 Found 85 hits with Last Name = 'pettersen' and Initial = 'j'
Found 85 hits with Last Name = 'pettersen' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50120502

(2-Amino-N-[(R)-2-(3a-benzyl-2-tert-butyl-3-oxo-2,3...)Show SMILES CC(C)(C)N1N=C2CCN(CC2(Cc2ccccc2)C1=O)C(=O)[C@@H](COCc1ccc(F)c(F)c1)NC(=O)C(C)(C)N |t:5| Show InChI InChI=1S/C31H39F2N5O4/c1-29(2,3)38-28(41)31(16-20-9-7-6-8-10-20)19-37(14-13-25(31)36-38)26(39)24(35-27(40)30(4,5)34)18-42-17-21-11-12-22(32)23(33)15-21/h6-12,15,24H,13-14,16-19,34H2,1-5H3,(H,35,40)/t24-,31?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... |
Bioorg Med Chem Lett 12: 3279-82 (2002)
BindingDB Entry DOI: 10.7270/Q23F4NZZ |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50120504
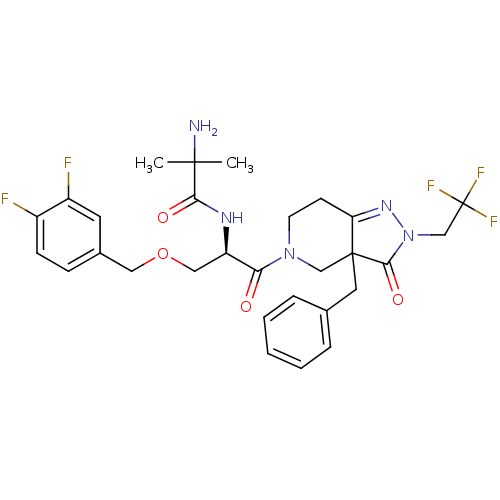
(2-Amino-N-[(R)-2-[3a-benzyl-3-oxo-2-(2,2,2-trifluo...)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccc(F)c(F)c1)C(=O)N1CCC2=NN(CC(F)(F)F)C(=O)C2(Cc2ccccc2)C1 |t:25| Show InChI InChI=1S/C29H32F5N5O4/c1-27(2,35)25(41)36-22(15-43-14-19-8-9-20(30)21(31)12-19)24(40)38-11-10-23-28(16-38,13-18-6-4-3-5-7-18)26(42)39(37-23)17-29(32,33)34/h3-9,12,22H,10-11,13-17,35H2,1-2H3,(H,36,41)/t22-,28?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... |
Bioorg Med Chem Lett 12: 3279-82 (2002)
BindingDB Entry DOI: 10.7270/Q23F4NZZ |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50120505
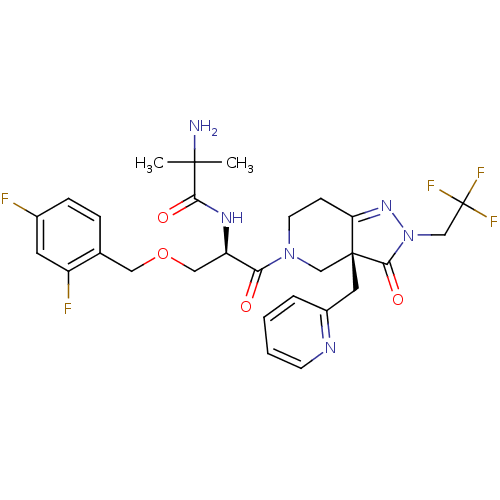
(2-Amino-N-{(R)-1-(2,4-difluoro-benzyloxymethyl)-2-...)Show SMILES CC(C)(N)C(=O)N[C@H](COCc1ccc(F)cc1F)C(=O)N1CCC2=NN(CC(F)(F)F)C(=O)[C@]2(Cc2ccccn2)C1 |t:25| Show InChI InChI=1S/C28H31F5N6O4/c1-26(2,34)24(41)36-21(14-43-13-17-6-7-18(29)11-20(17)30)23(40)38-10-8-22-27(15-38,12-19-5-3-4-9-35-19)25(42)39(37-22)16-28(31,32)33/h3-7,9,11,21H,8,10,12-16,34H2,1-2H3,(H,36,41)/t21-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... |
Bioorg Med Chem Lett 12: 3279-82 (2002)
BindingDB Entry DOI: 10.7270/Q23F4NZZ |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50083974
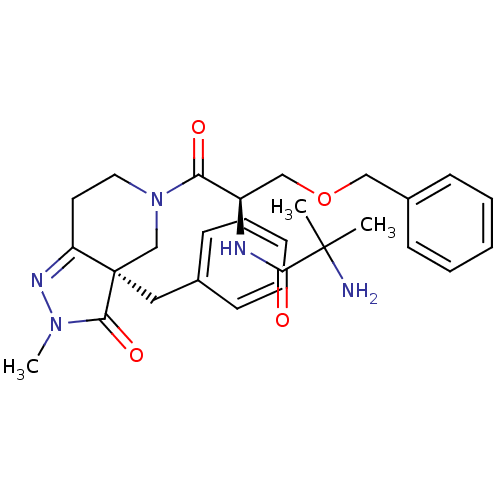
(2-Amino-N-[(R)-2-((R)-3a-benzyl-2-methyl-3-oxo-2,3...)Show SMILES CN1N=C2CCN(C[C@@]2(Cc2ccccc2)C1=O)C(=O)[C@@H](COCc1ccccc1)NC(=O)C(C)(C)N |t:2| Show InChI InChI=1S/C28H35N5O4/c1-27(2,29)25(35)30-22(18-37-17-21-12-8-5-9-13-21)24(34)33-15-14-23-28(19-33,26(36)32(3)31-23)16-20-10-6-4-7-11-20/h4-13,22H,14-19,29H2,1-3H3,(H,30,35)/t22-,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... |
Bioorg Med Chem Lett 12: 3279-82 (2002)
BindingDB Entry DOI: 10.7270/Q23F4NZZ |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Homo sapiens (Human)) | BDBM50120503
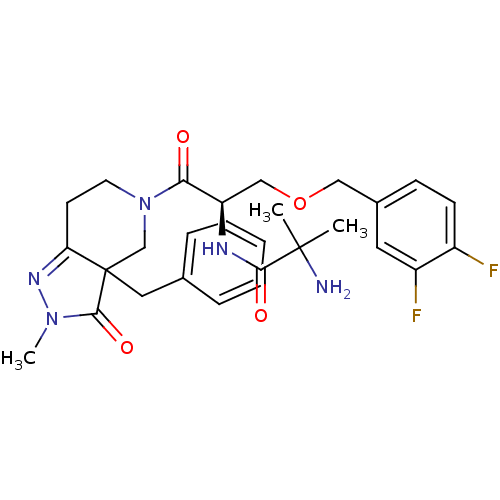
(2-Amino-N-[(R)-2-(3a-benzyl-2-methyl-3-oxo-2,3,3a,...)Show SMILES CN1N=C2CCN(CC2(Cc2ccccc2)C1=O)C(=O)[C@@H](COCc1ccc(F)c(F)c1)NC(=O)C(C)(C)N |t:2| Show InChI InChI=1S/C28H33F2N5O4/c1-27(2,31)25(37)32-22(16-39-15-19-9-10-20(29)21(30)13-19)24(36)35-12-11-23-28(17-35,26(38)34(3)33-23)14-18-7-5-4-6-8-18/h4-10,13,22H,11-12,14-17,31H2,1-3H3,(H,32,37)/t22-,28?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... |
Bioorg Med Chem Lett 12: 3279-82 (2002)
BindingDB Entry DOI: 10.7270/Q23F4NZZ |
More data for this
Ligand-Target Pair | |
Solute carrier family 5 member 4
(Homo sapiens (Human)) | BDBM50342885
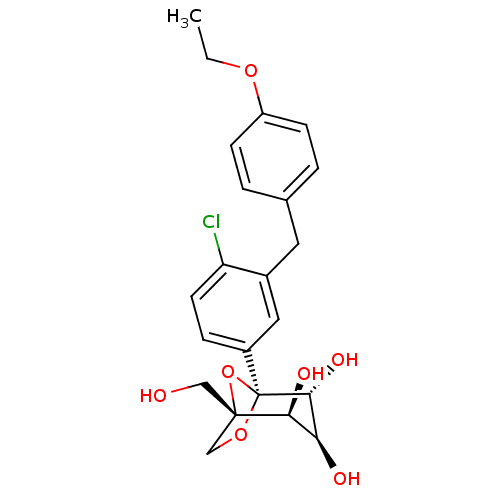
((1S,2S,3S,4R,5S)-5-[4-Chloro-3-(4-ethoxybenzyl)phe...)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@]23OC[C@](CO)(O2)[C@@H](O)[C@H](O)[C@H]3O)cc1 |r| Show InChI InChI=1S/C22H25ClO7/c1-2-28-16-6-3-13(4-7-16)9-14-10-15(5-8-17(14)23)22-20(27)18(25)19(26)21(11-24,30-22)12-29-22/h3-8,10,18-20,24-27H,2,9,11-12H2,1H3/t18-,19-,20+,21-,22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.877 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of methyl alpha-D-glucopyranoside uptake after 1 hr |
J Med Chem 54: 2952-60 (2011)
Article DOI: 10.1021/jm200049r
BindingDB Entry DOI: 10.7270/Q2NZ880H |
More data for this
Ligand-Target Pair | |
Solute carrier family 5 member 4
(Homo sapiens (Human)) | BDBM50342887

