem 52: 5241-52 (2010) Article DOI: 10.1021/jm900736e BindingDB Entry DOI: 10.7270/Q2PK0H54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50106479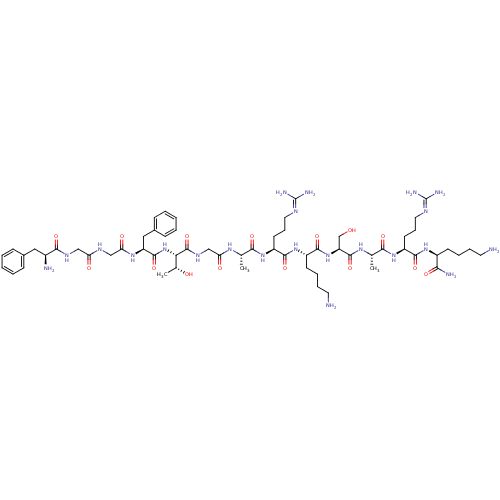 (CHEMBL384755 | FGGFTGARKSARK | H-FGGFTGARKSARK-NH2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louisiana State University Curated by PDSP Ki Database | J Pharmacol Exp Ther 314: 652-60 (2005) Article DOI: 10.1124/jpet.105.083436 BindingDB Entry DOI: 10.7270/Q2H41Q0Q | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50412340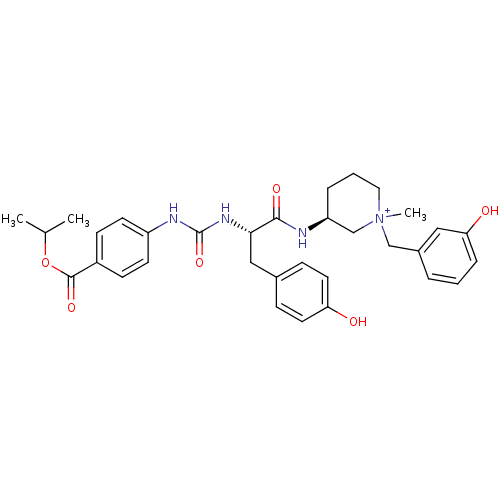 (CHEMBL540359) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human muscarinic acetylcholine M1 receptor expressed in CHO cells by scintillation proximity assay | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50345692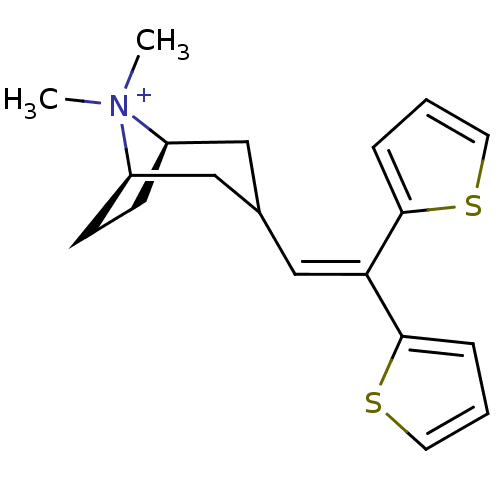 ((3-endo)-3-(2,2-Di-2-thienylethenyl)-8,8-dimethyl-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human muscarinic M1 receptor expressed in CHO cells by scintillation proximity assay | J Med Chem 52: 5241-52 (2010) Article DOI: 10.1021/jm900736e BindingDB Entry DOI: 10.7270/Q2PK0H54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50345692 ((3-endo)-3-(2,2-Di-2-thienylethenyl)-8,8-dimethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay | J Med Chem 52: 5241-52 (2010) Article DOI: 10.1021/jm900736e BindingDB Entry DOI: 10.7270/Q2PK0H54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM86695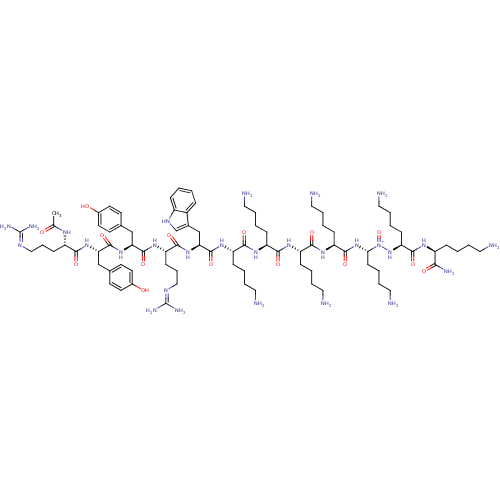 (Ac-RYYRWKKKKKKK-NH2 | ZP120) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louisiana State University Curated by PDSP Ki Database | J Pharmacol Exp Ther 314: 652-60 (2005) Article DOI: 10.1124/jpet.105.083436 BindingDB Entry DOI: 10.7270/Q2H41Q0Q | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50345692 ((3-endo)-3-(2,2-Di-2-thienylethenyl)-8,8-dimethyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human muscarinic M2 receptor expressed in CHO cells by scintillation proximity assay | J Med Chem 52: 5241-52 (2010) Article DOI: 10.1021/jm900736e