

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 20.5 | -45.6 | 23.1 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob... | J Med Chem 46: 2279-82 (2003) Article DOI: 10.1021/jm0340602 BindingDB Entry DOI: 10.7270/Q29Z9332 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10949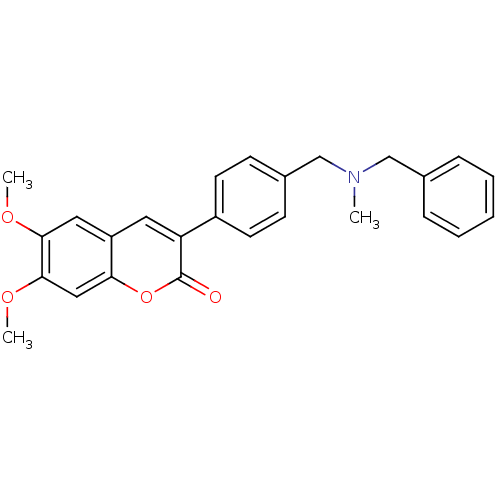 (3-(4-{[Benzyl(methyl)amino]methyl}-phenyl)-6,7-dim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21.7 | -45.5 | 44.5 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob... | J Med Chem 46: 2279-82 (2003) Article DOI: 10.1021/jm0340602 BindingDB Entry DOI: 10.7270/Q29Z9332 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50308280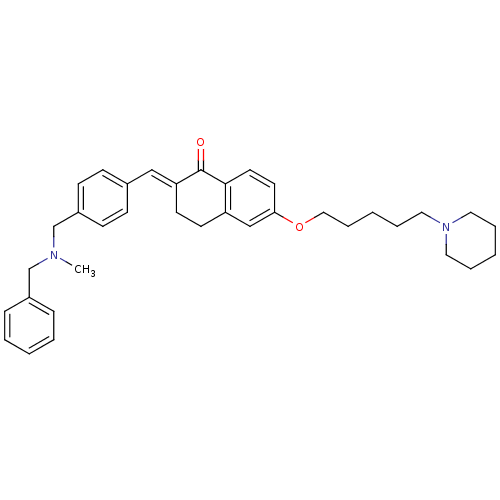 (2-{4-[(Benzylmethylamino)methyl]benzylidene}-6-(5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 61.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE-mediated hydrolysis of acetylcholine by Lineweaver-Burk plot analysis | Bioorg Med Chem 18: 1749-60 (2010) Article DOI: 10.1016/j.bmc.2010.01.071 BindingDB Entry DOI: 10.7270/Q2P26Z7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50218506 (3-{4-[(benzylmethylamino)methyl]phenyl}-6,7-dimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | J Med Chem 50: 4250-4 (2007) Article DOI: 10.1021/jm070100g BindingDB Entry DOI: 10.7270/Q2QC036V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50218521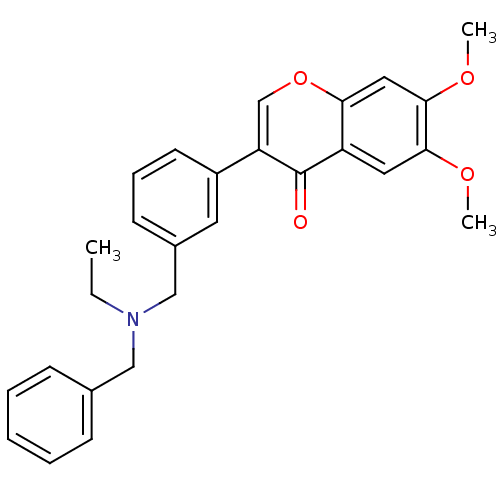 (3-{3-[(benzylethylamino)-methyl]-phenyl}-6,7-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | J Med Chem 50: 4250-4 (2007) Article DOI: 10.1021/jm070100g BindingDB Entry DOI: 10.7270/Q2QC036V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50218525 (6,7-dimethoxy-3-(4-{[(4-methoxybenzyl)methylamino]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | J Med Chem 50: 4250-4 (2007) Article DOI: 10.1021/jm070100g BindingDB Entry DOI: 