

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16318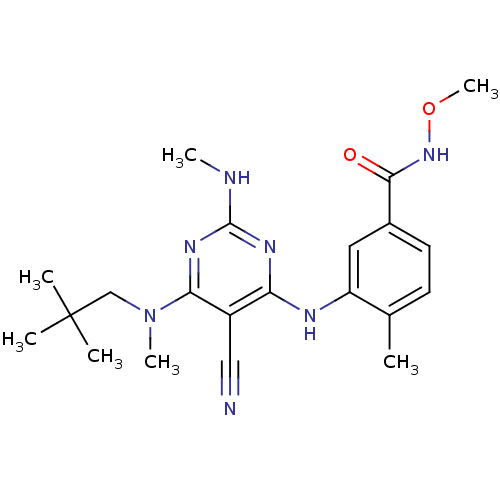 (3-({5-cyano-6-[(2,2-dimethylpropyl)(methyl)amino]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0470 | -58.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16319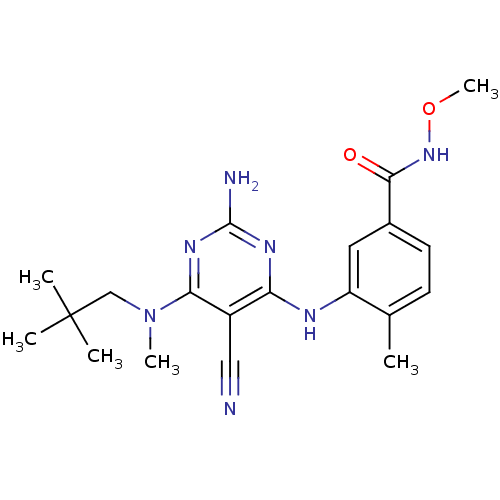 (3-({2-amino-5-cyano-6-[(2,2-dimethylpropyl)(methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | -58.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16329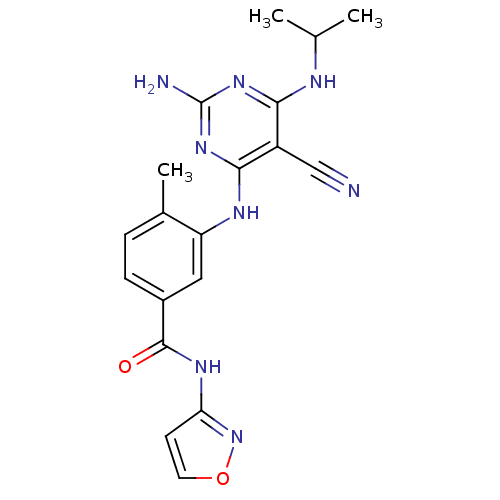 (3-({2-amino-5-cyano-6-[(1-methylethyl)amino]pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.0570 | -57.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16317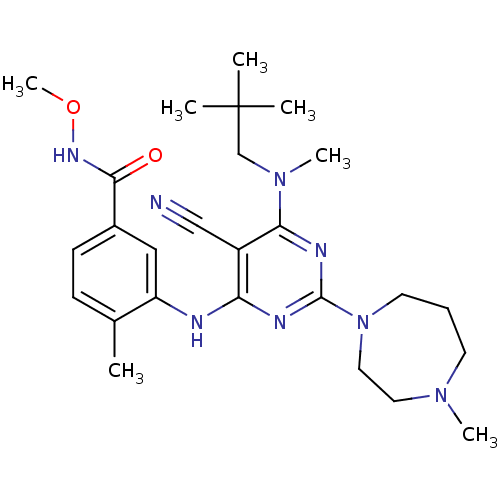 (3-({5-cyano-6-[(2,2-dimethylpropyl)(methyl)amino]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0570 | -57.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16320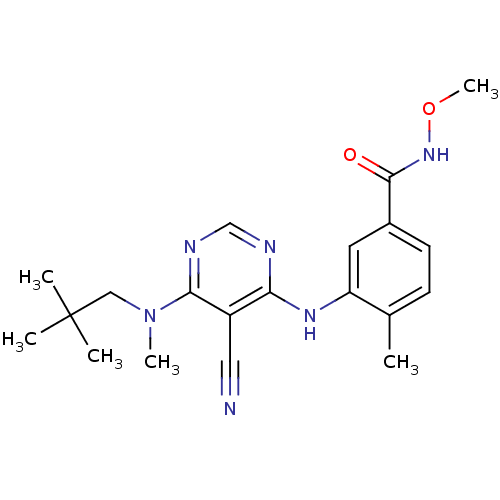 (3-({5-cyano-6-[(2,2-dimethylpropyl)(methyl)amino]p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16330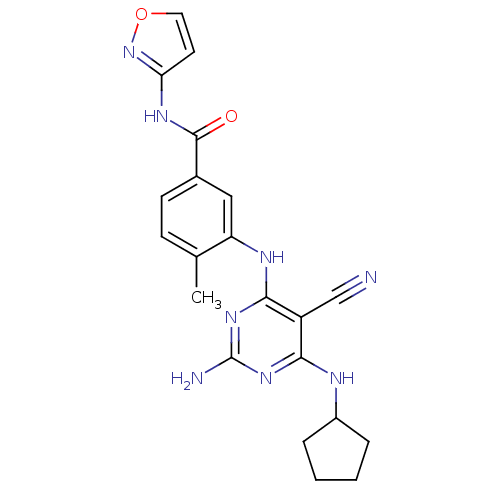 (3-{[2-amino-5-cyano-6-(cyclopentylamino)pyrimidin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376226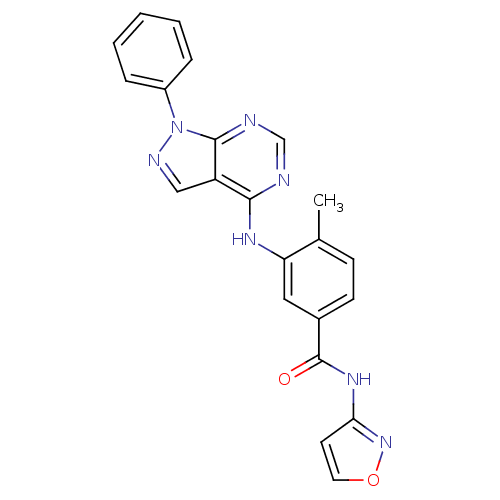 (CHEMBL259922) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant p38alpha-mediated myelin basic protein phosphorylation | Bioorg Med Chem Lett 18: 2652-7 (2008) Article DOI: 10.1016/j.bmcl.2008.03.019 BindingDB Entry DOI: 10.7270/Q2C53MR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376226 (CHEMBL259922) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant p38alpha-mediated myelin basic protein phosphorylation | Bioorg Med Chem Lett 18: 2652-7 (2008) Article DOI: 10.1016/j.bmcl.2008.03.019 BindingDB Entry DOI: 10.7270/Q2C53MR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16325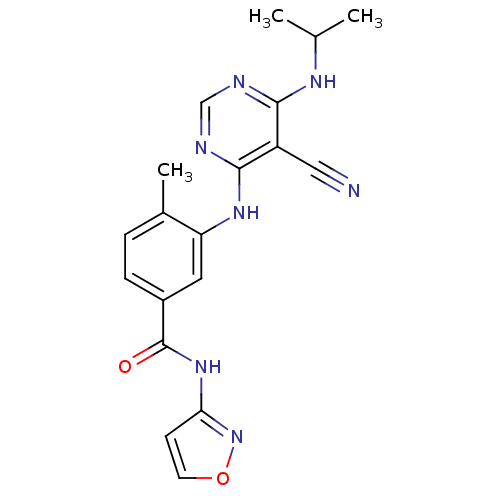 (3-({5-cyano-6-[(1-methylethyl)amino]pyrimidin-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.410 | -53.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376456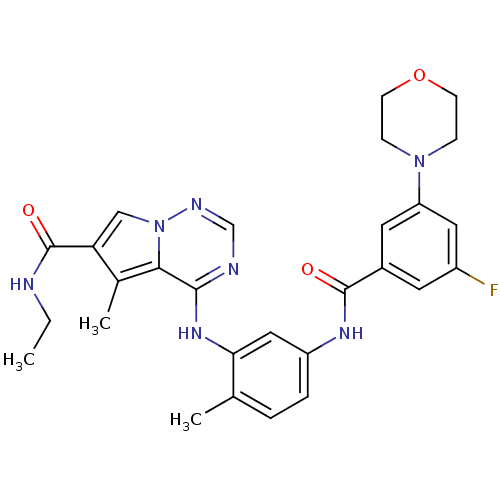 (CHEMBL262592) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of p38alpha | Bioorg Med Chem Lett 18: 2739-44 (2008) Article DOI: 10.1016/j.bmcl.2008.02.067 BindingDB Entry