

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50404821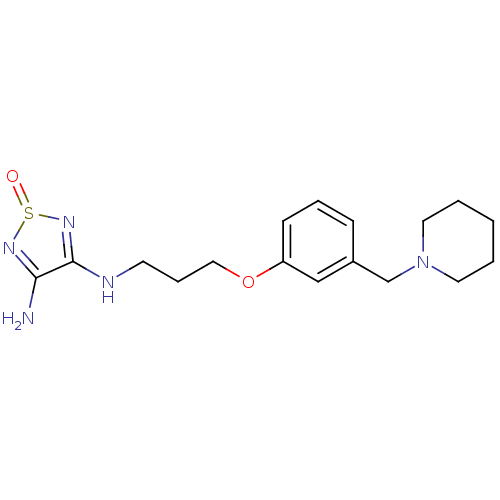 (CHEMBL306465) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Histamine H2 receptor by measuring its ability to block the histamine-stimulated adenylate cyclase of guinea pig hippocampal h... | J Med Chem 25: 207-10 (1982) BindingDB Entry DOI: 10.7270/Q2HH6M8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50404823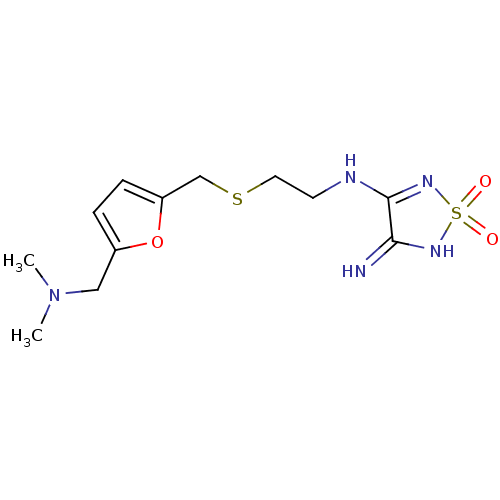 (CHEMBL63299) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Histamine H2 receptor by measuring its ability to block the histamine-stimulated adenylate cyclase of guinea pig hippocampal h... | J Med Chem 25: 207-10 (1982) BindingDB Entry DOI: 10.7270/Q2HH6M8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50404822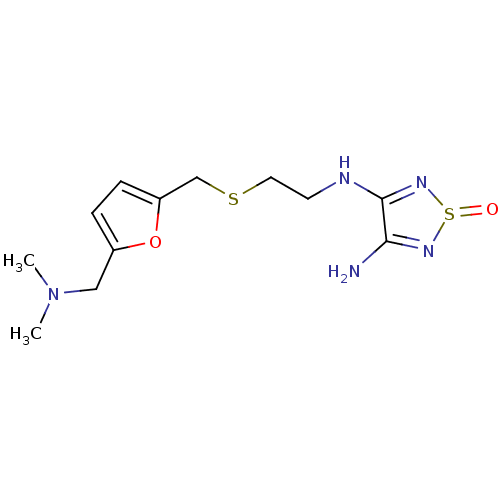 (CHEMBL8982) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Histamine H2 receptor by measuring its ability to block the histamine-stimulated adenylate cyclase of guinea pig hippocampal h... | J Med Chem 25: 207-10 (1982) BindingDB Entry DOI: 10.7270/Q2HH6M8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM22568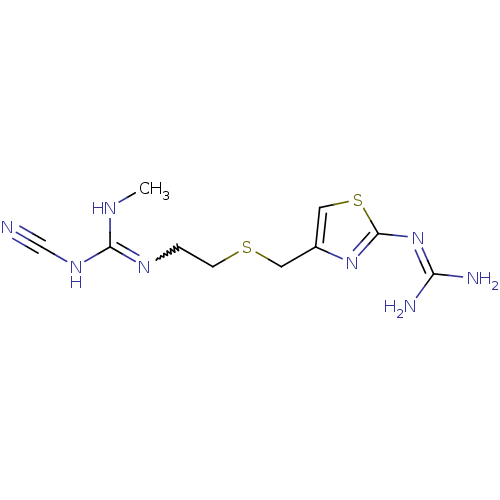 (1-cyano-3-{2-[({2-[(diaminomethylidene)amino]-1,3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated in vitro for Histamine H2 receptor inhibition using the dimaprit stimulated chronotropic response