

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50219206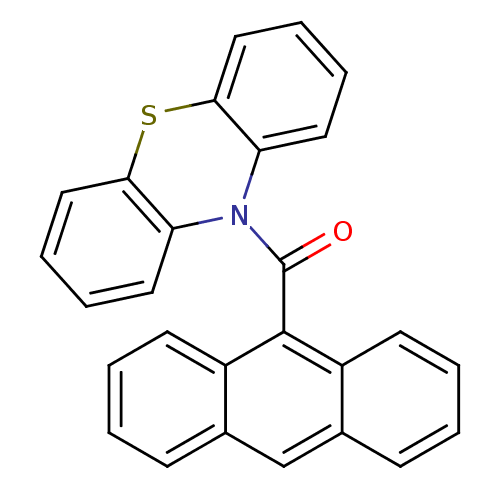 (Anthracen-9-yl (10H-phenothiazine-10yl) methanone,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova Curated by ChEMBL | Assay Description Inhibition of human plasma BChE | Eur J Med Chem 46: 2170-84 (2011) Article DOI: 10.1016/j.ejmech.2011.02.071 BindingDB Entry DOI: 10.7270/Q2BK1CPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50219206 (Anthracen-9-yl (10H-phenothiazine-10yl) methanone,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova Curated by ChEMBL | Assay Description Inhibition of human erythrocytes BChE | Eur J Med Chem 46: 2170-84 (2011) Article DOI: 10.1016/j.ejmech.2011.02.071 BindingDB Entry DOI: 10.7270/Q2BK1CPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50424045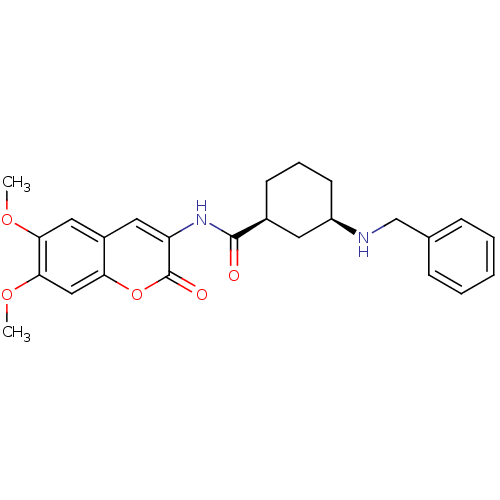 (CHEMBL2314726) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Mixed-type reversible inhibition of bovine acetylcholinesterase using S-acetylthiocholine as substrate incubated for 20 mins prior to substrate addit... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50585934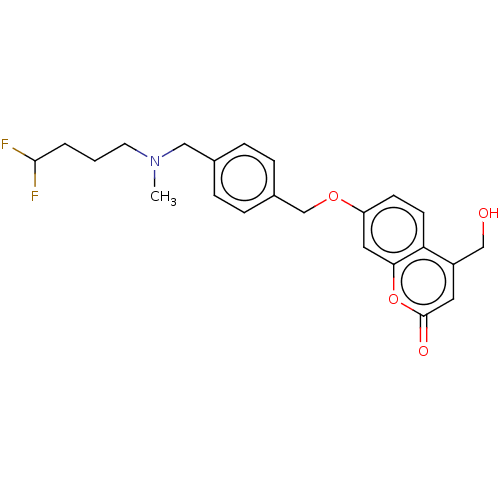 (CHEMBL5082824) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of human recombinant MAO-B expressed in supersomes using kynuramine as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01784 BindingDB Entry DOI: 10.7270/Q2BG2SWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari Aldo Moro Curated by ChEMBL | Assay Description Mixed type inhibition of human AChE using acetylthiocholineiodide as substrate measured for every 30 sec for 5 mins by Ellman's method | ACS Med Chem Lett 11: 869-876 (2020) Article DOI: 10.1021/acsmedchemlett.9b00656 BindingDB Entry DOI: 10.7270/Q2WS8XS4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50540764 (CHEMBL4648322) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari Aldo Moro Curated by ChEMBL | Assay Description Mixed type inhibition of human AChE using acetylthiocholineiodide as substrate measured for every 30 sec for 5 mins by Ellman's method | ACS Med Chem Lett 11: 869-876 (2020) Article DOI: 10.1021/acsmedchemlett.9b00656 BindingDB Entry DOI: 10.7270/Q2WS8XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50342853 (4-(6,7-Dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Granada Curated by ChEMBL | Assay Description Inhibition of bovine AChE at 30 nM using S-acetylthiocholine as as substrate by Lineweaver-Burk plot analysis | J Med Chem 54: 2627-45 (2011) Article DOI: 10.1021/jm101299d BindingDB Entry DOI: 10.7270/Q2SQ90QT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093085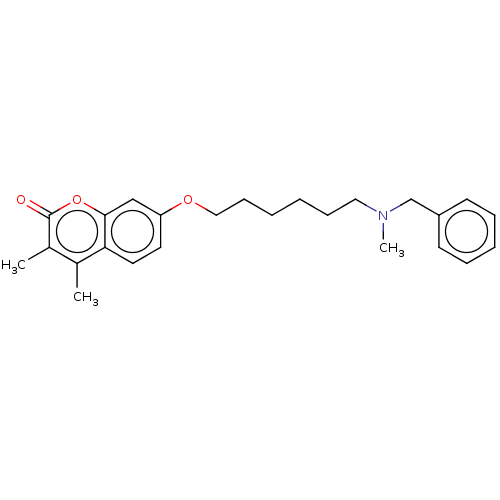 (CHEMBL3586582) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Mixed type inhibition of electric eel AChE by Lineweaver-Burk plot | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50424045 (CHEMBL2314726) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari Aldo Moro Curated by ChEMBL | Assay Description Mixed type inhibition of human AChE using acetylthiocholineiodide as substrate measured for every 30 sec for 5 mins by Ellman's method | ACS Med Chem Lett 11: 869-876 (2020) Article DOI: 10.1021/acsmedchemlett.9b00656 BindingDB Entry DOI: 10.7270/Q2WS8XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50532731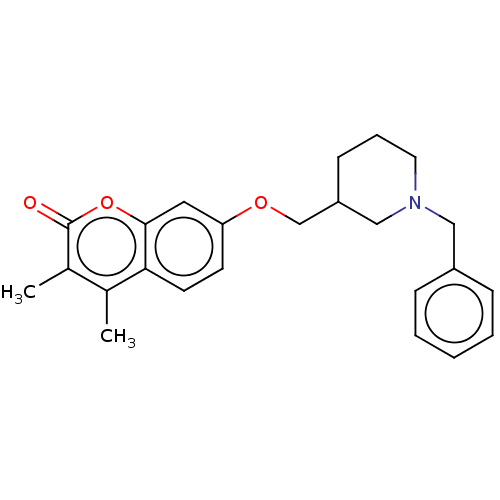 (CHEMBL4571763) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00001 BindingDB Entry DOI: 10.7270/Q2R49VSW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50424045 (CHEMBL2314726) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 93.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari Aldo Moro Curated by ChEMBL | Assay Description Mixed type inhibition of human AChE using acetylthiocholineiodide as substrate measured for every 30 sec for 5 mins by Ellman's method | ACS Med Chem Lett 11: 869-876 (2020) Article DOI: 10.1021/acsmedchemlett.9b00656 BindingDB Entry DOI: 10.7270/Q2WS8XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50524749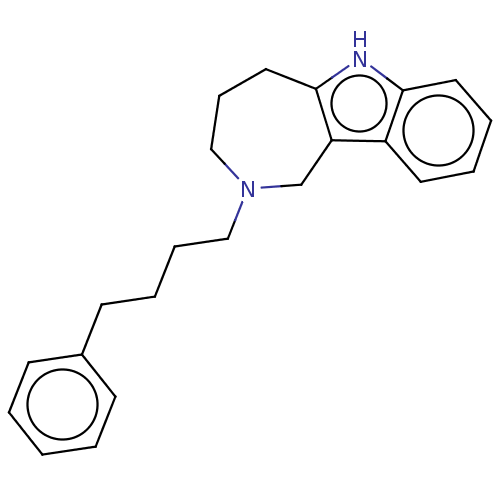 (CHEMBL4538834) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari Aldo Moro Curated by ChEMBL | Assay Description Mixed type inhibition of equine serum BChE assessed as Ki using butyrylthiocholine as substrate preincubated for 20 mins followed by substrate additi... | Eur J Med Chem 177: 414-424 (2019) Article DOI: 10.1016/j.ejmech.2019.05.062 BindingDB Entry DOI: 10.7270/Q2RF5ZGP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM19188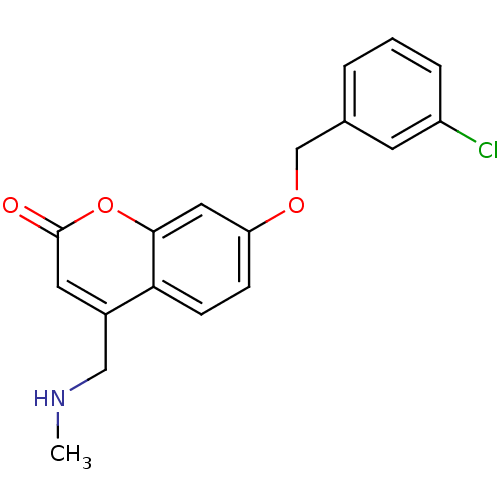 (7-(3-chlorobenzyloxy)-4-(methylamino)methyl-coumar...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 100 | -40.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Pavia | Assay Description MAO B activities were determined spectrophotometrically at 250 nm using benzylamine as substrate. Competitive Ki values were determined by measuring ... | J Med Chem 50: 5848-5852 (2007) Article DOI: 10.1021/jm070677y BindingDB Entry DOI: 10.7270/Q2DN43B4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50093227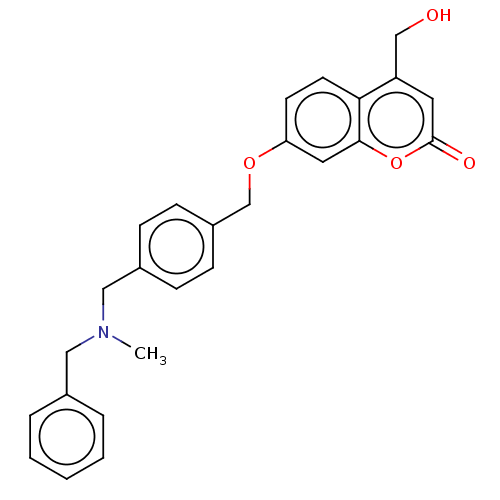 (CHEMBL3586608) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Mixed type inhibition of electric eel AChE by Lineweaver-Burk plot | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50532731 (CHEMBL4571763) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00001 BindingDB Entry DOI: 10.7270/Q2R49VSW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50532731 (CHEMBL4571763) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00001 BindingDB Entry DOI: 10.7270/Q2R49VSW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM19189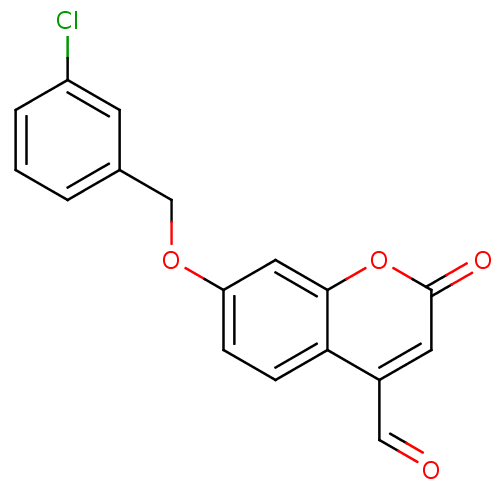 (7-(3-Chlorobenzyloxy)-4-carboxaldehyde-coumarin, 3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 400 | -36.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Pavia | Assay Description MAO B activities were determined spectrophotometrically at 250 nm using benzylamine as substrate. Competitive Ki values were determined by measuring ... | J Med Chem 50: 5848-5852 (2007) Article DOI: 10.1021/jm070677y BindingDB Entry DOI: 10.7270/Q2DN43B4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM19187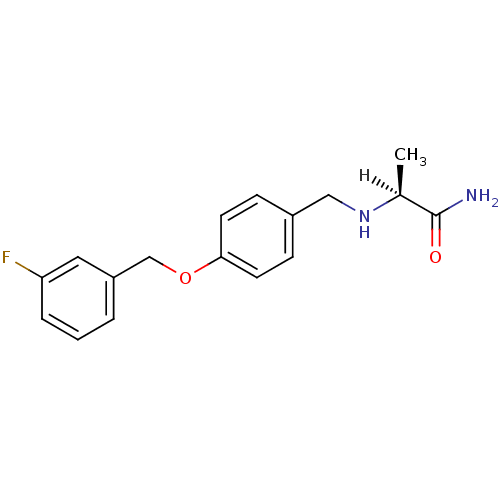 ((2S)-2-[({4-[(3-fluorophenyl)methoxy]phenyl}methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 450 | -36.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Pavia | Assay Description MAO B activities were determined spectrophotometrically at 250 nm using benzylamine as substrate. Competitive Ki values were determined by measuring ... | J Med Chem 50: 5848-5852 (2007) Article DOI: 10.1021/jm070677y BindingDB Entry DOI: 10.7270/Q2DN43B4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50532731 (CHEMBL4571763) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari "Aldo Moro" Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using S-acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition measured fo... | J Med Chem 59: 6791-806 (2016) Article DOI: 10.1021/acs.jmedchem.6b00562 BindingDB Entry DOI: 10.7270/Q25142Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50532731 (CHEMBL4571763) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari "Aldo Moro" Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using S-acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition measured fo... | J Med Chem 59: 6791-806 (2016) Article DOI: 10.1021/acs.jmedchem.6b00562 BindingDB Entry DOI: 10.7270/Q25142Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50585934 (CHEMBL5082824) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of human AChE by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01784 BindingDB Entry DOI: 10.7270/Q2BG2SWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50093227 (CHEMBL3586608) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00001 BindingDB Entry DOI: 10.7270/Q2R49VSW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM19189 (7-(3-Chlorobenzyloxy)-4-carboxaldehyde-coumarin, 3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.10E+4 | -28.