((1S,2S,3S,4R,5S)-5-[4-Chloro-3-(4-methoxybenzyl)ph...)Show SMILES COc1ccc(Cc2cc(ccc2Cl)[C@]23OC[C@](CO)(O2)[C@@H](O)[C@H](O)[C@H]3O)cc1 |r| Show InChI InChI=1S/C21H23ClO7/c1-27-15-5-2-12(3-6-15)8-13-9-14(4-7-16(13)22)21-19(26)17(24)18(25)20(10-23,29-21)11-28-21/h2-7,9,17-19,23-26H,8,10-11H2,1H3/t17-,18-,19+,20-,21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.882 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of methyl alpha-D-glucopyranoside uptake after 1 hr |
J Med Chem 54: 2952-60 (2011)
Article DOI: 10.1021/jm200049r
BindingDB Entry DOI: 10.7270/Q2NZ880H |
More data for this
Ligand-Target Pair | |
Solute carrier family 5 member 4
(Homo sapiens (Human)) | BDBM50342889
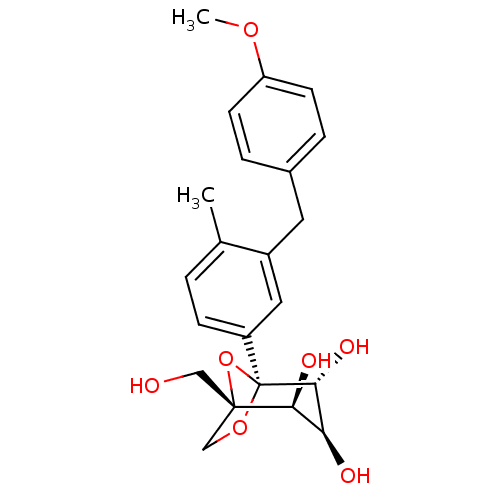
((1S,2S,3S,4R,5S)-1-(Hydroxymethyl)-5-[3-(4-methoxy...)Show SMILES COc1ccc(Cc2cc(ccc2C)[C@]23OC[C@](CO)(O2)[C@@H](O)[C@H](O)[C@H]3O)cc1 |r| Show InChI InChI=1S/C22H26O7/c1-13-3-6-16(10-15(13)9-14-4-7-17(27-2)8-5-14)22-20(26)18(24)19(25)21(11-23,29-22)12-28-22/h3-8,10,18-20,23-26H,9,11-12H2,1-2H3/t18-,19-,20+,21-,22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.07 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of methyl alpha-D-glucopyranoside uptake after 1 hr |
J Med Chem 54: 2952-60 (2011)
Article DOI: 10.1021/jm200049r
BindingDB Entry DOI: 10.7270/Q2NZ880H |
More data for this
Ligand-Target Pair | |
Solute carrier family 5 member 4
(Homo sapiens (Human)) | BDBM50342888
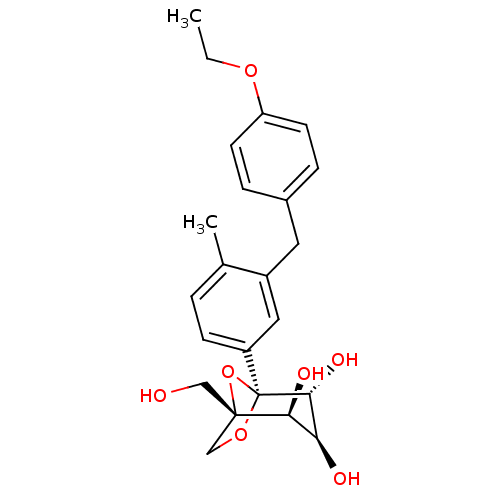
((1S,2S,3S,4R,5S)-5-[3-(4-Ethoxybenzyl)-4-methylphe...)Show SMILES CCOc1ccc(Cc2cc(ccc2C)[C@]23OC[C@](CO)(O2)[C@@H](O)[C@H](O)[C@H]3O)cc1 |r| Show InChI InChI=1S/C23H28O7/c1-3-28-18-8-5-15(6-9-18)10-16-11-17(7-4-14(16)2)23-21(27)19(25)20(26)22(12-24,30-23)13-29-23/h4-9,11,19-21,24-27H,3,10,12-13H2,1-2H3/t19-,20-,21+,22-,23-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of methyl alpha-D-glucopyranoside uptake after 1 hr |
J Med Chem 54: 2952-60 (2011)
Article DOI: 10.1021/jm200049r
BindingDB Entry DOI: 10.7270/Q2NZ880H |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 2
(Rattus norvegicus) | BDBM50342885