of the guinea pig atrium | J Med Chem 26: 140-4 (1983) BindingDB Entry DOI: 10.7270/Q2R212KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070791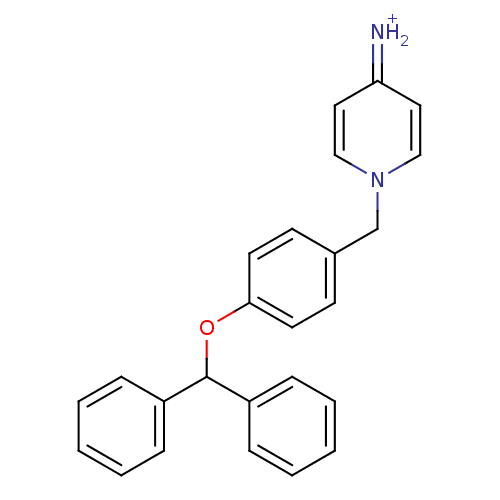 (4-Amino-1-(4-benzhydryloxy-benzyl)-pyridinium | CH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070787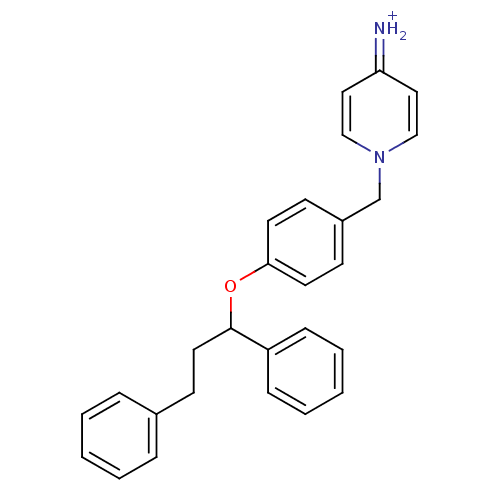 (4-Amino-1-[4-(1,3-diphenyl-propoxy)-benzyl]-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50404962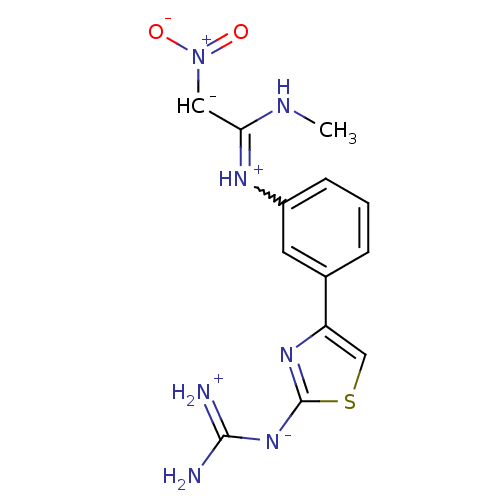 (CHEMBL33850) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated in vitro for Histamine H2 receptor inhibition using the dimaprit stimulated chronotropic response of the guinea pig atrium | J Med Chem 26: 140-4 (1983) BindingDB Entry DOI: 10.7270/Q2R212KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50404958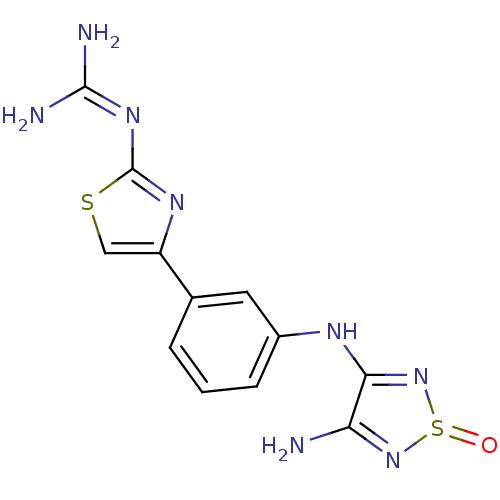 (CHEMBL284743) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated in vitro for Histamine H2 receptor inhibition using the dimaprit stimulated chronotropic response of the guinea pig atrium | J Med Chem 26: 140-4 (1983) BindingDB Entry DOI: 10.7270/Q2R212KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50404955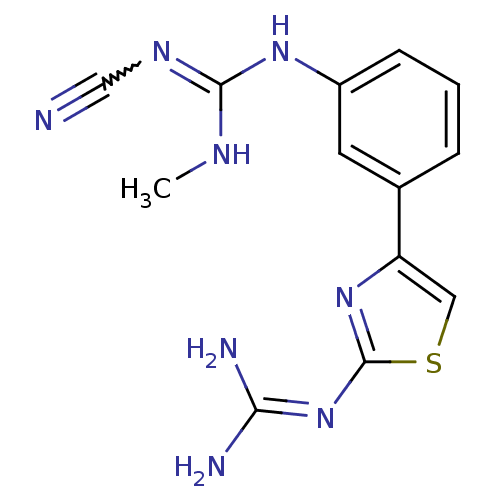 (CHEMBL441965) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated in vitro for Histamine H2 receptor inhibition using the dimaprit stimulated chronotropic response of the guinea