BindingDB Entry DOI: 10.7270/Q2PK0H54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50053265 ((6aR,9R,10aR)-3-((Z)-Hept-1-enyl)-9-hydroxymethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace specifically bound [3H]CP-55940 from a Cannabinoid receptor 1 enriched rat brain microsome prepara... | J Med Chem 39: 3790-6 (1996) Article DOI: 10.1021/jm950934b BindingDB Entry DOI: 10.7270/Q2TT4RM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50053266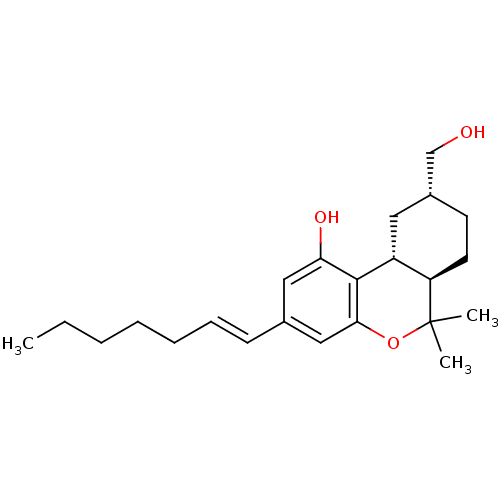 ((6aR,9R,10aR)-3-((E)-Hept-1-enyl)-9-hydroxymethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace specifically bound [3H]CP-55940 from a Cannabinoid receptor 1 enriched rat brain microsome prepara... | J Med Chem 39: 3790-6 (1996) Article DOI: 10.1021/jm950934b BindingDB Entry DOI: 10.7270/Q2TT4RM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50066705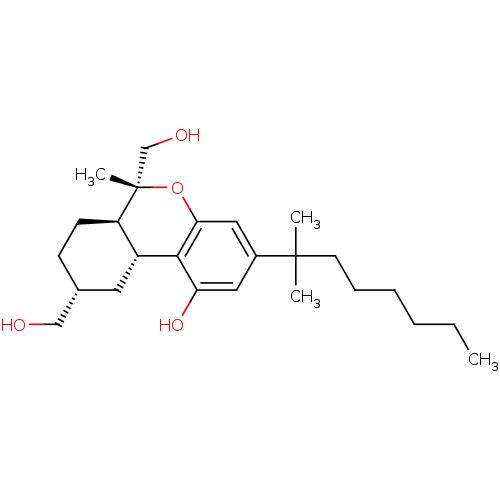 ((6R,6aR,9R,10aR)-3-(1,1-Dimethyl-heptyl)-6,9-bis-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Binding affinity of the compound to Cannabinoid receptor 2 from mouse spleen was measured using [3H]CP-55,940 as radioligand | J Med Chem 41: 3596-608 (1998) Article DOI: 10.1021/jm960677q BindingDB Entry DOI: 10.7270/Q26H4GHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50066705 ((6R,6aR,9R,10aR)-3-(1,1-Dimethyl-heptyl)-6,9-bis-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Binding affinity of the compound to Cannabinoid receptor 1 from rat forebrain synaptosomal membranes was measured using [3H]CP-55,940 as radioligand | J Med Chem 41: 3596-608 (1998) Article DOI: 10.1021/jm960677q BindingDB Entry DOI: 10.7270/Q26H4GHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50066709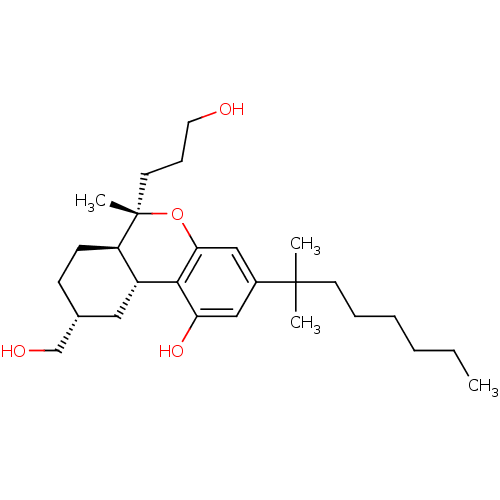 ((6S,6aR,9R,10aR)-3-(1,1-Dimethyl-heptyl)-9-hydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Binding affinity of the compound to Cannabinoid receptor 1 from rat forebrain synaptosomal membranes was measured using [3H]CP-55,940 as radioligand | J Med Chem 41: 3596-608 (1998) Article DOI: 