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Pavia | Assay Description MAO A activities were determined spectrophotometrically at 316 nm using kynuramine as substrate. Competitive Ki values were determined by measuring i... | J Med Chem 50: 5848-5852 (2007) Article DOI: 10.1021/jm070677y BindingDB Entry DOI: 10.7270/Q2DN43B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM19188 (7-(3-chlorobenzyloxy)-4-(methylamino)methyl-coumar...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.57E+4 | -27.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Pavia | Assay Description MAO A activities were determined spectrophotometrically at 316 nm using kynuramine as substrate. Competitive Ki values were determined by measuring i... | J Med Chem 50: 5848-5852 (2007) Article DOI: 10.1021/jm070677y BindingDB Entry DOI: 10.7270/Q2DN43B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM19187 ((2S)-2-[({4-[(3-fluorophenyl)methoxy]phenyl}methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.65E+5 | -19.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Pavia | Assay Description MAO A activities were determined spectrophotometrically at 316 nm using kynuramine as substrate. Competitive Ki values were determined by measuring i... | J Med Chem 50: 5848-5852 (2007) Article DOI: 10.1021/jm070677y BindingDB Entry DOI: 10.7270/Q2DN43B4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50262988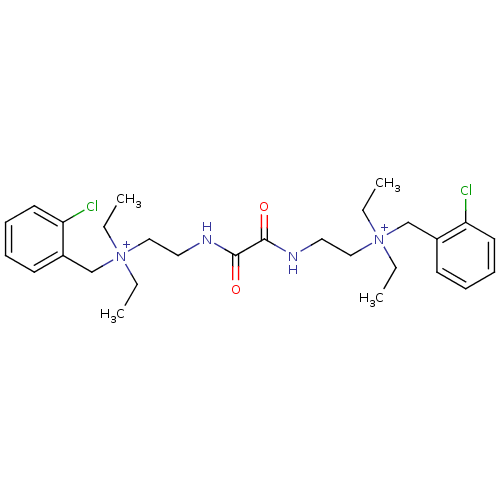 (CHEMBL1200541 | N-(2-chlorobenzyl)-2-(2-(2-((2-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description Inhibition of bovine AChE by Ellman's method | Bioorg Med Chem 16: 7450-6 (2008) Article DOI: 10.1016/j.bmc.2008.06.022 BindingDB Entry DOI: 10.7270/Q2NC611F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50262879 (3-hydroxy-5-(4-(3-hydroxy-5-(trimethylammonio)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description Inhibition of bovine AChE by Ellman's method | Bioorg Med Chem 16: 7450-6 (2008) Article DOI: 10.1016/j.bmc.2008.06.022 BindingDB Entry DOI: 10.7270/Q2NC611F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50262878 (3-hydroxy-5-(3-(3-hydroxy-5-(trimethylammonio)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description Inhibition of bovine AChE by Ellman's method | Bioorg Med Chem 16: 7450-6 (2008) Article DOI: 10.1016/j.bmc.2008.06.022 BindingDB Entry DOI: 10.7270/Q2NC611F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50523281 (CHEMBL4583650) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using ATCh as substrate preincubated for 20 mins followed by substrate addition and measured for 3 mins by Ellma... | Eur J Med Chem 168: 58-77 (2019) Article DOI: 10.1016/j.ejmech.2018.12.063 BindingDB Entry DOI: 10.7270/Q2J969S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM8611 (4-{5H,6H,7H,8H-imidazo[1,5-a]pyridin-5-yl}benzonit...