DOI: 10.7270/Q29887X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16324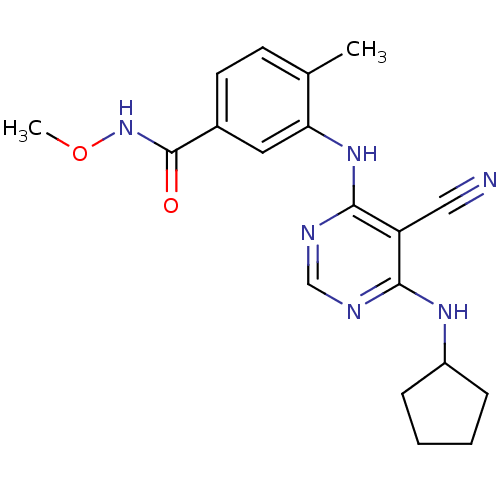 (3-{[5-cyano-6-(cyclopentylamino)pyrimidin-4-yl]ami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.420 | -53.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376433 (CHEMBL258895) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of p38alpha | Bioorg Med Chem Lett 18: 2739-44 (2008) Article DOI: 10.1016/j.bmcl.2008.02.067 BindingDB Entry DOI: 10.7270/Q29887X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376434 (CHEMBL408150) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of p38alpha | Bioorg Med Chem Lett 18: 2739-44 (2008) Article DOI: 10.1016/j.bmcl.2008.02.067 BindingDB Entry DOI: 10.7270/Q29887X6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50131114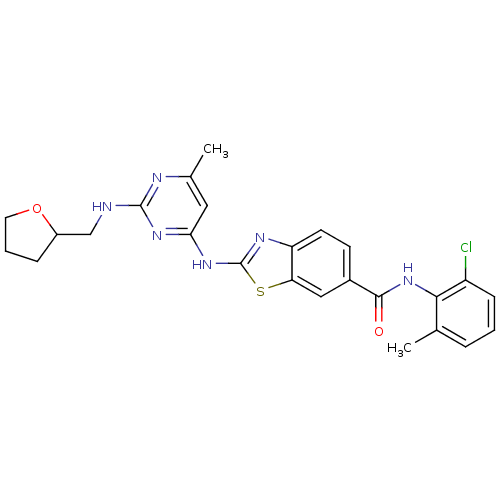 (2-{6-Methyl-2-[(tetrahydro-furan-2-ylmethyl)-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376435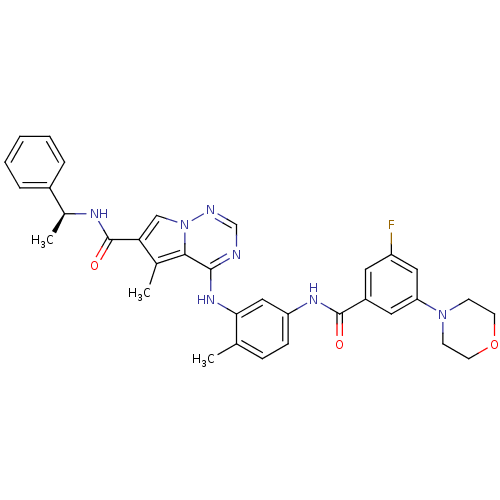 (CHEMBL261845) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of p38alpha | Bioorg Med Chem Lett 18: 2739-44 (2008) Article DOI: 