10.1021/jm960677q BindingDB Entry DOI: 10.7270/Q26H4GHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50066710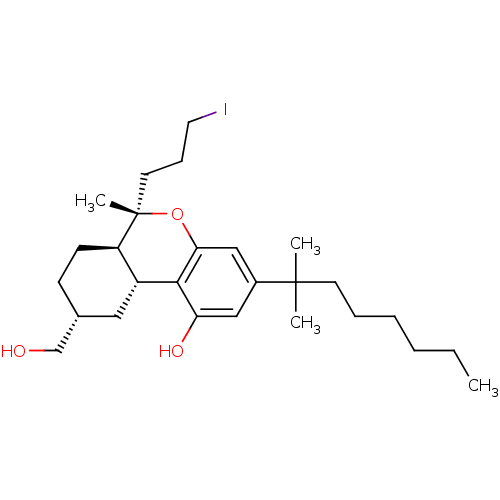 ((6S,6aR,9R,10aR)-3-(1,1-Dimethyl-heptyl)-9-hydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Binding affinity of the compound to Cannabinoid receptor 1 from rat forebrain synaptosomal membranes was measured using [3H]CP-55,940 as radioligand | J Med Chem 41: 3596-608 (1998) Article DOI: 10.1021/jm960677q BindingDB Entry DOI: 10.7270/Q26H4GHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50066712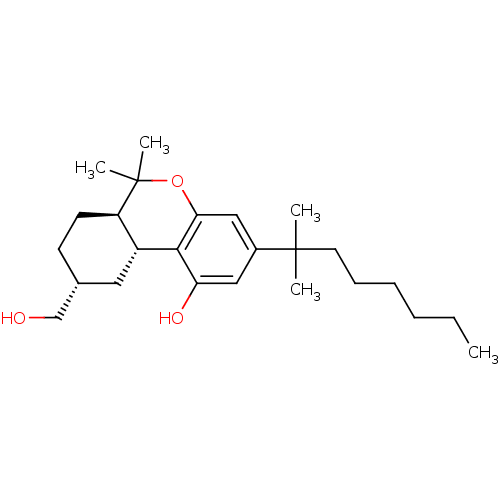 ((6aR,9R,10aR)-3-(1,1-Dimethyl-heptyl)-9-hydroxymet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Binding affinity of the compound to Cannabinoid receptor 2 from mouse spleen was measured using [3H]CP-55,940 as radioligand | J Med Chem 41: 3596-608 (1998) Article DOI: 10.1021/jm960677q BindingDB Entry DOI: 10.7270/Q26H4GHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50066706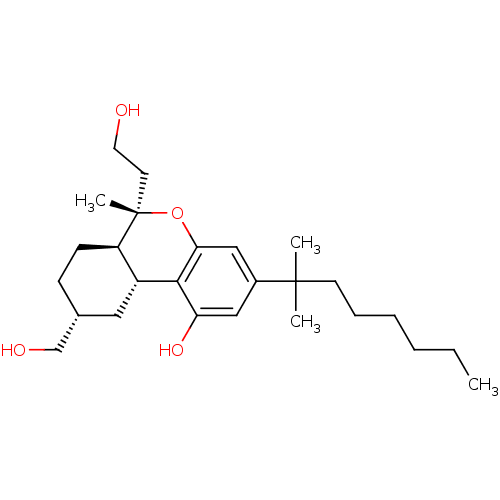 ((6S,6aR,9R,10aR)-3-(1,1-Dimethyl-heptyl)-6-(2-hydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Binding affinity of the compound to Cannabinoid receptor 2 from mouse spleen was measured using [3H]CP-55,940 as radioligand | J Med Chem 41: 3596-608 (1998) Article DOI: 10.1021/jm960677q BindingDB Entry DOI: 10.7270/Q26H4GHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50066712 ((6aR,9R,10aR)-3-(1,1-Dimethyl-heptyl)-9-hydroxymet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Binding affinity of the compound to Cannabinoid receptor 1 from rat forebrain synaptosomal membranes was measured using [3H]CP-55,940 as radioligand | J Med Chem 41: 3596-608 (1998) Article DOI: 10.1021/jm960677q BindingDB Entry DOI: 10.7270/Q26H4GHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50066706 ((6S,6aR,9R,10aR)-3-(1,1-Dimethyl-heptyl)-6-(2-hydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Binding affinity of the compound to Cannabinoid receptor 1 from rat forebrain synaptosomal membranes was