((1S,2S,3S,4R,5S)-5-[4-Chloro-3-(4-ethoxybenzyl)phe...)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@]23OC[C@](CO)(O2)[C@@H](O)[C@H](O)[C@H]3O)cc1 |r| Show InChI InChI=1S/C22H25ClO7/c1-2-28-16-6-3-13(4-7-16)9-14-10-15(5-8-17(14)23)22-20(27)18(25)19(26)21(11-24,30-22)12-29-22/h3-8,10,18-20,24-27H,2,9,11-12H2,1H3/t18-,19-,20+,21-,22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of rat SGLT2 |
J Med Chem 54: 2952-60 (2011)
Article DOI: 10.1021/jm200049r
BindingDB Entry DOI: 10.7270/Q2NZ880H |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276748
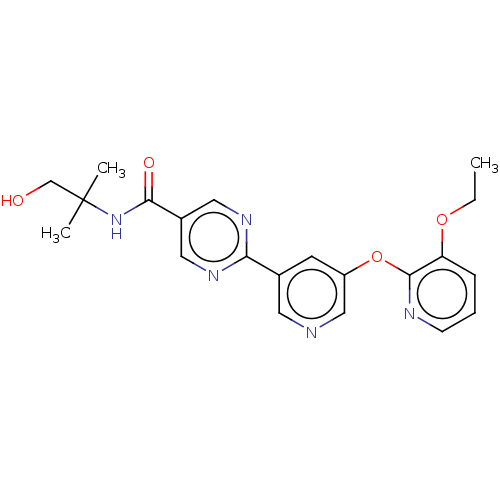
(US10071992, Example 3.5 | US10071992, Example 5 | ...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC(C)(C)CO Show InChI InChI=1S/C21H23N5O4/c1-4-29-17-6-5-7-23-20(17)30-16-8-14(9-22-12-16)18-24-10-15(11-25-18)19(28)26-21(2,3)13-27/h5-12,27H,4,13H2,1-3H3,(H,26,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Solute carrier family 5 member 4
(Homo sapiens (Human)) | BDBM50313368
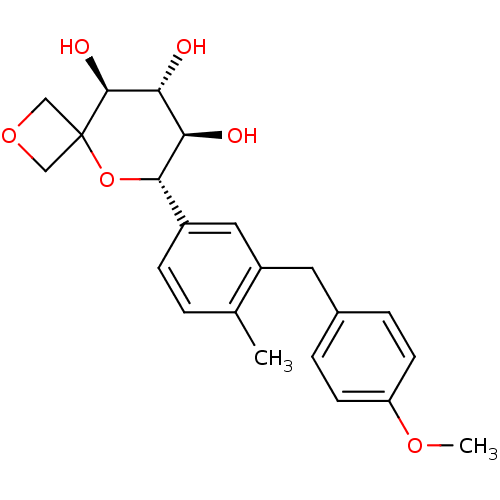
((6S,7R,8R,9S)-6-(3-(4-methoxybenzyl)-4-methylpheny...)Show SMILES COc1ccc(Cc2cc(ccc2C)[C@@H]2OC3(COC3)[C@@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C22H26O6/c1-13-3-6-15(10-16(13)9-14-4-7-17(26-2)8-5-14)20-18(23)19(24)21(25)22(28-20)11-27-12-22/h3-8,10,18-21,23-25H,9,11-12H2,1-2H3/t18-,19-,20+,21+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.98 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of methyl alpha-D-glucopyranoside uptake after 1 hr |
J Med Chem 54: 2952-60 (2011)
Article DOI: 10.1021/jm200049r
BindingDB Entry DOI: 10.7270/Q2NZ880H |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276749
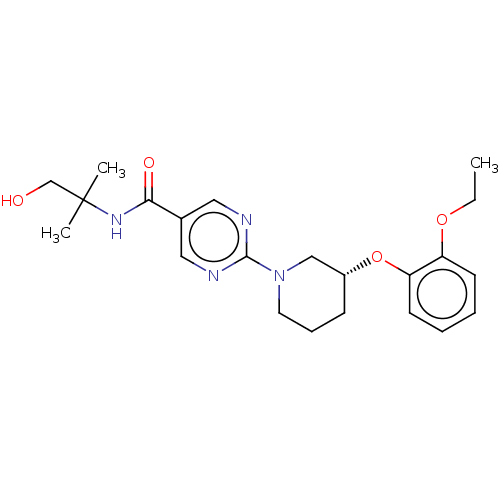
(US10188653, Example 19.21 | US11034678, WO20151406...)Show SMILES CCOc1ccccc1O[C@@H]1CCCN(C1)c1ncc(cn1)C(=O)NC(C)(C)CO |r| Show InChI InChI=1S/C22H30N4O4/c1-4-29-18-9-5-6-10-19(18)30-17-8-7-11-26(14-17)21-23-12-16(13-24-21)20(28)25-22(2,3)15-27/h5-6,9-10,12-13,17,27H,4,7-8,11,14-15H2,1-3H3,(H,25,28)/t17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50588577