10.1016/j.bmcl.2008.02.067 BindingDB Entry DOI: 10.7270/Q29887X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16323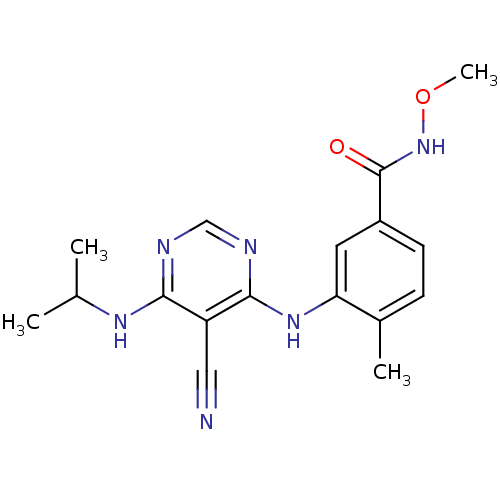 (3-({5-cyano-6-[(1-methylethyl)amino]pyrimidin-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.610 | -52.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13357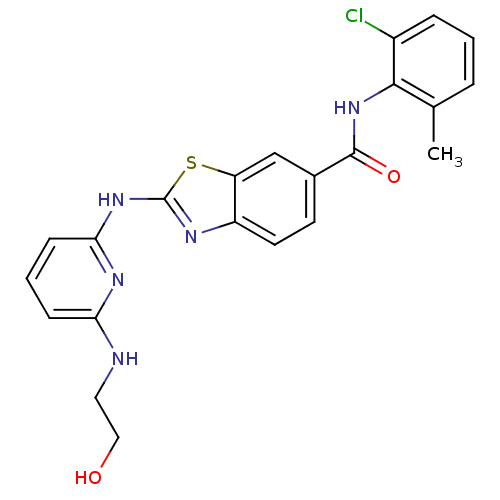 (CHEMBL312933 | N-(2-chloro-6-methylphenyl)-2-({6-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16322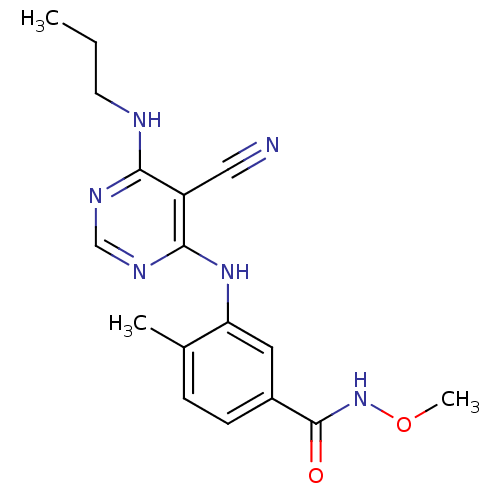 (3-{[5-cyano-6-(propylamino)pyrimidin-4-yl]amino}-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.970 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376432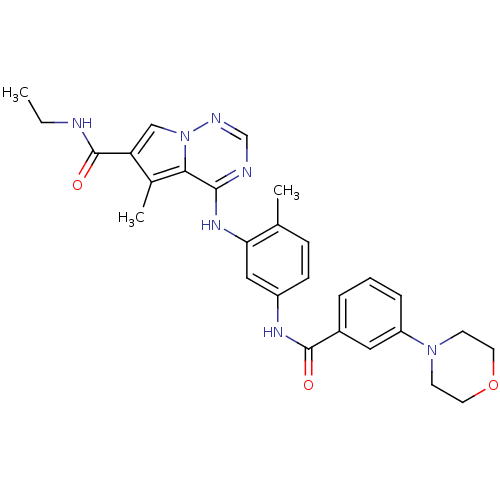 (CHEMBL258748) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of p38alpha | Bioorg Med Chem Lett 18: 2739-44 (2008) Article DOI: 10.1016/j.bmcl.2008.02.067 BindingDB Entry DOI: 10.7270/Q29887X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50131114 (2-{6-Methyl-2-[(tetrahydro-furan-2-ylmethyl)-amino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Fyn protein kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16331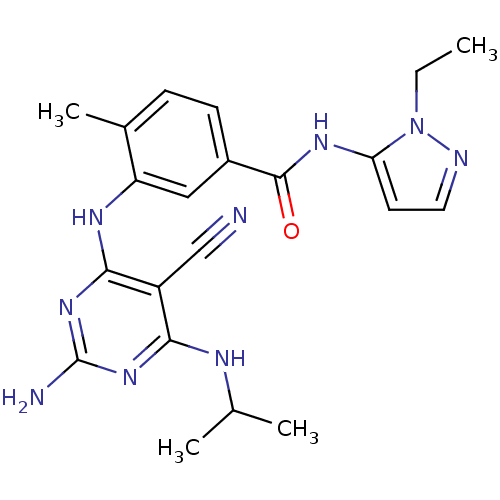 (3-({2-amino-5-cyano-6-[(1-methylethyl)amino]pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | -49.