measured using [3H]CP-55,940 as radioligand | J Med Chem 41: 3596-608 (1998) Article DOI: 10.1021/jm960677q BindingDB Entry DOI: 10.7270/Q26H4GHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50412340 (CHEMBL540359) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human muscarinic acetylcholine M2 receptor expressed in CHO cells coexpressed with Gqi5 by scintillatio... | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50066709 ((6S,6aR,9R,10aR)-3-(1,1-Dimethyl-heptyl)-9-hydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Binding affinity of the compound to Cannabinoid receptor 2 from mouse spleen was measured using [3H]CP-55,940 as radioligand | J Med Chem 41: 3596-608 (1998) Article DOI: 10.1021/jm960677q BindingDB Entry DOI: 10.7270/Q26H4GHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50066710 ((6S,6aR,9R,10aR)-3-(1,1-Dimethyl-heptyl)-9-hydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Binding affinity of the compound to Cannabinoid receptor 2 from mouse spleen was measured using [3H]CP-55,940 as radioligand | J Med Chem 41: 3596-608 (1998) Article DOI: 10.1021/jm960677q BindingDB Entry DOI: 10.7270/Q26H4GHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50422013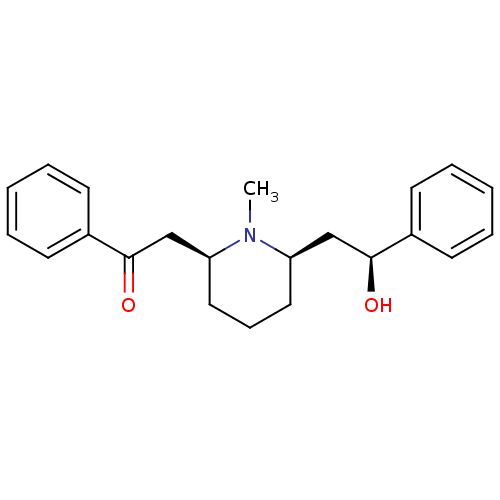 (LOBELINE | Lobeline Hydrochloride) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Binding affinity to alpha4beta2 (unknown origin) | Eur J Med Chem 102: 425-44 (2015) Article DOI: 10.1016/j.ejmech.2015.07.024 BindingDB Entry DOI: 10.7270/Q2765H4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50422013 (LOBELINE | Lobeline Hydrochloride) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Binding affinity to alpha4beta2 (unknown origin) | Eur J Med Chem 102: 425-44 (2015) Article DOI: 10.1016/j.ejmech.2015.07.024 BindingDB Entry DOI: 10.7270/Q2765H4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50053266 ((6aR,9R,10aR)-3-((E)-Hept-1-enyl)-9-hydroxymethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace specifically bound [3H]CP-55940 from a Cannabinoid receptor 2 enriched