(CHEMBL5176366)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC1CCCOC1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276750
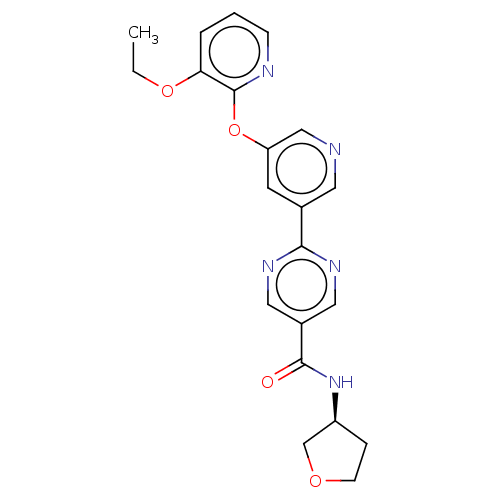
((S)-2-(5-((3-ethoxypyridin-2-yl)oxy)pyridin-3-yl)-...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@H]1CCOC1 |r| Show InChI InChI=1S/C21H21N5O4/c1-2-29-18-4-3-6-23-21(18)30-17-8-14(9-22-12-17)19-24-10-15(11-25-19)20(27)26-16-5-7-28-13-16/h3-4,6,8-12,16H,2,5,7,13H2,1H3,(H,26,27)/t16-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276760
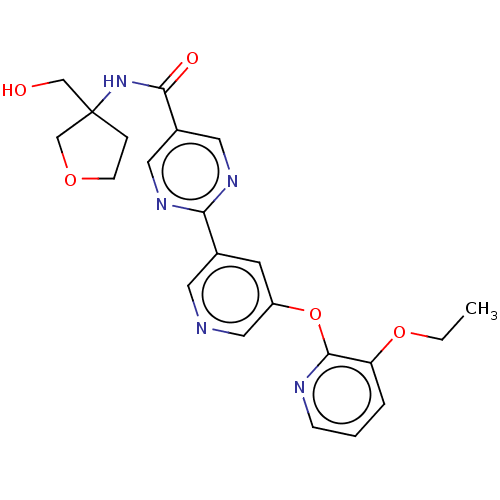
(US10071992, Example 6a)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC1(CO)CCOC1 Show InChI InChI=1S/C22H23N5O5/c1-2-31-18-4-3-6-24-21(18)32-17-8-15(9-23-12-17)19-25-10-16(11-26-19)20(29)27-22(13-28)5-7-30-14-22/h3-4,6,8-12,28H,2,5,7,13-14H2,1H3,(H,27,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276760