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita` degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of human CYP11B2 expressed in Chinese hamster V79MZ cells using [1,2-3H]11-deoxycorticosterone/11-deoxycorticosterone | J Med Chem 54: 1613-25 (2011) Article DOI: 10.1021/jm101120u BindingDB Entry DOI: 10.7270/Q24X583Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50262926 (3-(4-(3,4-dimethyl-2-oxo-2H-chromen-7-yloxy)butoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description Inhibition of bovine AChE by Ellman's method | Bioorg Med Chem 16: 7450-6 (2008) Article DOI: 10.1016/j.bmc.2008.06.022 BindingDB Entry DOI: 10.7270/Q2NC611F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase (unknown origin) | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50262880 (3-(3-(3,4-dimethyl-2-oxo-2H-chromen-7-yloxy)propox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Bari Curated by ChEMBL | Assay Description Inhibition of bovine AChE by Ellman's method | Bioorg Med Chem 16: 7450-6 (2008) Article DOI: 10.1016/j.bmc.2008.06.022 BindingDB Entry DOI: 10.7270/Q2NC611F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari "Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA expressed in microsomes of baculovirus-infected insect cell using kynuramine as substrate preincubated for 20 mi... | J Med Chem 59: 6791-806 (2016) Article DOI: 10.1021/acs.jmedchem.6b00562 BindingDB Entry DOI: 10.7270/Q25142Q8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari "Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAOA expressed in microsomes of baculovirus-infected insect cell using kynuramine as substrate preincubated for 20 mi... | J Med Chem 59: 6791-806 (2016) Article DOI: 10.1021/acs.jmedchem.6b00562 BindingDB Entry DOI: 10.7270/Q25142Q8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM19188 (7-(3-chlorobenzyloxy)-4-(methylamino)methyl-coumar...