mouse spleen preparation. | J Med Chem 39: 3790-6 (1996) Article DOI: 10.1021/jm950934b BindingDB Entry DOI: 10.7270/Q2TT4RM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50053267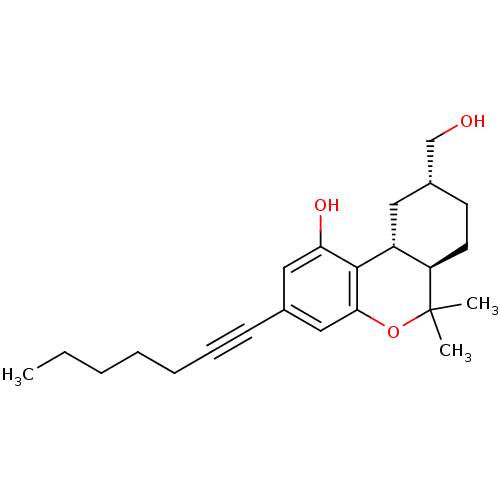 ((6aR,9R,10aR)-3-Hept-1-ynyl-9-hydroxymethyl-6,6-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace specifically bound [3H]CP-55940 from a Cannabinoid receptor 1 enriched rat brain microsome prepara... | J Med Chem 39: 3790-6 (1996) Article DOI: 10.1021/jm950934b BindingDB Entry DOI: 10.7270/Q2TT4RM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50053268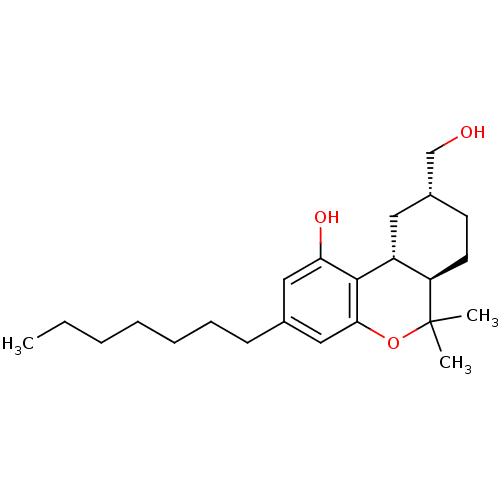 ((6aR,9R,10aR)-3-Heptyl-9-hydroxymethyl-6,6-dimethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace specifically bound [3H]CP-55940 from a Cannabinoid receptor 1 enriched rat brain microsome prepara... | J Med Chem 39: 3790-6 (1996) Article DOI: 10.1021/jm950934b BindingDB Entry DOI: 10.7270/Q2TT4RM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50053265 ((6aR,9R,10aR)-3-((Z)-Hept-1-enyl)-9-hydroxymethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace specifically bound [3H]CP-55940 from a Cannabinoid receptor 2 enriched mouse spleen preparation. | J Med Chem 39: 3790-6 (1996) Article DOI: 10.1021/jm950934b BindingDB Entry DOI: 10.7270/Q2TT4RM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50066708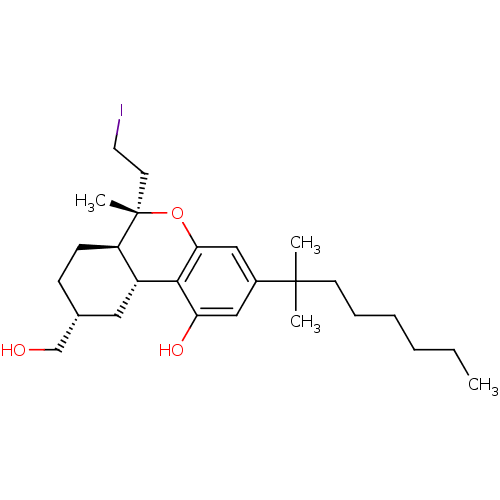 ((6S,6aR,9R,10aR)-3-(1,1-Dimethyl-heptyl)-9-hydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Binding affinity of the compound to Cannabinoid receptor 2 from mouse spleen was measured using [3H]CP-55,940 as radioligand | J Med Chem 41: 3596-608 (1998) Article DOI: 10.1021/jm960677q BindingDB Entry DOI: 10.7270/Q26H4GHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50066707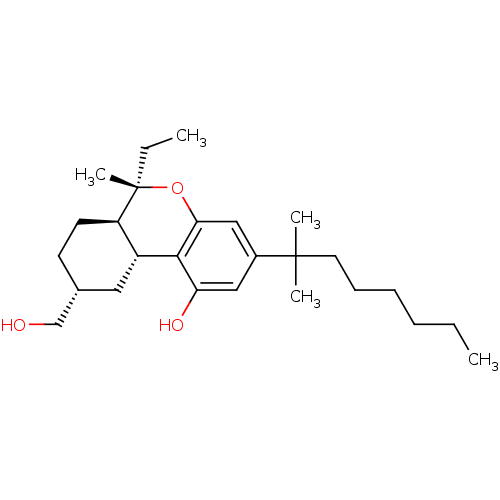 ((6S,6aR,9R,10aR)-3-(1,1-Dimethyl-heptyl)-6-ethyl-9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Binding affinity of the compound to Cannabinoid receptor 1 from rat forebrain synaptosomal membranes was measured using [3H]CP-55,940 as radioligand | J Med Chem 41: 3596-608 (1998) Article DOI: 10.1021/jm960677q BindingDB Entry DOI: 10.7270/Q26H4GHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50053268 ((6aR,9R,10aR)-3-Heptyl-9-hydroxymethyl-6,6-dimethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace specifically bound [3H]CP-55940 from a Cannabinoid receptor 2 enriched mouse spleen preparation. | J Med Chem 39: 3790-6 (1996) Article DOI: 10.1021/jm950934b BindingDB Entry DOI: 10.7270/Q2TT4RM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50066711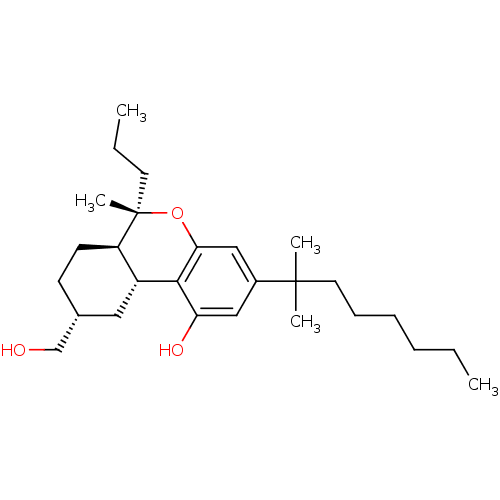 ((6S,6aR,9R,10aR)-3-(1,1-Dimethyl-heptyl)-9-hydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Binding affinity of the compound to Cannabinoid receptor 1 from rat forebrain synaptosomal membranes was measured using [3H]CP-55,940 as radioligand | J Med Chem 41: 3596-608 (1998) Article DOI: 10.1021/jm960677q BindingDB Entry DOI: 10.7270/Q26H4GHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50066707 ((6S,6aR,9R,10aR)-3-(1,1-Dimethyl-heptyl)-6-ethyl-9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Binding affinity of the compound to Cannabinoid receptor 2 from mouse spleen was measured using [3H]CP-55,940 as radioligand | J Med Chem 41: 3596-608 (1998) Article DOI: 10.1021/jm960677q BindingDB Entry DOI: 10.7270/Q26H4GHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50412340 (CHEMBL540359) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human muscarinic acetylcholine M3 receptor expressed in CHO cells by scintillation proximity assay | J Med Chem 51: 4866-9 (2008) Article DOI: 10.1021/jm800634k BindingDB Entry DOI: 10.7270/Q2MG7QQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50066711 ((6S,6aR,9R,10aR)-3-(1,1-Dimethyl-heptyl)-9-hydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 38.