pig atrium | J Med Chem 26: 140-4 (1983) BindingDB Entry DOI: 10.7270/Q2R212KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50404956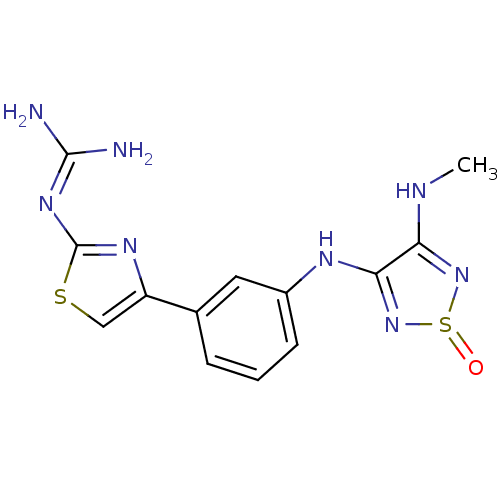 (CHEMBL284556) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated in vitro for Histamine H2 receptor inhibition using the dimaprit stimulated chronotropic response of the guinea pig atrium | J Med Chem 26: 140-4 (1983) BindingDB Entry DOI: 10.7270/Q2R212KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM22893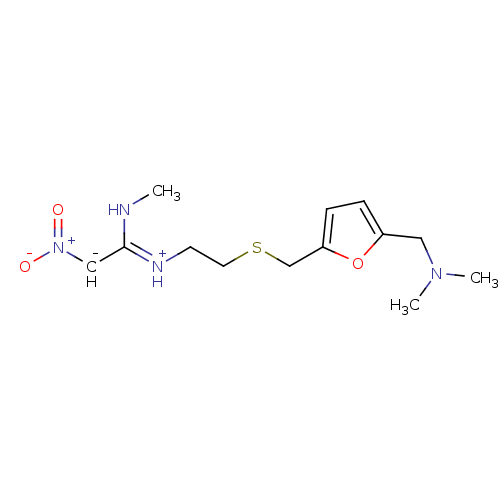 (CHEMBL512 | Ranitidine | ZANTAC | dimethyl[(5-{[(2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Histamine H2 receptor by measuring its ability to block the histamine-stimulated adenylate cyclase of guinea pig hippocampal h... | J Med Chem 25: 207-10 (1982) BindingDB Entry DOI: 10.7270/Q2HH6M8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50403559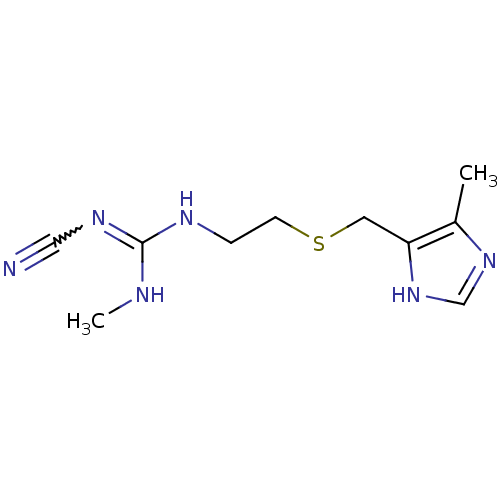 (Brumetadina | CIMETIDINE) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated in vitro for Histamine H2 receptor inhibition using the dimaprit stimulated chronotropic response of the guinea pig atrium | J Med Chem 26: 140-4 (1983) BindingDB Entry DOI: 10.7270/Q2R212KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50403559 (Brumetadina | CIMETIDINE) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of Histamine H2 receptor by measuring