10.7270/Q2QC036V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50218504 (3-{3-[(benzylmethylamino)methyl]phenyl}-6,7-dimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | J Med Chem 50: 4250-4 (2007) Article DOI: 10.1021/jm070100g BindingDB Entry DOI: 10.7270/Q2QC036V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50218507 (6,7-dimethoxy-3-(4-{[(3-nitrobenzyl)methylamino]me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | J Med Chem 50: 4250-4 (2007) Article DOI: 10.1021/jm070100g BindingDB Entry DOI: 10.7270/Q2QC036V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50218508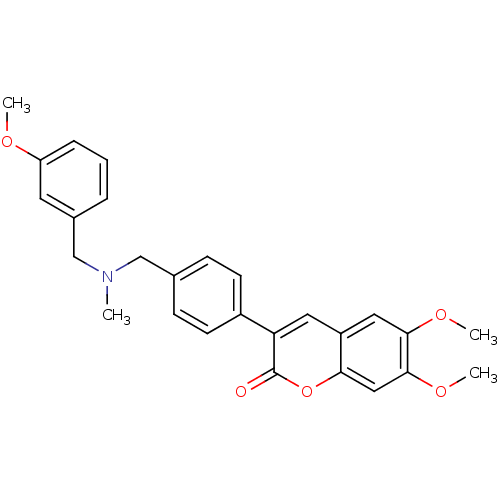 (6,7-dimethoxy-3-(4-{[(3-methoxybenzyl)methylamino]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | J Med Chem 50: 4250-4 (2007) Article DOI: 10.1021/jm070100g BindingDB Entry DOI: 10.7270/Q2QC036V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50218522 (6-amino-3-{4-[(benzylmethylamino)methyl]phenyl}-ch...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | J Med Chem 50: 4250-4 (2007) Article DOI: 10.1021/jm070100g BindingDB Entry DOI: 10.7270/Q2QC036V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50218519 (6,7-dimethoxy-3-(4-{[(2-nitrobenzyl)methylamino]me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | J Med Chem 50: 4250-4 (2007) Article DOI: 10.1021/jm070100g BindingDB Entry DOI: 10.7270/Q2QC036V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50218511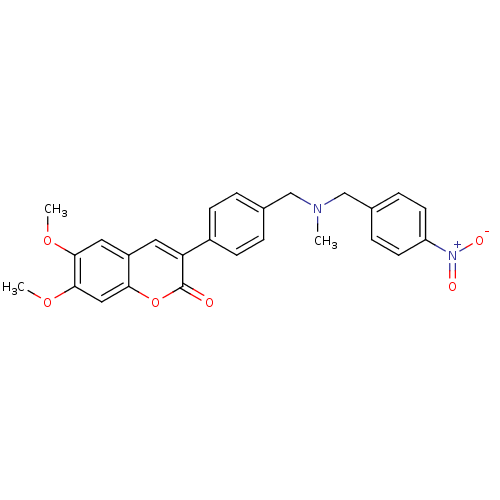 (6,7-dimethoxy-3-(4-{[(4-nitrobenzyl)methylamino]me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | J Med Chem 50: 4250-4 (2007) Article DOI: 10.1021/jm070100g BindingDB Entry DOI: 10.7270/Q2QC036V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50218524 (6-[(benzylmethylamino)methyl]-2,3-dimethoxyxanthen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | J Med Chem 50: 4250-4 (2007) Article DOI: 10.1021/jm070100g BindingDB