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Binding affinity of the compound to Cannabinoid receptor 2 from mouse spleen was measured using [3H]CP-55,940 as radioligand | J Med Chem 41: 3596-608 (1998) Article DOI: 10.1021/jm960677q BindingDB Entry DOI: 10.7270/Q26H4GHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM60994 ((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace specifically bound [3H]CP-55940 from a Cannabinoid receptor 2 enriched mouse spleen preparation. | J Med Chem 39: 3790-6 (1996) Article DOI: 10.1021/jm950934b BindingDB Entry DOI: 10.7270/Q2TT4RM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50066708 ((6S,6aR,9R,10aR)-3-(1,1-Dimethyl-heptyl)-9-hydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Binding affinity of the compound to Cannabinoid receptor 1 from rat forebrain synaptosomal membranes was measured using [3H]CP-55,940 as radioligand | J Med Chem 41: 3596-608 (1998) Article DOI: 10.1021/jm960677q BindingDB Entry DOI: 10.7270/Q26H4GHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine-binding protein (Lymnaea stagnalis) | BDBM50425041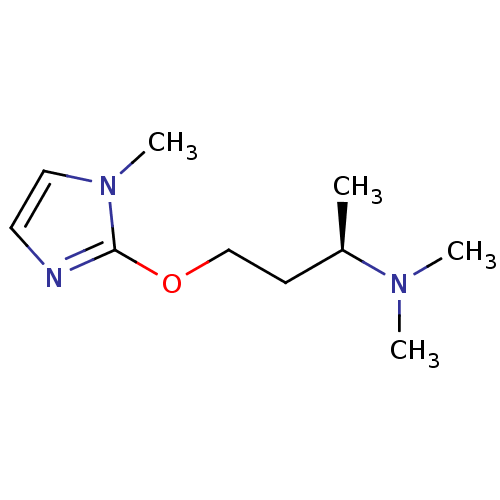 (CHEMBL2312565) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from Lymnaea stagnalis AChBP linked to ion channel portion of 5-HT3A receptor expressed in HEK293 cells after 4 hrs b... | J Med Chem 56: 940-51 (2013) Article DOI: 10.1021/jm301409f BindingDB Entry DOI: 10.7270/Q2RJ4KST | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM60994 ((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace specifically bound [3H]CP-55940 from a Cannabinoid receptor 1 enriched rat brain microsome prepara... | J Med Chem 39: 3790-6 (1996) Article DOI: 10.1021/jm950934b BindingDB Entry DOI: 10.7270/Q2TT4RM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5/beta-2/gamma-2 (Homo sapiens (Human)) | BDBM50588336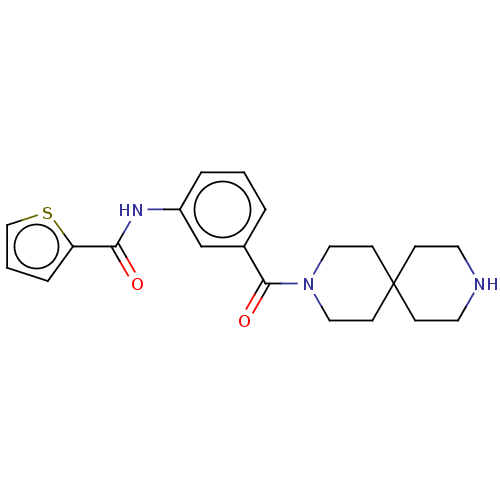 (CHEMBL5186739) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00290 BindingDB Entry DOI: 10.7270/Q2SB49PD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50053267 ((6aR,9R,10aR)-3-Hept-1-ynyl-9-hydroxymethyl-6,6-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hawaii Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace specifically bound [3H]CP-55940 from a