its ability to block the histamine-stimulated adenylate cyclase of guinea pig hippocampal h... | J Med Chem 25: 207-10 (1982) BindingDB Entry DOI: 10.7270/Q2HH6M8R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070786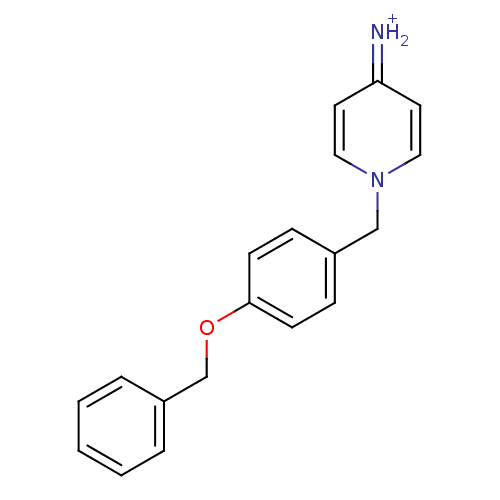 (4-Amino-1-(4-benzyloxy-benzyl)-pyridinium | CHEMBL...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50404961 (CHEMBL284379) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated in vitro for Histamine H2 receptor inhibition using the dimaprit stimulated chronotropic response of the guinea pig atrium | J Med Chem 26: 140-4 (1983) BindingDB Entry DOI: 10.7270/Q2R212KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070793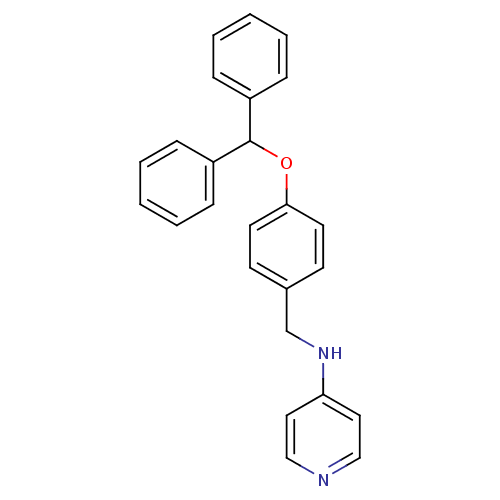 ((4-Benzhydryloxy-benzyl)-pyridin-4-yl-amine | CHEM...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070790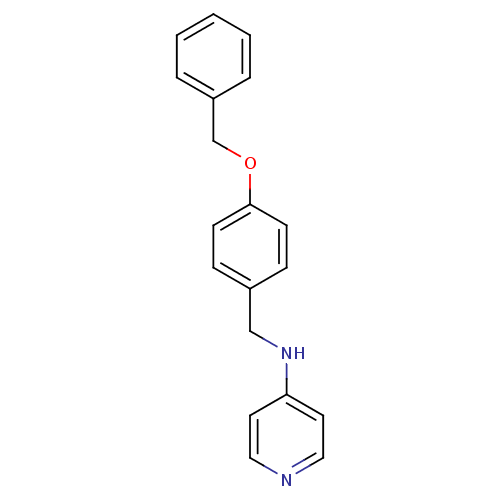 ((4-Benzyloxy-benzyl)-pyridin-4-yl-amine | CHEMBL47...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070788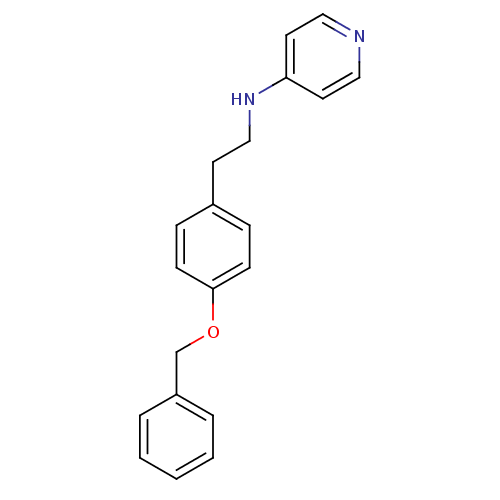 (CHEMBL48029 | N-(4-(benzyloxy)phenethyl)pyridin-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070792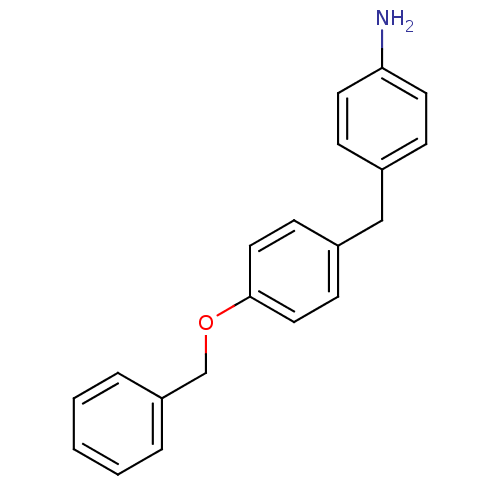 (4-(4-Benzyloxy-benzyl)-phenylamine | CHEMBL45648) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070792 (4-(4-Benzyloxy-benzyl)-phenylamine | CHEMBL45648) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against