Entry DOI: 10.7270/Q2QC036V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50218529 (6,7-dimethoxy-3-(4-{[(4-methylbenzyl)methylamino]m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | J Med Chem 50: 4250-4 (2007) Article DOI: 10.1021/jm070100g BindingDB Entry DOI: 10.7270/Q2QC036V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50218526 (6,7-dimethoxy-3-(4-{[(3-methylbenzyl)methylamino]m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | J Med Chem 50: 4250-4 (2007) Article DOI: 10.1021/jm070100g BindingDB Entry DOI: 10.7270/Q2QC036V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50218516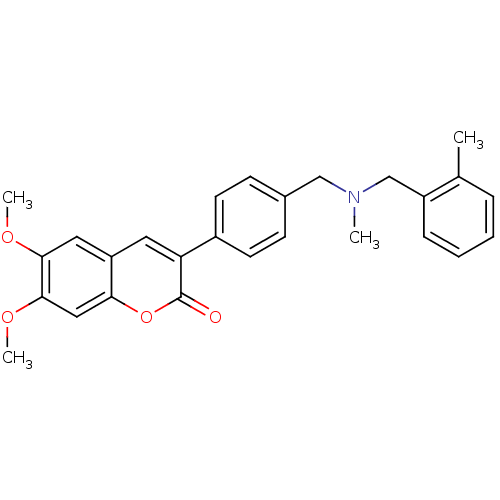 (6,7-dimethoxy-3-(4-{[(2-methylbenzyl)methylamino]m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | J Med Chem 50: 4250-4 (2007) Article DOI: 10.1021/jm070100g BindingDB Entry DOI: 10.7270/Q2QC036V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50218523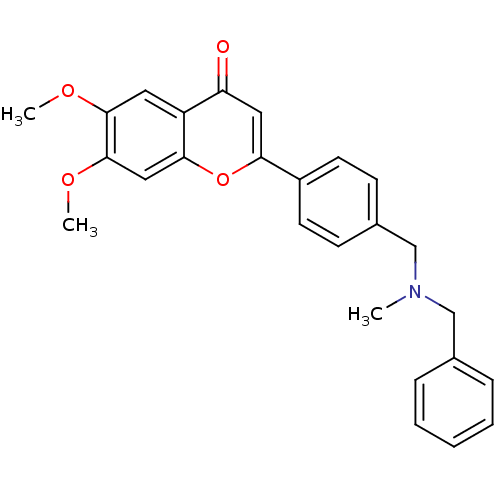 (2-{4-[(benzylmethylamino)methyl]phenyl}-6,7-dimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | J Med Chem 50: 4250-4 (2007) Article DOI: 10.1021/jm070100g BindingDB Entry DOI: 10.7270/Q2QC036V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50218503 (3-{3-[(benzylmethylamino)methyl]phenyl}-7-methoxyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | J Med Chem 50: 4250-4 (2007) Article DOI: 10.1021/jm070100g BindingDB Entry DOI: 10.7270/Q2QC036V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50218514 (2-{4-[(benzylmethylamino)methyl]phenyl}-7-methoxyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | J Med Chem 50: 4250-4 (2007) Article DOI: 10.1021/jm070100g BindingDB Entry DOI: 10.7270/Q2QC036V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50218510 (3-{4-[(benzylmethylamino)methyl]phenyl}-6-nitrochr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | J Med Chem 50: 4250-4 (2007) Article DOI: 