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bari Curated by ChEMBL | Assay Description Inhibition of MAO-B from Wistar rat brain by radioenzymatic assay in presence of human platelet rich plasma | J Med Chem 52: 6685-706 (2009) Article DOI: 10.1021/jm9010127 BindingDB Entry DOI: 10.7270/Q2DR2VJ6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50038051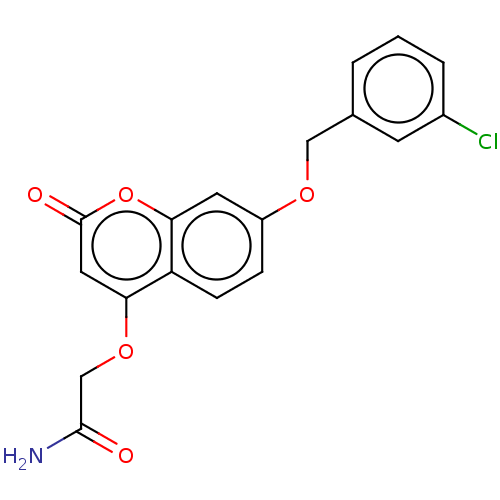 (CHEMBL3094016) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari "Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat MAO-B using Kynuramine as substrate assessed as formation of 4-hydroxyquinoline preincubated for 5 mins prior to sub... | Eur J Med Chem 70: 723-39 (2013) Article DOI: 10.1016/j.ejmech.2013.09.034 BindingDB Entry DOI: 10.7270/Q2WM1HCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50038051 (CHEMBL3094016) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari "Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat MAO-B using Kynuramine as substrate assessed as formation of 4-hydroxyquinoline preincubated for 5 mins prior to sub... | Eur J Med Chem 70: 723-39 (2013) Article DOI: 10.1016/j.ejmech.2013.09.034 BindingDB Entry DOI: 10.7270/Q2WM1HCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50038051 (CHEMBL3094016) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat MAO-B in brain mitochondrial homogenate assessed as 4-hydroxyquinoline by spectrophotometric method | Eur J Med Chem 89: 98-105 (2014) Article DOI: 10.1016/j.ejmech.2014.10.029 BindingDB Entry DOI: 10.7270/Q2FX7C3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari "Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of recombinant human MAO-A expressed in baculovirus infected insect cell microsomes using kynuramine as substrate measured after 30 mins b... | Eur J Med Chem 161: 292-309 (2019) Article DOI: 10.1016/j.ejmech.2018.10.016 BindingDB Entry DOI: 10.7270/Q2X351QR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari Aldo Moro Curated by ChEMBL | Assay Description Inhibition of bovine acetylcholinesterase using acetylcholine iodide as substrate incubated for 20 mins prior to substrate addition meausred after 3 ... | Bioorg Med Chem 21: 146-52 (2012) Article DOI: 10.1016/j.bmc.2012.10.045 BindingDB Entry DOI: 10.7270/Q2QV3NTH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM15581 (CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-A using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50339668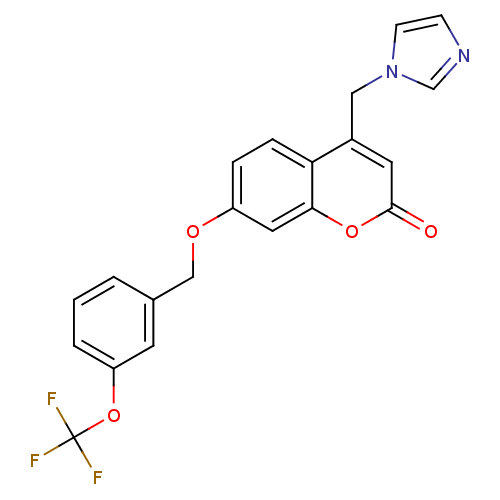 (4-(1H-Imidazol-1-ylmethyl)-7-{[3-(trifluoromethoxy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human CYP11B1 expressed in hamster V79MZh11B1 cells using [1,2-3H]-11-deoxycorticosterone substrate incubated for 25 mins by HPLC metho... | Eur J Med Chem 89: 106-14 (2014) Article DOI: 10.1016/j.ejmech.2014.10.021 BindingDB Entry DOI: 10.7270/Q2B56MC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10972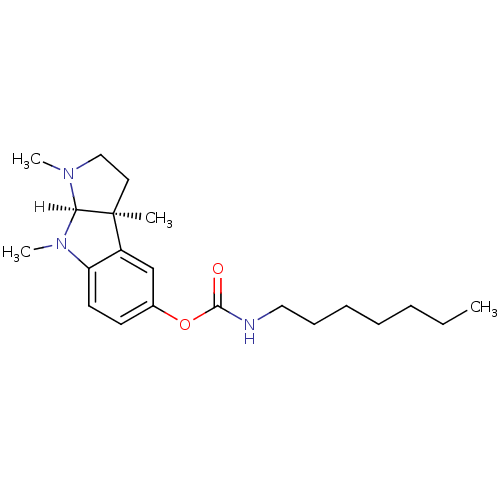 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova Curated