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16326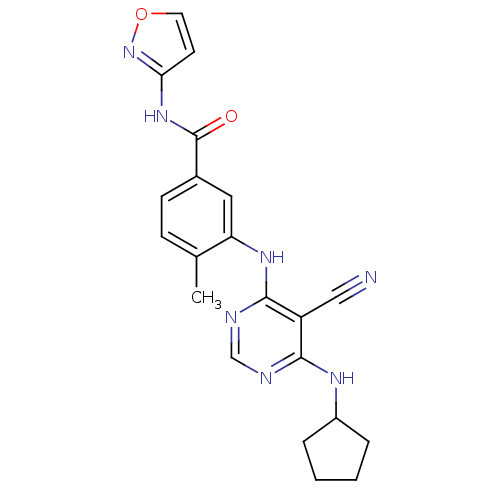 (3-{[5-cyano-6-(cyclopentylamino)pyrimidin-4-yl]ami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16332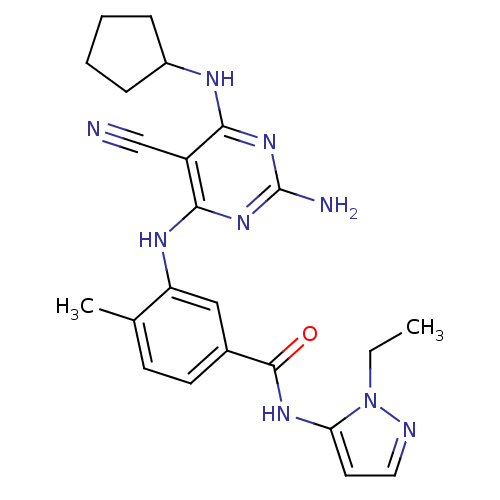 (3-{[2-amino-5-cyano-6-(cyclopentylamino)pyrimidin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90 | -49.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM13357 (CHEMBL312933 | N-(2-chloro-6-methylphenyl)-2-({6-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Fyn protein kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50131114 (2-{6-Methyl-2-[(tetrahydro-furan-2-ylmethyl)-amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376457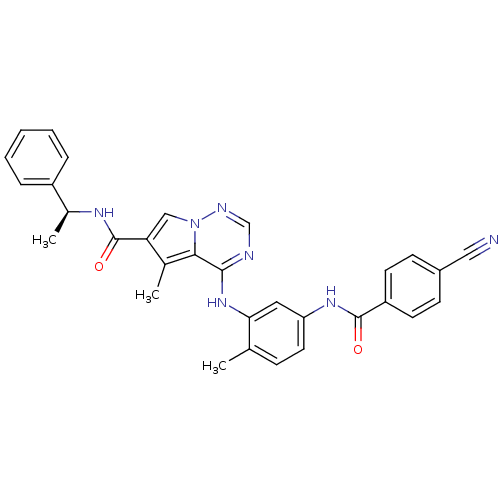 (CHEMBL259596) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of p38alpha | Bioorg Med Chem Lett 18: 2739-44 (2008) Article DOI: 10.1016/j.bmcl.2008.02.067 BindingDB Entry DOI: 