(US10071992, Example 6a)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC1(CO)CCOC1 Show InChI InChI=1S/C22H23N5O5/c1-2-31-18-4-3-6-24-21(18)32-17-8-15(9-23-12-17)19-25-10-16(11-26-19)20(29)27-22(13-28)5-7-30-14-22/h3-4,6,8-12,28H,2,5,7,13-14H2,1H3,(H,27,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50588576
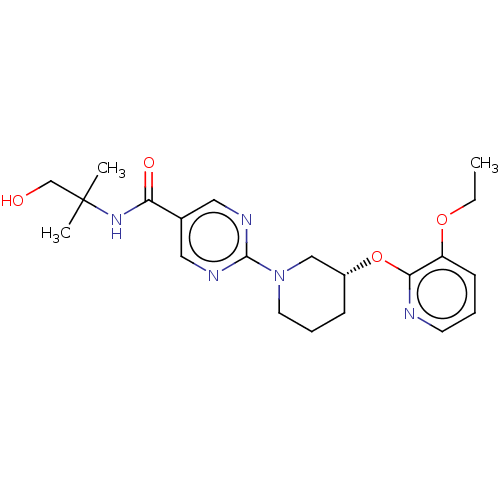
(CHEMBL5207410)Show SMILES CCOc1cccnc1O[C@@H]1CCCN(C1)c1ncc(cn1)C(=O)NC(C)(C)CO |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM503720
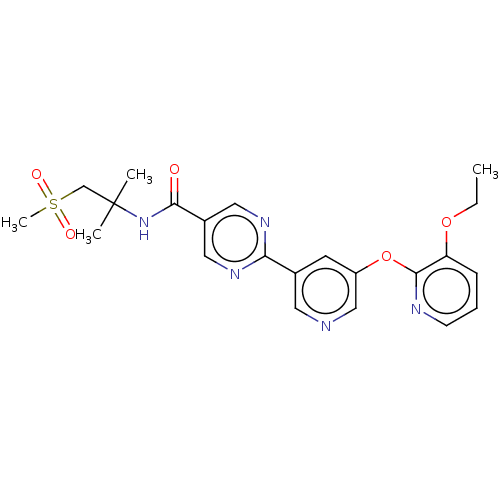
(US11034678, Example 3.6)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC(C)(C)CS(C)(=O)=O | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Solute carrier family 5 member 4
(Homo sapiens (Human)) | BDBM50342886
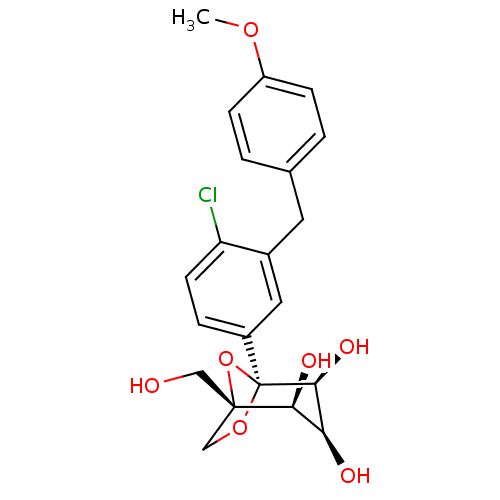
((1S,2S,3S,4S,5S)-5-[4-Chloro-3-(4-methoxybenzyl)-p...)Show SMILES COc1ccc(Cc2cc(ccc2Cl)[C@]23OC[C@](CO)(O2)[C@@H](O)[C@H](O)[C@@H]3O)cc1 |r| Show InChI InChI=1S/C21H23ClO7/c1-27-15-5-2-12(3-6-15)8-13-9-14(4-7-16(13)22)21-19(26)17(24)18(25)20(10-23,29-21)11-28-21/h2-7,9,17-19,23-26H,8,10-11H2,1H3/t17-,18-,19-,20-,21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 43.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of methyl alpha-D-glucopyranoside uptake after 1 hr |
J Med Chem 54: 2952-60 (2011)
Article DOI: 10.1021/jm200049r
BindingDB Entry DOI: 10.7270/Q2NZ880H |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50588580
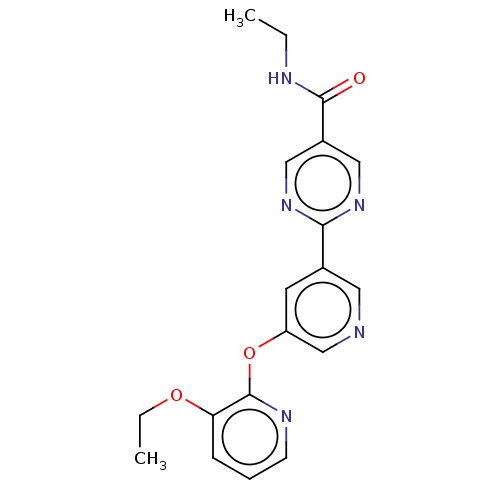
(CHEMBL5176043) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Solute carrier family 5 member 4
(Homo sapiens (Human)) | BDBM50342884
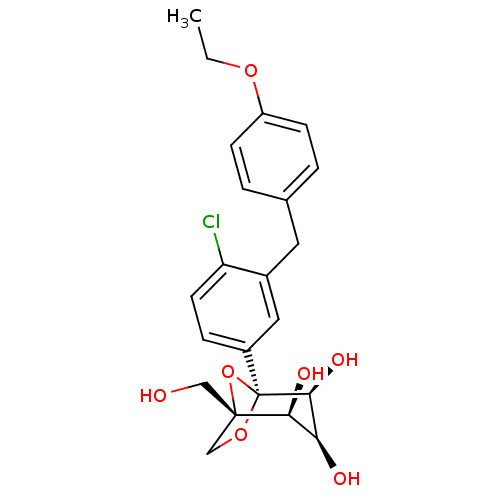
((1S,2S,3S,4S,5S)-5-[4-Chloro-3-(4-ethoxybenzyl)-ph...)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@]23OC[C@](CO)(O2)[C@@H](O)[C@H](O)[C@@H]3O)cc1 |r| Show InChI InChI=1S/C22H25ClO7/c1-2-28-16-6-3-13(4-7-16)9-14-10-15(5-8-17(14)23)22-20(27)18(25)19(26)21(11-24,30-22)12-29-22/h3-8,10,18-20,24-27H,2,9,11-12H2,1H3/t18-,19-,20-,21-,22-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 89.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT2 expressed in CHO cells assessed as inhibition of methyl alpha-D-glucopyranoside uptake after 1 hr |
J Med Chem 54: 2952-60 (2011)
Article DOI: 10.1021/jm200049r
BindingDB Entry DOI: 10.7270/Q2NZ880H |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM323589
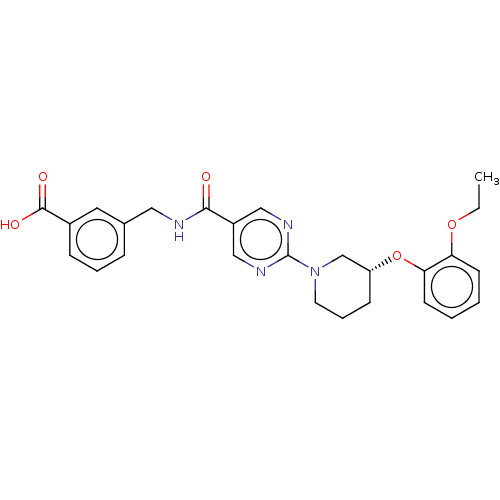
(US10188653, Example 1 | US9789110, 1)Show SMILES CCOc1ccccc1O[C@@H]1CCCN(C1)c1ncc(cn1)C(=O)NCc1cccc(c1)C(O)=O |r| Show InChI InChI=1S/C26H28N4O5/c1-2-34-22-10-3-4-11-23(22)35-21-9-6-12-30(17-21)26-28-15-20(16-29-26)24(31)27-14-18-7-5-8-19(13-18)25(32)33/h3-5,7-8,10-11,13,15-16,21H,2,6,9,12,14,17H2,1H3,(H,27,31)(H,32,33)/t21-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276754
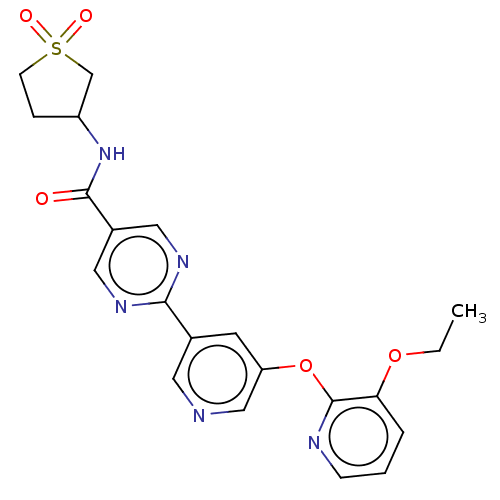
(N-(1,1-dioxidotetrahydro- thiophen-3-yl)-2-(5-((3-...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC1CCS(=O)(=O)C1 Show InChI InChI=1S/C21H21N5O5S/c1-2-30-18-4-3-6-23-21(18)31-17-8-14(9-22-12-17)19-24-10-15(11-25-19)20(27)26-16-5-7-32(28,29)13-16/h3-4,6,8-12,16H,2,5,7,13H2,1H3,(H,26,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 196 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM276751
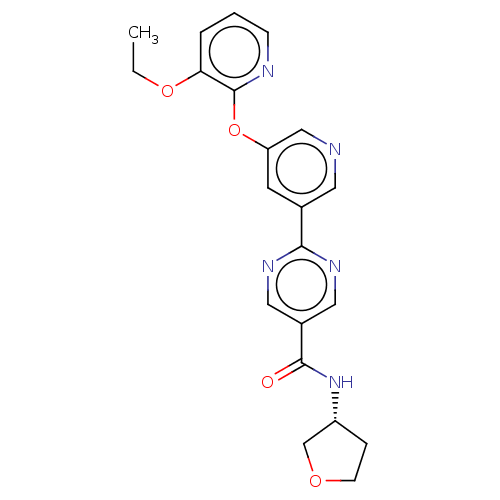
((R)-2-(5-((3-Ethoxypyridin-2-yl)oxy)pyridin-3-yl)-...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@@H]1CCOC1 |r| Show InChI InChI=1S/C21H21N5O4/c1-2-29-18-4-3-6-23-21(18)30-17-8-14(9-22-12-17)19-24-10-15(11-25-19)20(27)26-16-5-7-28-13-16/h3-4,6,8-12,16H,2,5,7,13H2,1H3,(H,26,27)/t16-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50588578
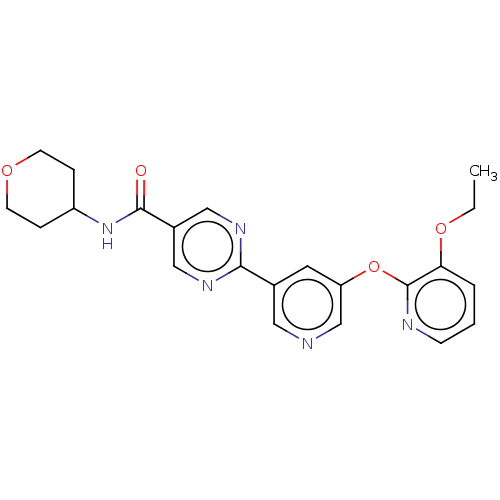
(CHEMBL5171697)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC1CCOCC1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 353 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50588579

