

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8611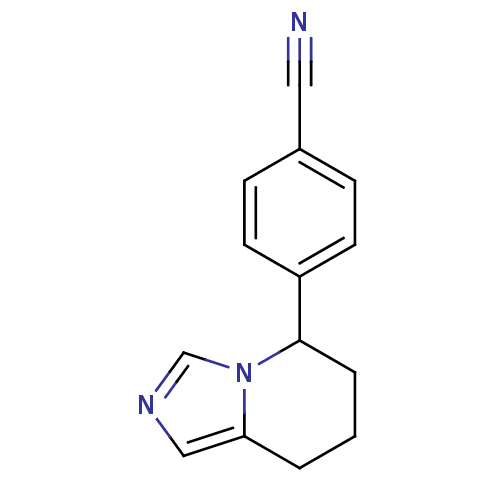 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50438994 (CHEMBL2420683) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using 1,2[3H]-progesterone as substrate in presence of NADPH | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50438994 (CHEMBL2420683) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50438995 (CHEMBL2420682) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50438993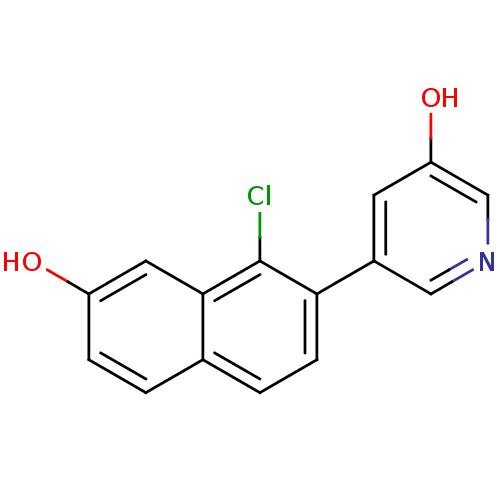 (CHEMBL2420684) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using 1,2[3H]-progesterone as substrate in presence of NADPH | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50439002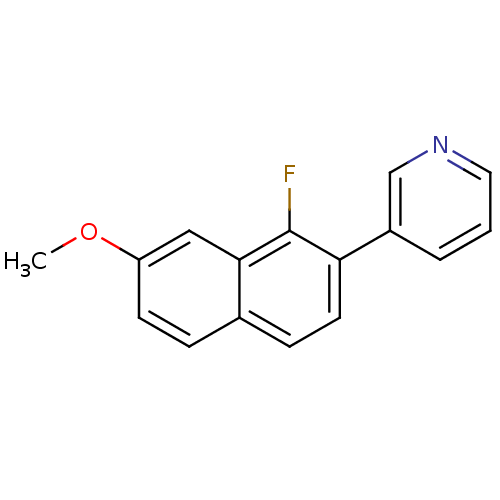 (CHEMBL2420675) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50438991 (CHEMBL2420686) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50438996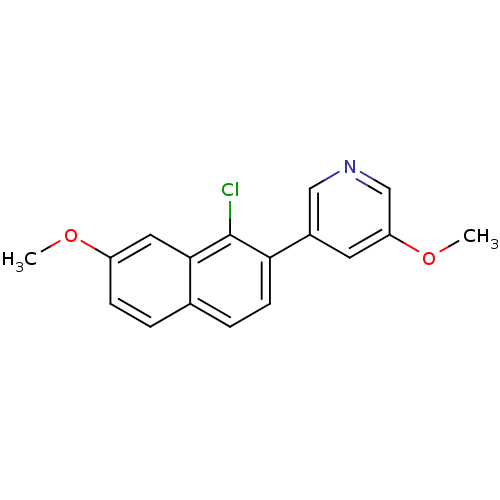 (CHEMBL2420681) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50438999 (CHEMBL2420678) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50439003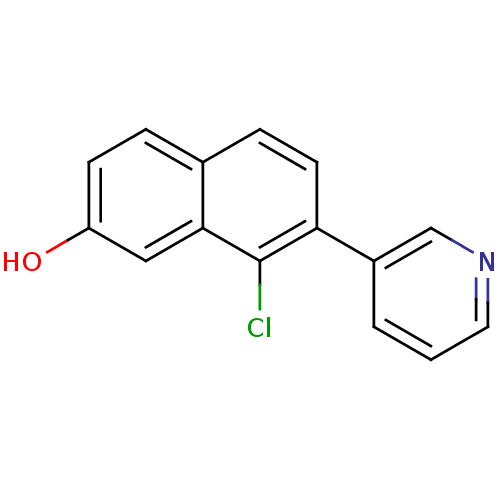 (CHEMBL2420674) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50438993 (CHEMBL2420684) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM8913 (3-(1-Chloro-7-methoxy-2-naphthyl)pyridine | 3-(1-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50439000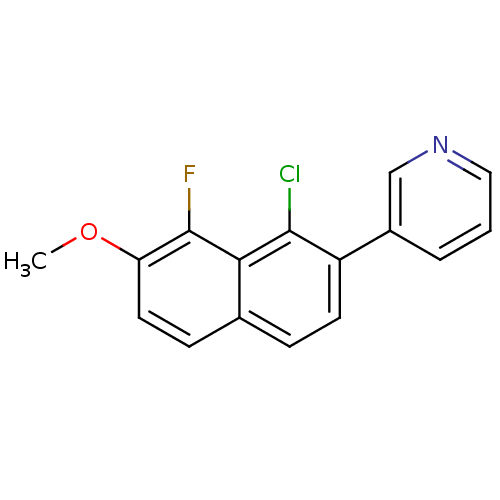 (CHEMBL2420677) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8904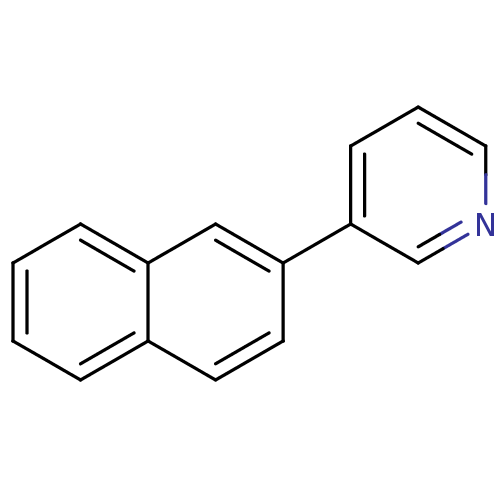 (3-(2-Naphthyl)pyridine | 3-(naphthalen-2-yl)pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8913 (3-(1-Chloro-7-methoxy-2-naphthyl)pyridine | 3-(1-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50439001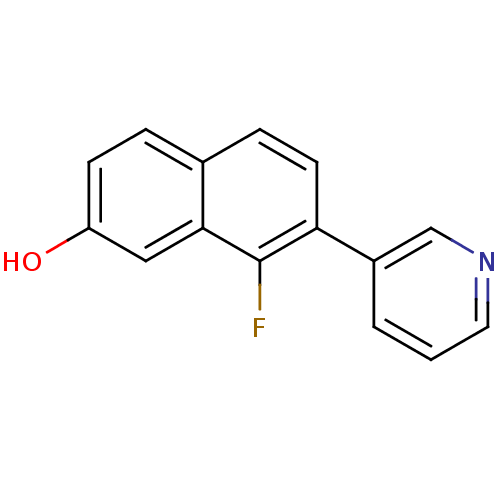 (CHEMBL2420676) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50438997 (CHEMBL2420680) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50438998 (CHEMBL2420679) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50438990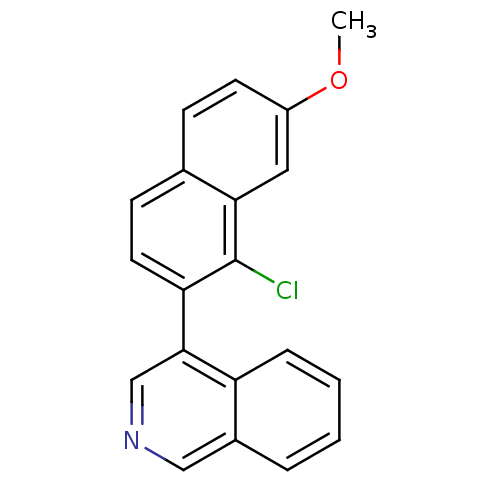 (CHEMBL2420687) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50439003 (CHEMBL2420674) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using 1,2[3H]-progesterone as substrate in presence of NADPH | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25457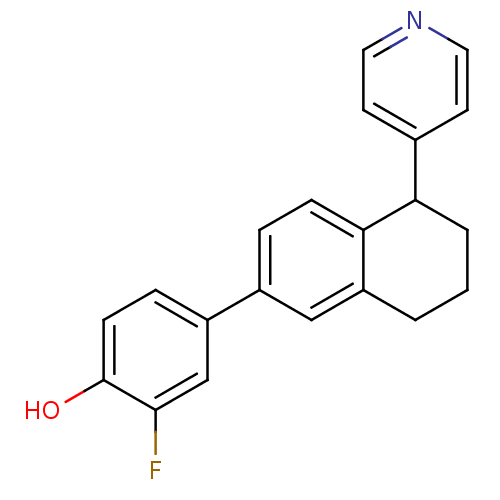 (2-fluoro-4-[5-(pyridin-4-yl)-5,6,7,8-tetrahydronap...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50438989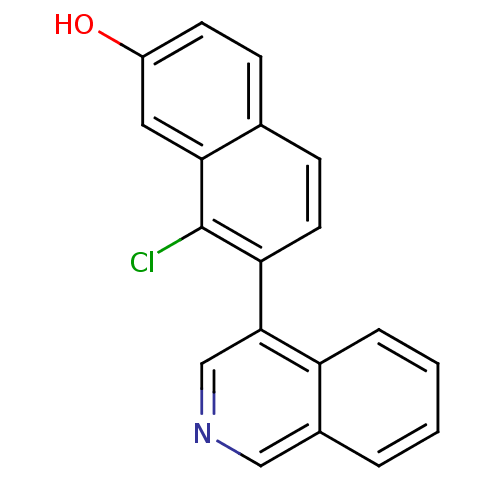 (CHEMBL2420688) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50439004 (CHEMBL193863) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using 1,2[3H]-progesterone as substrate in presence of NADPH | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli | Bioorg Med Chem Lett 18: 267-73 (2008) Article DOI: 10.1016/j.bmcl.2007.10.079 BindingDB Entry DOI: 10.7270/Q2833RSP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli co-expressed with NADPH-P450 reductase | Bioorg Med Chem 16: 1992-2010 (2008) Article DOI: 10.1016/j.bmc.2007.10.094 BindingDB Entry DOI: 10.7270/Q29W0GBN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50438992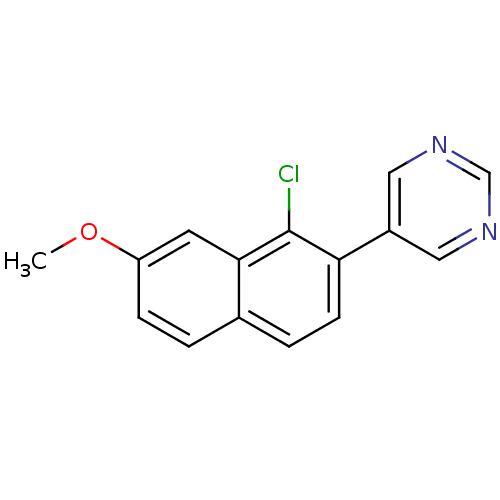 (CHEMBL2420685) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description To measure CYP3A4 activity, the product of testosterone 6-hydroxylation, 6beta-hydroxytestosterone was determined. After incubation, the reaction was... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50438996 (CHEMBL2420681) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using 1,2[3H]-progesterone as substrate in presence of NADPH | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50439002 (CHEMBL2420675) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using 1,2[3H]-progesterone as substrate in presence of NADPH | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM25448 (4-[6-(4-fluorophenyl)-3,4-dihydronaphthalen-1-yl]p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25456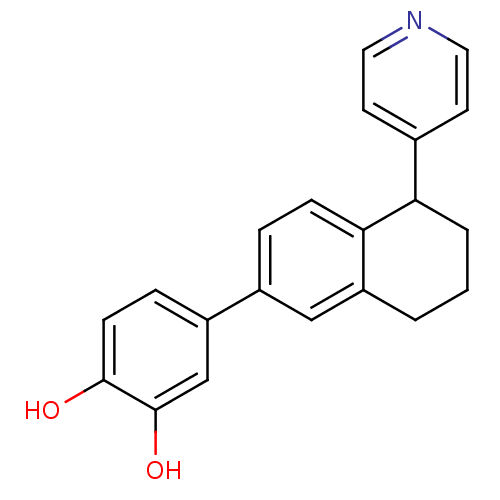 (4-[5-(pyridin-4-yl)-5,6,7,8-tetrahydronaphthalen-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50439000 (CHEMBL2420677) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using 1,2[3H]-progesterone as substrate in presence of NADPH | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM25446 (4-[6-(4-fluorophenyl)-1H-inden-3-yl]pyridine | Abi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description To measure CYP3A4 activity, the product of testosterone 6-hydroxylation, 6beta-hydroxytestosterone was determined. After incubation, the reaction was... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25454 (4-[6-(4-fluorophenyl)-1,2,3,4-tetrahydronaphthalen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 163 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50438995 (CHEMBL2420682) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli using 1,2[3H]-progesterone as substrate in presence of NADPH | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25451 (2-fluoro-4-[5-(pyridin-4-yl)-7,8-dihydronaphthalen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 188 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM25458 ((1S,2R,5S,10R,11S,15S)-2,15-dimethyl-14-(pyridin-3...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat testicular CYP17 | Bioorg Med Chem 16: 1992-2010 (2008) Article DOI: 10.1016/j.bmc.2007.10.094 BindingDB Entry DOI: 10.7270/Q29W0GBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50438999 (CHEMBL2420678) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM25453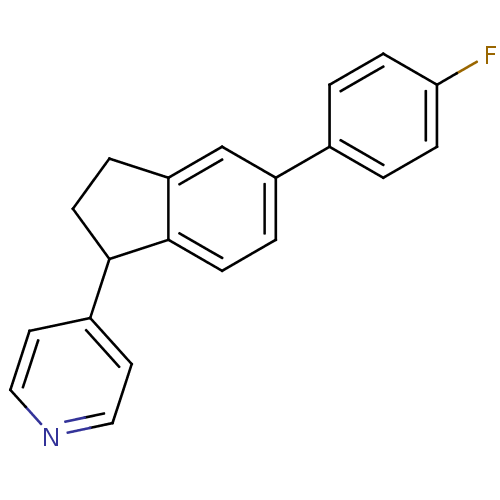 (4-[5-(4-fluorophenyl)-2,3-dihydro-1H-inden-1-yl]py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 233 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Saarland University | Assay Description The 17 alpha-hydroxylase activity of CYP 17 was determined by measuring the conversion of progesterone into 17 alpha-hydroxyprogesterone and the bypr... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50439003 (CHEMBL2420674) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 235 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50371480 (CHEMBL403808) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 236 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli co-expressed with NADPH-P450 reductase | Bioorg Med Chem 16: 1992-2010 (2008) Article DOI: 10.1016/j.bmc.2007.10.094 BindingDB Entry DOI: 10.7270/Q29W0GBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50438989 (CHEMBL2420688) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50438995 (CHEMBL2420682) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University& Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZh cells using [1,2-3H]-11-deoxycorticosterone as substrate | J Med Chem 56: 6101-7 (2013) Article DOI: 10.1021/jm400484p BindingDB Entry DOI: 10.7270/Q2CJ8FX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50371479 (CHEMBL403852) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 263 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University Curated by ChEMBL | Assay Description Inhibition of human CYP17 expressed in Escherichia coli co-expressed with NADPH-P450 reductase | Bioorg Med Chem 16: 1992-2010 (2008) Article DOI: 10.1016/j.bmc.2007.10.094 BindingDB Entry DOI: 10.7270/Q29W0GBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM25441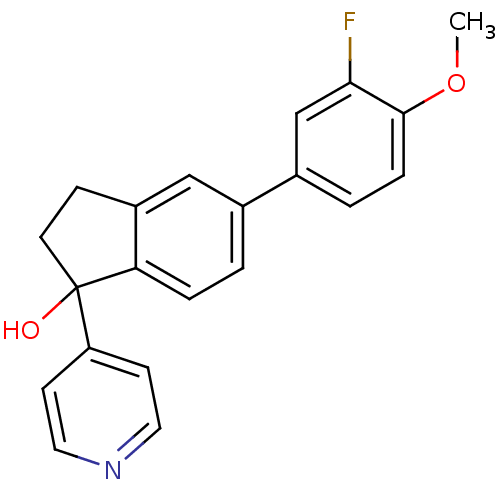 (5-(3-fluoro-4-methoxyphenyl)-1-(pyridin-4-yl)-2,3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 291 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by monitoring the conversion of deoxycorticosterone to corticosterone in the presence of inhibitor compounds. The pro... | J Med Chem 51: 5009-18 (2008) Article DOI: 10.1021/jm800355c BindingDB Entry DOI: 10.7270/Q2TB156N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 164 total ) | Next | Last >> |