10.1021/jm070100g BindingDB Entry DOI: 10.7270/Q2QC036V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50218509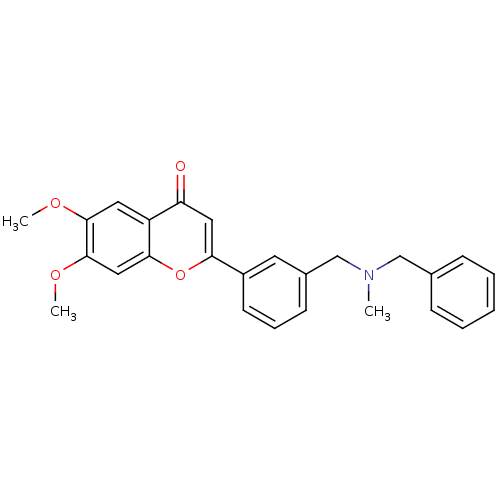 (2-{3-[(benzylmethylamino)methyl]phenyl}-6,7-dimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >0 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant BChE | J Med Chem 50: 4250-4 (2007) Article DOI: 10.1021/jm070100g BindingDB Entry DOI: 10.7270/Q2QC036V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10692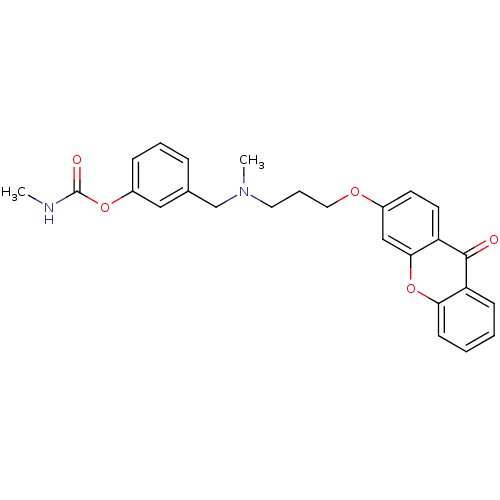 (3-{[methyl({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl})...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AchE | Bioorg Med Chem 15: 575-85 (2006) Article DOI: 10.1016/j.bmc.2006.09.026 BindingDB Entry DOI: 10.7270/Q2JS9Q2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10692 (3-{[methyl({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl})...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10693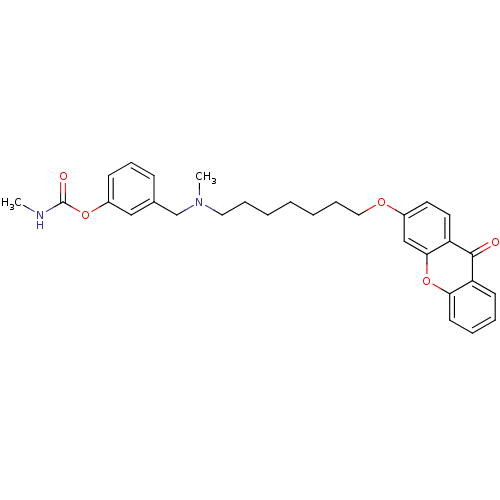 (3-{[methyl({7-[(9-oxo-9H-xanthen-3-yl)oxy]heptyl})...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 3810-20 (2001) Article DOI: 10.1021/jm010914b BindingDB Entry DOI: 10.7270/Q2MG7MR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10693 (3-{[methyl({7-[(9-oxo-9H-xanthen-3-yl)oxy]heptyl})...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10693 (3-{[methyl({7-[(9-oxo-9H-xanthen-3-yl)oxy]heptyl})...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AchE | Bioorg Med Chem 15: 575-85 (2006) Article DOI: 10.1016/j.bmc.2006.09.026 BindingDB Entry DOI: 10.7270/Q2JS9Q2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10697 (3-{[methyl(7-{[(2Z)-3-oxo-2-(3,4,5-trimethoxybenzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10710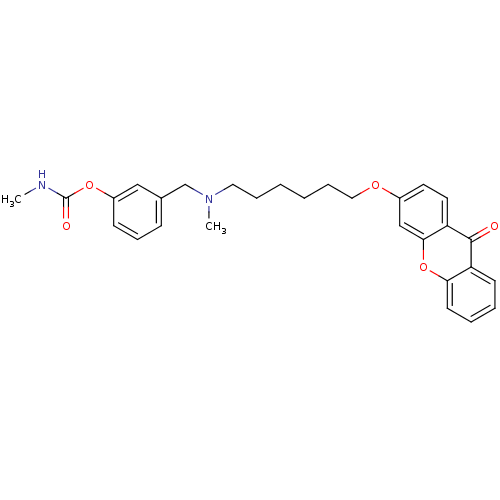 (3-{[methyl({6-[(9-oxo-9H-xanthen-3-yl)oxy]hexyl})a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 3810-20 (2001) Article DOI: 10.1021/jm010914b BindingDB Entry DOI: 10.7270/Q2MG7MR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10704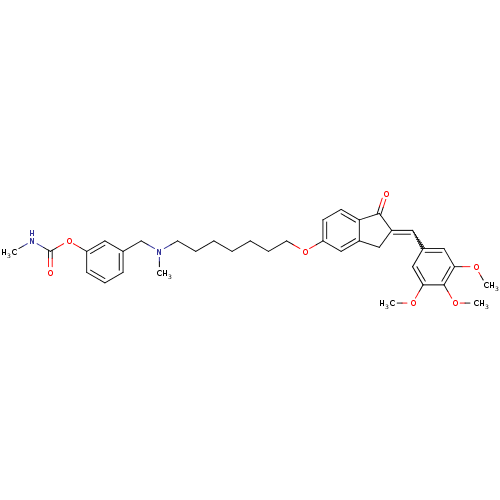 (3-{[methyl(7-{[(2E)-1-oxo-2-(3,4,5-trimethoxybenzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10701 (3-{[(7-{[(2Z)-2-benzylidene-3-oxo-2,3-dihydro-1-be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.95 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10699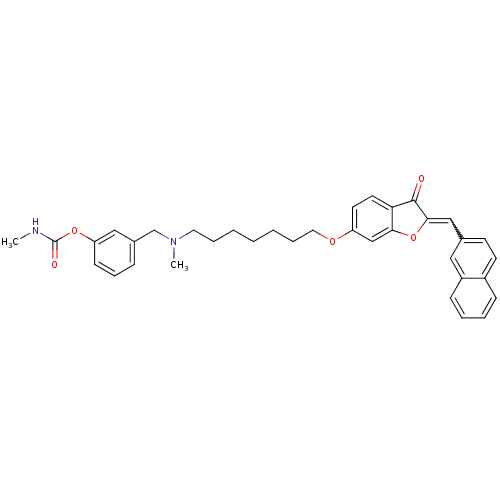 (3-{[methyl(7-{[(2Z)-2-(2-naphthylmethylene)-3-oxo-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.09 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9914 (3-[(R)-1H-imidazol-1-yl(4-nitrophenyl)methyl]-4H-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bologna | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta,2beta-3H]testosterone during aromatization. After incubation, the ... | J Med Chem 48: 7282-9 (2005) Article DOI: 10.1021/jm058042r BindingDB Entry DOI: 10.7270/Q2JS9NN9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10698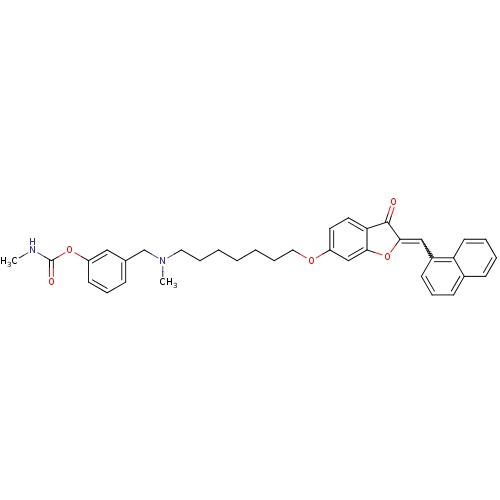 (3-{[methyl(7-{[(2Z)-2-(1-naphthylmethylene)-3-oxo-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.76 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10694 (3-{[methyl(3-{[(2Z)-3-oxo-2-(3,4,5-trimethoxybenzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.99 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10725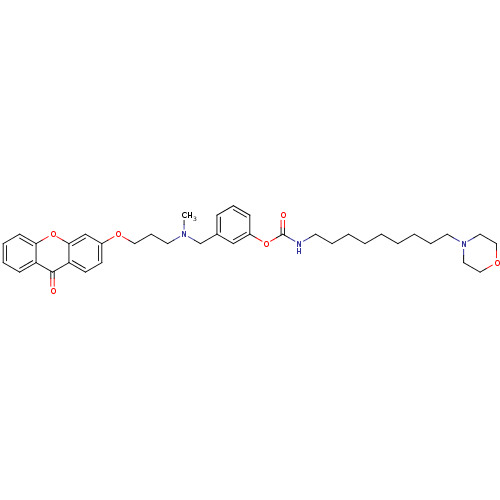 (3-{[methyl({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl})...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 3810-20 (2001) Article DOI: 10.1021/jm010914b BindingDB Entry DOI: 10.7270/Q2MG7MR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10696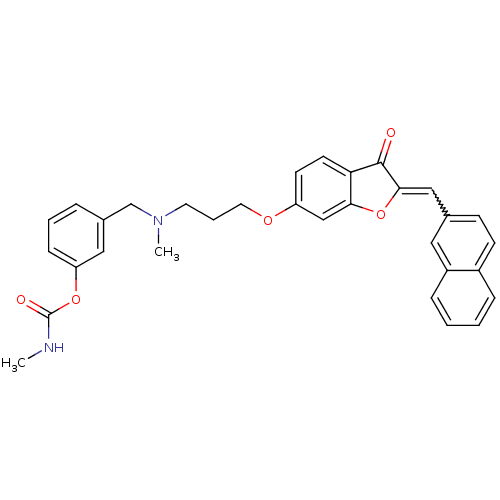 (3-{[methyl(3-{[(2Z)-2-(2-naphthylmethylene)-3-oxo-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.41 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10724 (3-{[methyl({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl})...