(CHEMBL5173066)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC1CN(C)C(=O)C1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM50342889

((1S,2S,3S,4R,5S)-1-(Hydroxymethyl)-5-[3-(4-methoxy...)Show SMILES COc1ccc(Cc2cc(ccc2C)[C@]23OC[C@](CO)(O2)[C@@H](O)[C@H](O)[C@H]3O)cc1 |r| Show InChI InChI=1S/C22H26O7/c1-13-3-6-16(10-15(13)9-14-4-7-17(27-2)8-5-14)22-20(26)18(24)19(25)21(11-23,29-22)12-28-22/h3-8,10,18-20,23-26H,9,11-12H2,1-2H3/t18-,19-,20+,21-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 506 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT1 expressed in CHO cells assessed as inhibition of methyl alpha-D-glucopyranoside uptake after 1 hr |
J Med Chem 54: 2952-60 (2011)
Article DOI: 10.1021/jm200049r
BindingDB Entry DOI: 10.7270/Q2NZ880H |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM50342887

((1S,2S,3S,4R,5S)-5-[4-Chloro-3-(4-methoxybenzyl)ph...)Show SMILES COc1ccc(Cc2cc(ccc2Cl)[C@]23OC[C@](CO)(O2)[C@@H](O)[C@H](O)[C@H]3O)cc1 |r| Show InChI InChI=1S/C21H23ClO7/c1-27-15-5-2-12(3-6-15)8-13-9-14(4-7-16(13)22)21-19(26)17(24)18(25)20(10-23,29-21)11-28-21/h2-7,9,17-19,23-26H,8,10-11H2,1H3/t17-,18-,19+,20-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 546 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT1 expressed in CHO cells assessed as inhibition of methyl alpha-D-glucopyranoside uptake after 1 hr |
J Med Chem 54: 2952-60 (2011)
Article DOI: 10.1021/jm200049r
BindingDB Entry DOI: 10.7270/Q2NZ880H |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 2
(Rattus norvegicus) | BDBM276750

((S)-2-(5-((3-ethoxypyridin-2-yl)oxy)pyridin-3-yl)-...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@H]1CCOC1 |r| Show InChI InChI=1S/C21H21N5O4/c1-2-29-18-4-3-6-23-21(18)30-17-8-14(9-22-12-17)19-24-10-15(11-25-19)20(27)26-16-5-7-28-13-16/h3-4,6,8-12,16H,2,5,7,13H2,1H3,(H,26,27)/t16-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 833 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM50342888