by ChEMBL | Assay Description Inhibition of human plasma BChE | Eur J Med Chem 46: 2170-84 (2011) Article DOI: 10.1016/j.ejmech.2011.02.071 BindingDB Entry DOI: 10.7270/Q2BK1CPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10972 ((3aS,8aR)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova Curated by ChEMBL | Assay Description Inhibition of human erythrocytes BChE | Eur J Med Chem 46: 2170-84 (2011) Article DOI: 10.1016/j.ejmech.2011.02.071 BindingDB Entry DOI: 10.7270/Q2BK1CPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50038179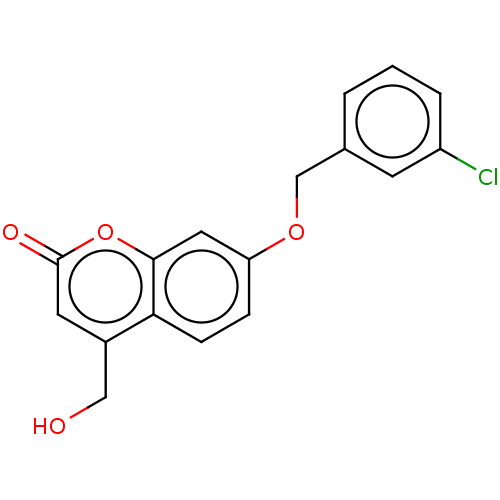 (CHEMBL3094037) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari "Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat MAO-B using Kynuramine as substrate assessed as formation of 4-hydroxyquinoline preincubated for 5 mins prior to sub... | Eur J Med Chem 70: 723-39 (2013) Article DOI: 10.1016/j.ejmech.2013.09.034 BindingDB Entry DOI: 10.7270/Q2WM1HCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50038179 (CHEMBL3094037) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat MAO-B in brain mitochondrial homogenate assessed as 4-hydroxyquinoline by spectrophotometric method | Eur J Med Chem 89: 98-105 (2014) Article DOI: 10.1016/j.ejmech.2014.10.029 BindingDB Entry DOI: 10.7270/Q2FX7C3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50093335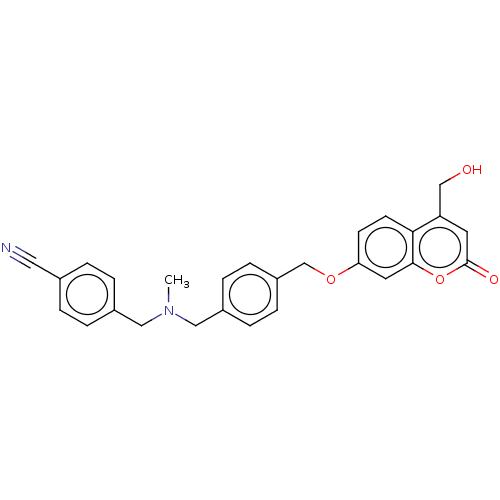 (CHEMBL3586611) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of human recombinant MAO-B using kynuramine substrate by spectrophotometric assay | J Med Chem 58: 5561-78 (2015) Article DOI: 10.1021/acs.jmedchem.5b00599 BindingDB Entry DOI: 10.7270/Q26T0PCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50038156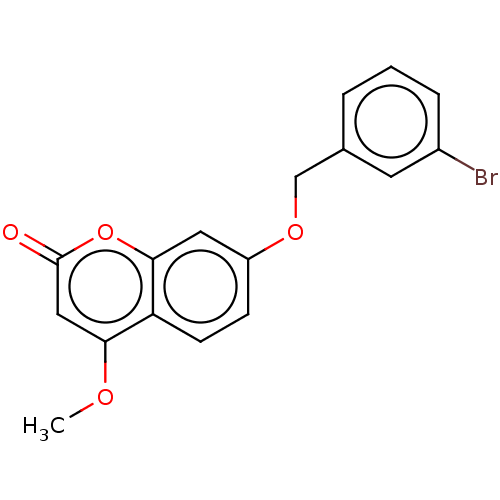 (CHEMBL3093993) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari "Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat MAO-B using Kynuramine as substrate assessed as formation of 4-hydroxyquinoline preincubated for 5 mins prior to sub... | Eur J Med Chem 70: 723-39 (2013) Article DOI: 10.1016/j.ejmech.2013.09.034 BindingDB Entry DOI: 10.7270/Q2WM1HCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50038156 (CHEMBL3093993) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Bari"Aldo Moro" Curated by ChEMBL | Assay Description Inhibition of Sprague-Dawley rat MAO-B in brain mitochondrial homogenate assessed as 4-hydroxyquinoline by spectrophotometric method | Eur J Med Chem 89: 98-105 (2014) Article DOI: 10.1016/j.ejmech.2014.10.029 BindingDB Entry DOI: 10.7270/Q2FX7C3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1517 total ) | Next | Last >> |