Cannabinoid receptor 2 enriched mouse spleen preparation. | J Med Chem 39: 3790-6 (1996) Article DOI: 10.1021/jm950934b BindingDB Entry DOI: 10.7270/Q2TT4RM1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine-binding protein (Lymnaea stagnalis) | BDBM50425042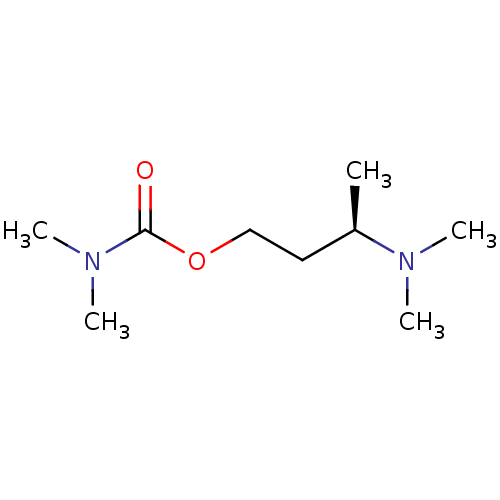 ((R)-3-(Dimethylamino)Butyl Dimethylcarbamate | CHE...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from Lymnaea stagnalis AChBP linked to ion channel portion of 5-HT3A receptor expressed in HEK293 cells after 4 hrs b... | J Med Chem 56: 940-51 (2013) Article DOI: 10.1021/jm301409f BindingDB Entry DOI: 10.7270/Q2RJ4KST | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-3/beta-2/gamma-2 (Homo sapiens (Human)) | BDBM50588336 (CHEMBL5186739) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00290 BindingDB Entry DOI: 10.7270/Q2SB49PD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine-binding protein (Lymnaea stagnalis) | BDBM82070 (CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from Lymnaea stagnalis AChBP linked to ion channel portion of 5-HT3A receptor expressed in HEK293 cells after 4 hrs b... | J Med Chem 56: 940-51 (2013) Article DOI: 10.1021/jm301409f BindingDB Entry DOI: 10.7270/Q2RJ4KST | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-4/beta-1/delta (Homo sapiens (Human)) | BDBM50588336 (CHEMBL5186739) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00290 BindingDB Entry DOI: 10.7270/Q2SB49PD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-4/beta-1/delta (Homo sapiens (Human)) | BDBM50588339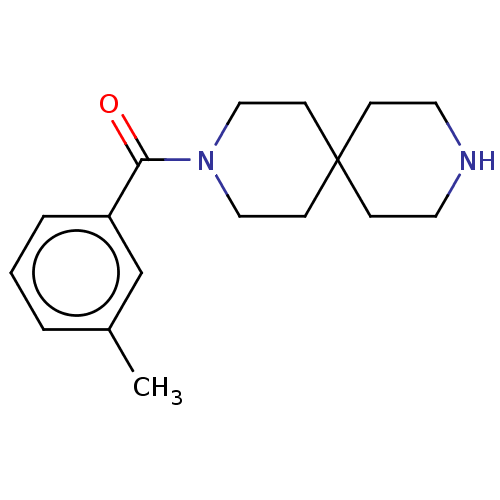 (CHEMBL5187612) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00290 BindingDB Entry DOI: 10.7270/Q2SB49PD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-3/beta-2/gamma-2 (Homo sapiens (Human)) | BDBM50588337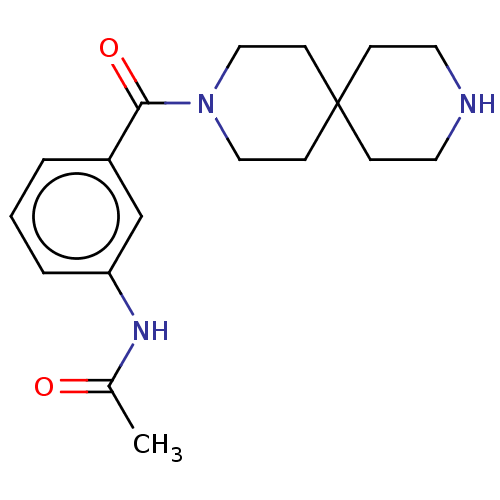 (CHEMBL5177970) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00290 BindingDB Entry DOI: 10.7270/Q2SB49PD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2623 total ) | Next | Last >> |