((1S,2S,3S,4R,5S)-5-[3-(4-Ethoxybenzyl)-4-methylphe...)Show SMILES CCOc1ccc(Cc2cc(ccc2C)[C@]23OC[C@](CO)(O2)[C@@H](O)[C@H](O)[C@H]3O)cc1 |r| Show InChI InChI=1S/C23H28O7/c1-3-28-18-8-5-15(6-9-18)10-16-11-17(7-4-14(16)2)23-21(27)19(25)20(26)22(12-24,30-23)13-29-23/h4-9,11,19-21,24-27H,3,10,12-13H2,1-2H3/t19-,20-,21+,22-,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 958 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT1 expressed in CHO cells assessed as inhibition of methyl alpha-D-glucopyranoside uptake after 1 hr |
J Med Chem 54: 2952-60 (2011)
Article DOI: 10.1021/jm200049r
BindingDB Entry DOI: 10.7270/Q2NZ880H |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM50313368

((6S,7R,8R,9S)-6-(3-(4-methoxybenzyl)-4-methylpheny...)Show SMILES COc1ccc(Cc2cc(ccc2C)[C@@H]2OC3(COC3)[C@@H](O)[C@H](O)[C@H]2O)cc1 |r| Show InChI InChI=1S/C22H26O6/c1-13-3-6-15(10-16(13)9-14-4-7-17(26-2)8-5-14)20-18(23)19(24)21(25)22(28-20)11-27-12-22/h3-8,10,18-21,23-25H,9,11-12H2,1-2H3/t18-,19-,20+,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT1 expressed in CHO cells assessed as inhibition of methyl alpha-D-glucopyranoside uptake after 1 hr |
J Med Chem 54: 2952-60 (2011)
Article DOI: 10.1021/jm200049r
BindingDB Entry DOI: 10.7270/Q2NZ880H |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50444409
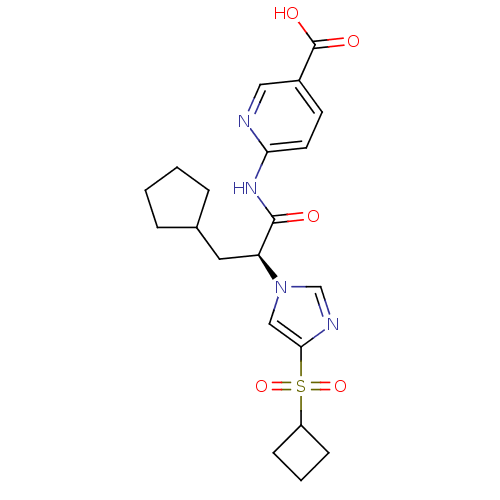
(CHEMBL3091581)Show SMILES OC(=O)c1ccc(NC(=O)[C@H](CC2CCCC2)n2cnc(c2)S(=O)(=O)C2CCC2)nc1 |r| Show InChI InChI=1S/C21H26N4O5S/c26-20(24-18-9-8-15(11-22-18)21(27)28)17(10-14-4-1-2-5-14)25-12-19(23-13-25)31(29,30)16-6-3-7-16/h8-9,11-14,16-17H,1-7,10H2,(H,27,28)(H,22,24,26)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of glucokinase in Wistar rat hepatocytes assessed as nuclear to cytosolic translocation of protein by immunofluorescence assay |
Bioorg Med Chem Lett 23: 6588-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.057
BindingDB Entry DOI: 10.7270/Q2QZ2CFX |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM50342885

((1S,2S,3S,4R,5S)-5-[4-Chloro-3-(4-ethoxybenzyl)phe...)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@]23OC[C@](CO)(O2)[C@@H](O)[C@H](O)[C@H]3O)cc1 |r| Show InChI InChI=1S/C22H25ClO7/c1-2-28-16-6-3-13(4-7-16)9-14-10-15(5-8-17(14)23)22-20(27)18(25)19(26)21(11-24,30-22)12-29-22/h3-8,10,18-20,24-27H,2,9,11-12H2,1H3/t18-,19-,20+,21-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT1 expressed in CHO cells assessed as inhibition of methyl alpha-D-glucopyranoside uptake after 1 hr |
J Med Chem 54: 2952-60 (2011)
Article DOI: 10.1021/jm200049r
BindingDB Entry DOI: 10.7270/Q2NZ880H |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM276748

(US10071992, Example 3.5 | US10071992, Example 5 | ...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC(C)(C)CO Show InChI InChI=1S/C21H23N5O4/c1-4-29-17-6-5-7-23-20(17)30-16-8-14(9-22-12-16)18-24-10-15(11-25-18)19(28)26-21(2,3)13-27/h5-12,27H,4,13H2,1-3H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM276750

((S)-2-(5-((3-ethoxypyridin-2-yl)oxy)pyridin-3-yl)-...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@H]1CCOC1 |r| Show InChI InChI=1S/C21H21N5O4/c1-2-29-18-4-3-6-23-21(18)30-17-8-14(9-22-12-17)19-24-10-15(11-25-19)20(27)26-16-5-7-28-13-16/h3-4,6,8-12,16H,2,5,7,13H2,1H3,(H,26,27)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM50342886

((1S,2S,3S,4S,5S)-5-[4-Chloro-3-(4-methoxybenzyl)-p...)Show SMILES COc1ccc(Cc2cc(ccc2Cl)[C@]23OC[C@](CO)(O2)[C@@H](O)[C@H](O)[C@@H]3O)cc1 |r| Show InChI InChI=1S/C21H23ClO7/c1-27-15-5-2-12(3-6-15)8-13-9-14(4-7-16(13)22)21-19(26)17(24)18(25)20(10-23,29-21)11-28-21/h2-7,9,17-19,23-26H,8,10-11H2,1H3/t17-,18-,19-,20-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT1 expressed in CHO cells assessed as inhibition of methyl alpha-D-glucopyranoside uptake after 1 hr |
J Med Chem 54: 2952-60 (2011)
Article DOI: 10.1021/jm200049r
BindingDB Entry DOI: 10.7270/Q2NZ880H |
More data for this
Ligand-Target Pair | |
Sodium/glucose cotransporter 1
(Homo sapiens (Human)) | BDBM50342884