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 3810-20 (2001) Article DOI: 10.1021/jm010914b BindingDB Entry DOI: 10.7270/Q2MG7MR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10723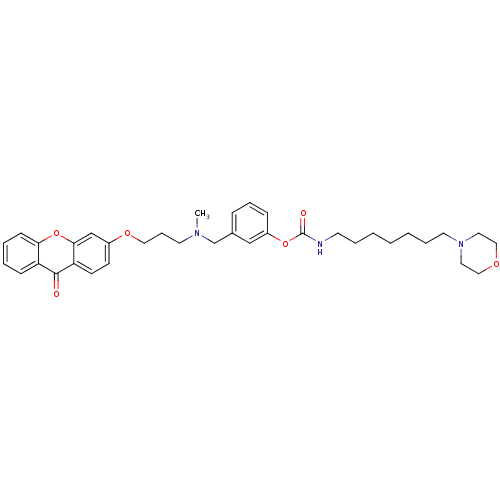 (3-{[methyl({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl})...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 3810-20 (2001) Article DOI: 10.1021/jm010914b BindingDB Entry DOI: 10.7270/Q2MG7MR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10706 (3-{[methyl(7-{[(6E)-5-oxo-6-(3,4,5-trimethoxybenzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.94 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50216367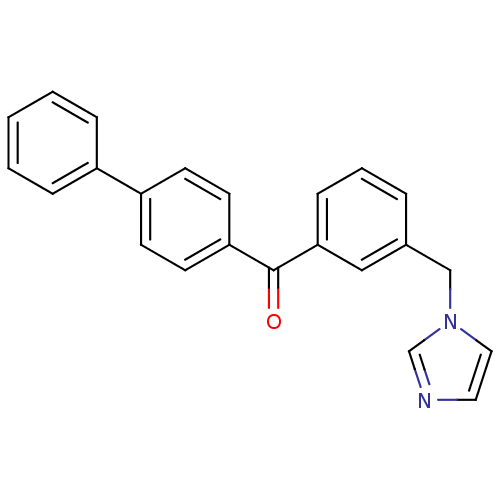 (CHEMBL243961 | biphenyl-4-yl-(3-imidazol-1-ylmethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes | J Med Chem 50: 3420-2 (2007) Article DOI: 10.1021/jm0702938 BindingDB Entry DOI: 10.7270/Q2TD9X34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10715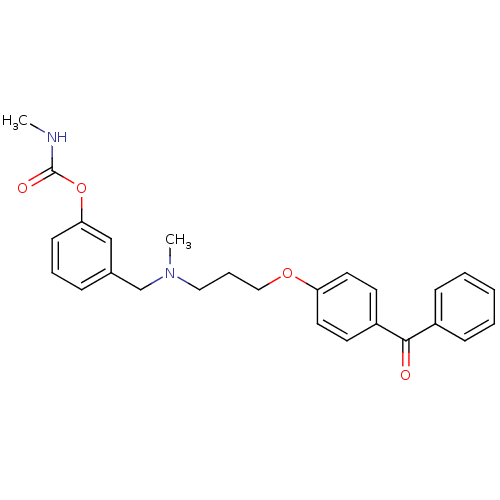 (3-({[3-(4-benzoylphenoxy)propyl](methyl)amino}meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 3810-20 (2001) Article DOI: 10.1021/jm010914b BindingDB Entry DOI: 10.7270/Q2MG7MR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10712 (3-{[methyl({8-[(9-oxo-9H-xanthen-3-yl)oxy]octyl})a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 3810-20 (2001) Article DOI: 10.1021/jm010914b BindingDB Entry DOI: 10.7270/Q2MG7MR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10695 (3-{[methyl(3-{[(2Z)-2-(1-naphthylmethylene)-3-oxo-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.86 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM50216364 ((3-((1H-imidazol-1-yl)methyl)phenyl)(phenyl)methan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of CYP19 in human placental microsomes | J Med Chem 50: 3420-2 (2007) Article DOI: 10.1021/jm0702938 BindingDB Entry DOI: 10.7270/Q2TD9X34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10722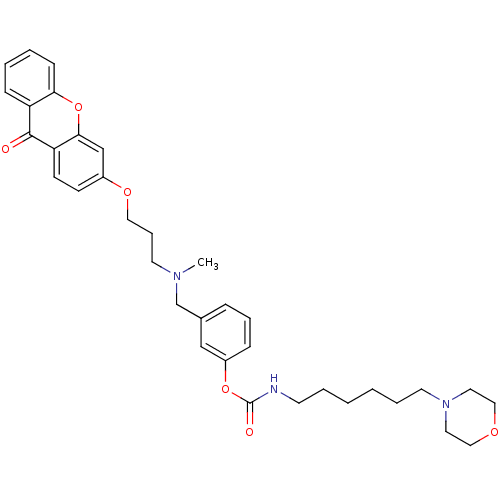 (3-{[methyl({3-[(9-oxo-9H-xanthen-3-yl)oxy]propyl})...