trypsin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50404957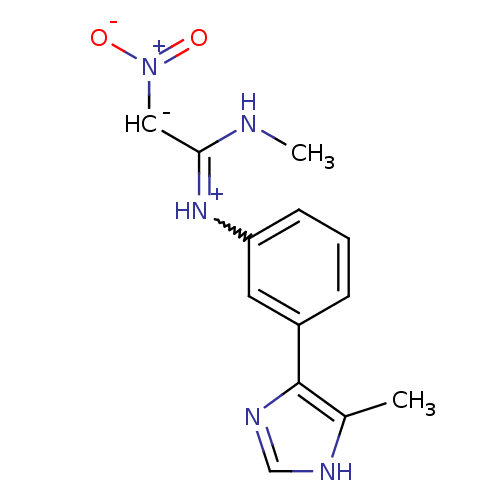 (CHEMBL33996) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated in vitro for Histamine H2 receptor inhibition using the dimaprit stimulated chronotropic response of the guinea pig atrium | J Med Chem 26: 140-4 (1983) BindingDB Entry DOI: 10.7270/Q2R212KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070787 (4-Amino-1-[4-(1,3-diphenyl-propoxy)-benzyl]-pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against trypsin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50404959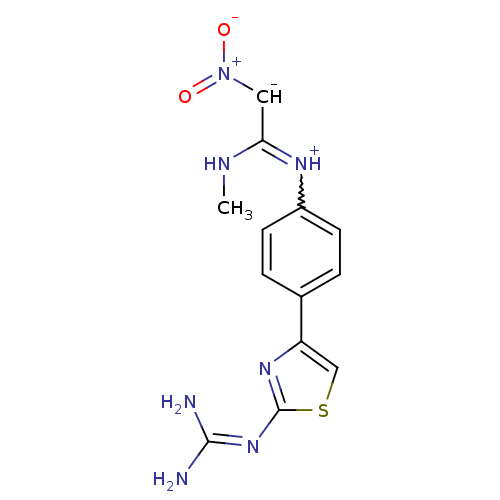 (CHEMBL33108) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated in vitro for Histamine H2 receptor inhibition using the dimaprit stimulated chronotropic response of the guinea pig atrium | J Med Chem 26: 140-4 (1983) BindingDB Entry DOI: 10.7270/Q2R212KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50404960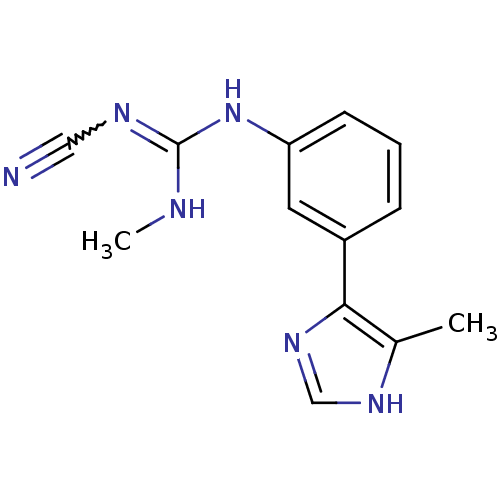 (CHEMBL32409) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Evaluated in vitro for Histamine H2 receptor inhibition using the dimaprit stimulated chronotropic response of the guinea pig atrium | J Med Chem 26: 140-4 (1983) BindingDB Entry DOI: 10.7270/Q2R212KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070793 ((4-Benzhydryloxy-benzyl)-pyridin-4-yl-amine | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against trypsin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070786 (4-Amino-1-(4-benzyloxy-benzyl)-pyridinium | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against trypsin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070790 ((4-Benzyloxy-benzyl)-pyridin-4-yl-amine | CHEMBL47...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against trypsin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070789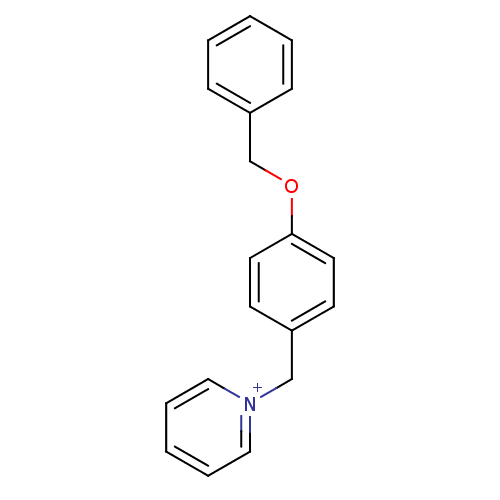 (1-(4-Benzyloxy-benzyl)-pyridinium | CHEMBL296280) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070791 (4-Amino-1-(4-benzhydryloxy-benzyl)-pyridinium | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against trypsin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50070788 (CHEMBL48029 | N-(4-(benzyloxy)phenethyl)pyridin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 5.68E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against trypsin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070794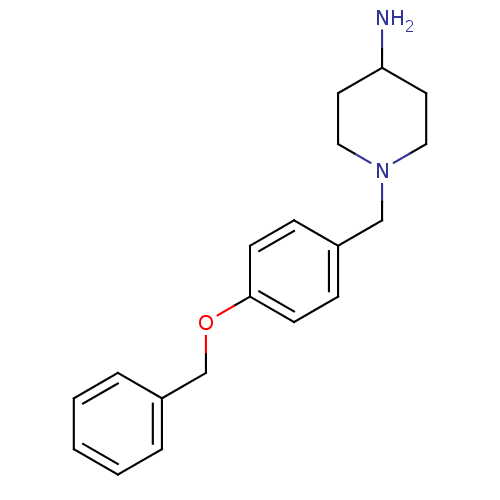 (1-(4-Benzyloxy-benzyl)-piperidin-4-ylamine | CHEMB...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.81E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 8: 1697-702 (1999) BindingDB Entry DOI: 10.7270/Q26T0KRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50368446 (CHEMBL1203299) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity at bovine brain Alpha-1 adrenergic receptor in a filtration-based assay using [3H]prazosin as the radioli... | J Med Chem 35: 3973-6 (1992) BindingDB Entry DOI: 10.7270/Q29P327D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50003331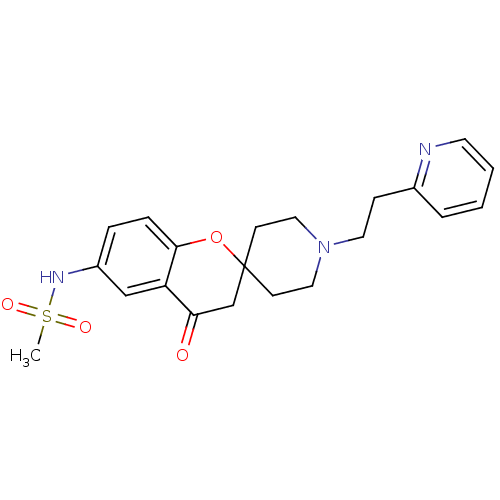 (CHEMBL101322 | CHEMBL1204312 | N-[4-oxo-1'-[2-(2-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity at bovine brain Alpha-1 adrenergic receptor in a filtration-based assay using [3H]prazosin as the radioli... | J Med Chem 35: 3973-6 (1992) BindingDB Entry DOI: 10.7270/Q29P327D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50368441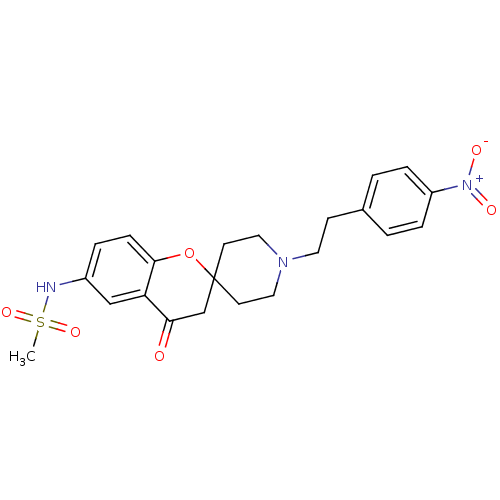 (CHEMBL1203302) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity at bovine brain Alpha-1 adrenergic receptor determined in a filtration-based assay using [3H]prazosin as ... | J Med Chem 35: 3973-6 (1992) BindingDB Entry DOI: 10.7270/Q29P327D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50368442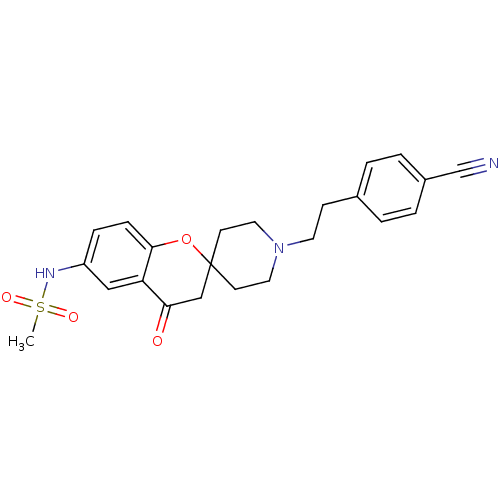 (CHEMBL1203297) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity at bovine brain Alpha-1 adrenergic receptor in a filtration-based assay using [3H]prazosin as the radioli... | J Med Chem 35: 3973-6 (1992) BindingDB Entry DOI: 10.7270/Q29P327D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50003335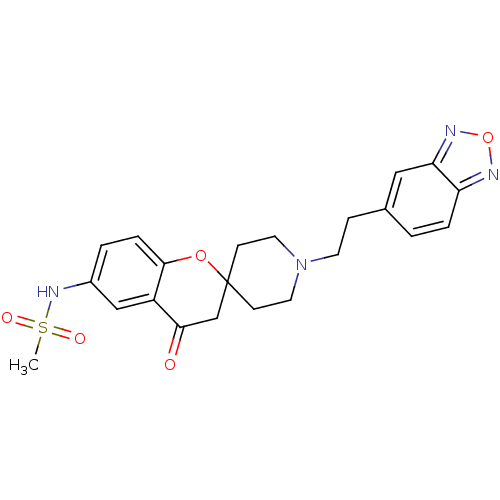 (CHEMBL1204345 | CHEMBL129583 | L-691121 | N-[1''-(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity at bovine brain Alpha-1 adrenergic receptor in a filtration-based assay using [3H]prazosin as the radioli... | J Med Chem 35: 3973-6 (1992) BindingDB Entry DOI: 10.7270/Q29P327D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50368447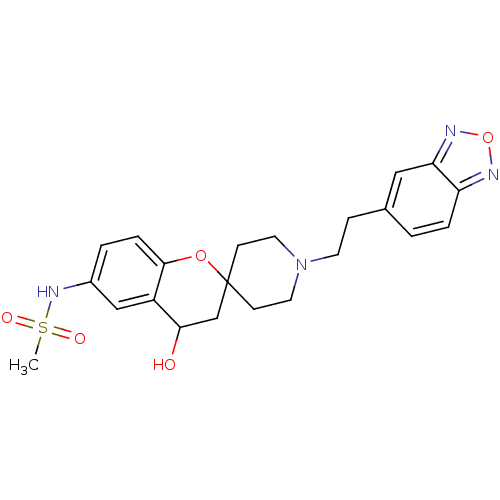 (CHEMBL1203301) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity at bovine brain Alpha-1 adrenergic receptor in a filtration-based assay using [3H]prazosin as the radioli... | J Med Chem 35: 3973-6 (1992) BindingDB Entry DOI: 10.7270/Q29P327D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50368445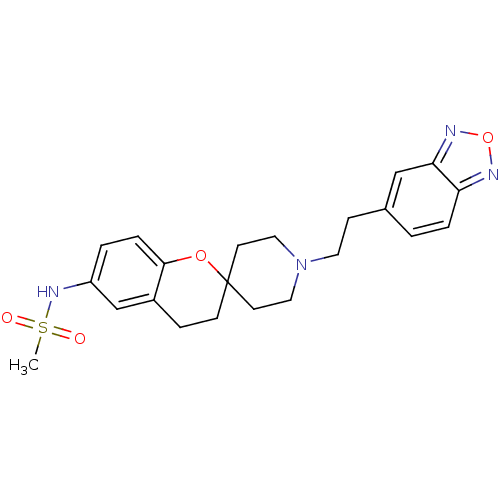 (CHEMBL1203300) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity at bovine brain Alpha-1 adrenergic receptor in a filtration-based assay using [3H]prazosin as the radioli... | J Med Chem 35: 3973-6 (1992) BindingDB Entry DOI: 10.7270/Q29P327D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50368443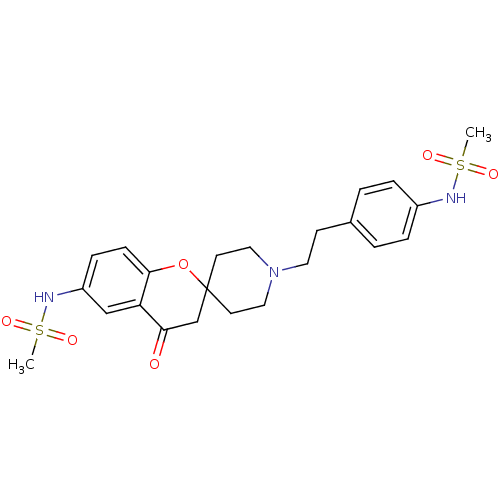 (CHEMBL1203296) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity at bovine brain Alpha-1 adrenergic receptor in a filtration-based assay using [3H]prazosin as the radioli... | J Med Chem 35: 3973-6 (1992) BindingDB Entry DOI: 10.7270/Q29P327D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||