((1S,2S,3S,4S,5S)-5-[4-Chloro-3-(4-ethoxybenzyl)-ph...)Show SMILES CCOc1ccc(Cc2cc(ccc2Cl)[C@]23OC[C@](CO)(O2)[C@@H](O)[C@H](O)[C@@H]3O)cc1 |r| Show InChI InChI=1S/C22H25ClO7/c1-2-28-16-6-3-13(4-7-16)9-14-10-15(5-8-17(14)23)22-20(27)18(25)19(26)21(11-24,30-22)12-29-22/h3-8,10,18-20,24-27H,2,9,11-12H2,1H3/t18-,19-,20-,21-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human SGLT1 expressed in CHO cells assessed as inhibition of methyl alpha-D-glucopyranoside uptake after 1 hr |
J Med Chem 54: 2952-60 (2011)
Article DOI: 10.1021/jm200049r
BindingDB Entry DOI: 10.7270/Q2NZ880H |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM276748

(US10071992, Example 3.5 | US10071992, Example 5 | ...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC(C)(C)CO Show InChI InChI=1S/C21H23N5O4/c1-4-29-17-6-5-7-23-20(17)30-16-8-14(9-22-12-16)18-24-10-15(11-25-18)19(28)26-21(2,3)13-27/h5-12,27H,4,13H2,1-3H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM276748

(US10071992, Example 3.5 | US10071992, Example 5 | ...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC(C)(C)CO Show InChI InChI=1S/C21H23N5O4/c1-4-29-17-6-5-7-23-20(17)30-16-8-14(9-22-12-16)18-24-10-15(11-25-18)19(28)26-21(2,3)13-27/h5-12,27H,4,13H2,1-3H3,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens) | BDBM276748

(US10071992, Example 3.5 | US10071992, Example 5 | ...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC(C)(C)CO Show InChI InChI=1S/C21H23N5O4/c1-4-29-17-6-5-7-23-20(17)30-16-8-14(9-22-12-16)18-24-10-15(11-25-18)19(28)26-21(2,3)13-27/h5-12,27H,4,13H2,1-3H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM276748

(US10071992, Example 3.5 | US10071992, Example 5 | ...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC(C)(C)CO Show InChI InChI=1S/C21H23N5O4/c1-4-29-17-6-5-7-23-20(17)30-16-8-14(9-22-12-16)18-24-10-15(11-25-18)19(28)26-21(2,3)13-27/h5-12,27H,4,13H2,1-3H3,(H,26,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM276748

(US10071992, Example 3.5 | US10071992, Example 5 | ...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC(C)(C)CO Show InChI InChI=1S/C21H23N5O4/c1-4-29-17-6-5-7-23-20(17)30-16-8-14(9-22-12-16)18-24-10-15(11-25-18)19(28)26-21(2,3)13-27/h5-12,27H,4,13H2,1-3H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM276748

(US10071992, Example 3.5 | US10071992, Example 5 | ...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC(C)(C)CO Show InChI InChI=1S/C21H23N5O4/c1-4-29-17-6-5-7-23-20(17)30-16-8-14(9-22-12-16)18-24-10-15(11-25-18)19(28)26-21(2,3)13-27/h5-12,27H,4,13H2,1-3H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4/3A5
(Homo sapiens (Human)) | BDBM276748

(US10071992, Example 3.5 | US10071992, Example 5 | ...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC(C)(C)CO Show InChI InChI=1S/C21H23N5O4/c1-4-29-17-6-5-7-23-20(17)30-16-8-14(9-22-12-16)18-24-10-15(11-25-18)19(28)26-21(2,3)13-27/h5-12,27H,4,13H2,1-3H3,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM276748

(US10071992, Example 3.5 | US10071992, Example 5 | ...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC(C)(C)CO Show InChI InChI=1S/C21H23N5O4/c1-4-29-17-6-5-7-23-20(17)30-16-8-14(9-22-12-16)18-24-10-15(11-25-18)19(28)26-21(2,3)13-27/h5-12,27H,4,13H2,1-3H3,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM276750

((S)-2-(5-((3-ethoxypyridin-2-yl)oxy)pyridin-3-yl)-...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@H]1CCOC1 |r| Show InChI InChI=1S/C21H21N5O4/c1-2-29-18-4-3-6-23-21(18)30-17-8-14(9-22-12-17)19-24-10-15(11-25-19)20(27)26-16-5-7-28-13-16/h3-4,6,8-12,16H,2,5,7,13H2,1H3,(H,26,27)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Beta-1,4-mannosyl-glycoprotein 4-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM276748

(US10071992, Example 3.5 | US10071992, Example 5 | ...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC(C)(C)CO Show InChI InChI=1S/C21H23N5O4/c1-4-29-17-6-5-7-23-20(17)30-16-8-14(9-22-12-16)18-24-10-15(11-25-18)19(28)26-21(2,3)13-27/h5-12,27H,4,13H2,1-3H3,(H,26,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Beta-1,4-mannosyl-glycoprotein 4-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM276750

((S)-2-(5-((3-ethoxypyridin-2-yl)oxy)pyridin-3-yl)-...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)N[C@H]1CCOC1 |r| Show InChI InChI=1S/C21H21N5O4/c1-2-29-18-4-3-6-23-21(18)30-17-8-14(9-22-12-17)19-24-10-15(11-25-19)20(27)26-16-5-7-28-13-16/h3-4,6,8-12,16H,2,5,7,13H2,1H3,(H,26,27)/t16-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase
(Homo sapiens (Human)) | BDBM276748

(US10071992, Example 3.5 | US10071992, Example 5 | ...)Show SMILES CCOc1cccnc1Oc1cncc(c1)-c1ncc(cn1)C(=O)NC(C)(C)CO Show InChI InChI=1S/C21H23N5O4/c1-4-29-17-6-5-7-23-20(17)30-16-8-14(9-22-12-16)18-24-10-15(11-25-18)19(28)26-21(2,3)13-27/h5-12,27H,4,13H2,1-3H3,(H,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01200
BindingDB Entry DOI: 10.7270/Q23N27CP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data