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 3810-20 (2001) Article DOI: 10.1021/jm010914b BindingDB Entry DOI: 10.7270/Q2MG7MR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10702 (3-{[(7-{[(2Z)-2-[(3,4-dichlorophenyl)methylidene]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10705 (3-{[methyl(7-{[(3E)-4-oxo-3-(3,4,5-trimethoxybenzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.81 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 48: 4444-56 (2005) Article DOI: 10.1021/jm049515h BindingDB Entry DOI: 10.7270/Q2R78CF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8987 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The appearance of product was monitored at 412 nm for 5 min us... | J Med Chem 43: 2007-18 (2000) Article DOI: 10.1021/jm990971t BindingDB Entry DOI: 10.7270/Q2057D4R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50218515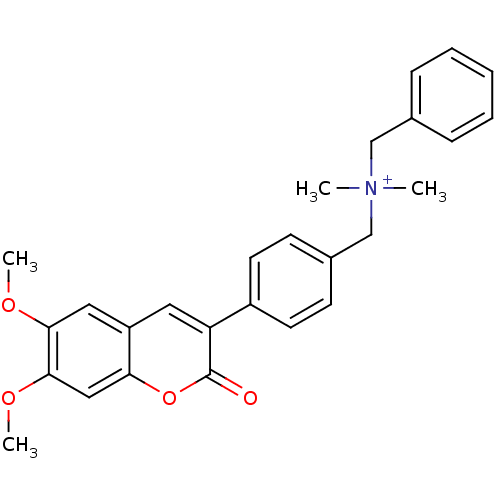 (CHEMBL396199 | benzyl-[4-(6,7-dimethoxy-2-oxo-2H-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE | J Med Chem 50: 4250-4 (2007) Article DOI: 10.1021/jm070100g BindingDB Entry DOI: 10.7270/Q2QC036V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10713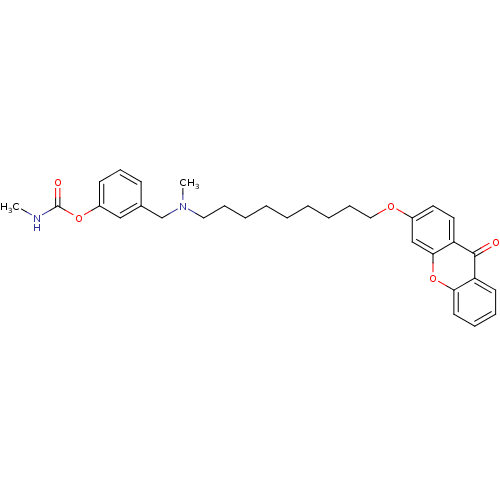 (3-{[methyl({9-[(9-oxo-9H-xanthen-3-yl)oxy]nonyl})a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.7 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Bologna | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 44: 3810-20 (2001) Article DOI: 10.1021/jm010914b BindingDB Entry DOI: 10.7270/Q2MG7MR0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 368 total ) | Next | Last >> |