10.7270/Q29887X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16316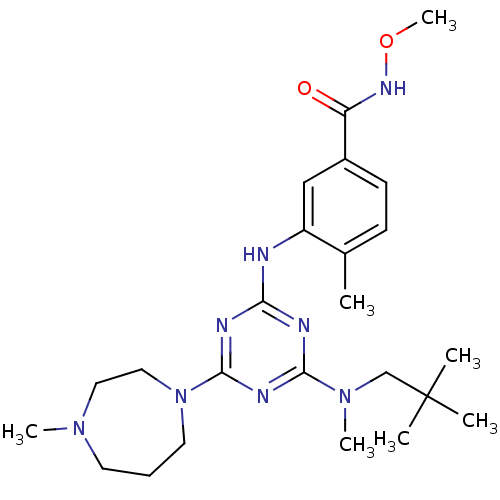 (3-({4-[(2,2-dimethylpropyl)(methyl)amino]-6-(4-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70 | -47.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50129307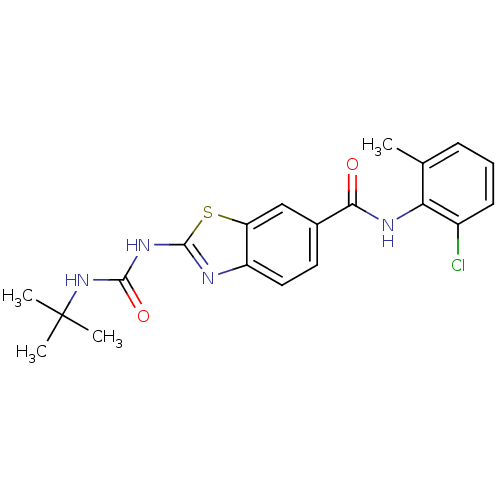 (2-(3-tert-Butyl-ureido)-benzothiazole-6-carboxylic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50131131 (2-(6-Methylamino-pyrimidin-4-ylamino)-benzothiazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50131131 (2-(6-Methylamino-pyrimidin-4-ylamino)-benzothiazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Fyn protein kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM13357 (CHEMBL312933 | N-(2-chloro-6-methylphenyl)-2-({6-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16328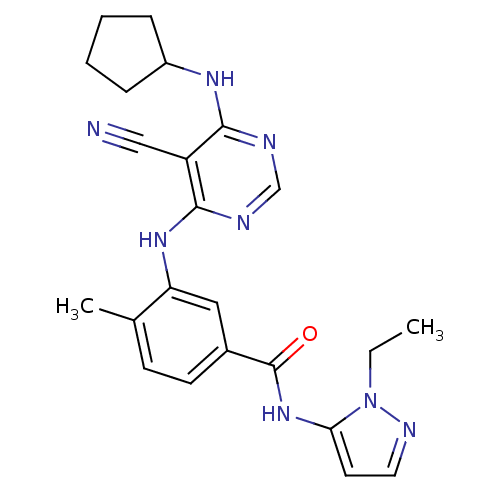 (3-{[5-cyano-6-(cyclopentylamino)pyrimidin-4-yl]ami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | -44.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16327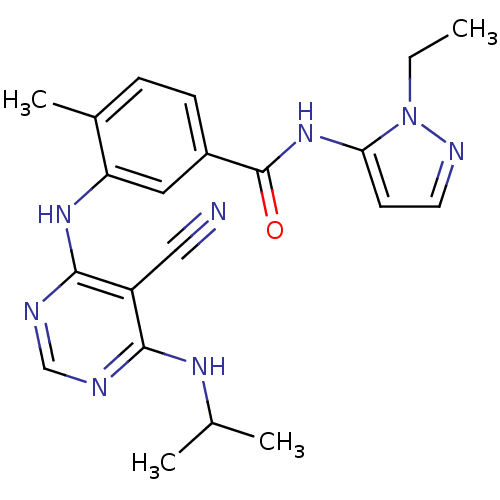 (3-({5-cyano-6-[(1-methylethyl)amino]pyrimidin-4-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM16321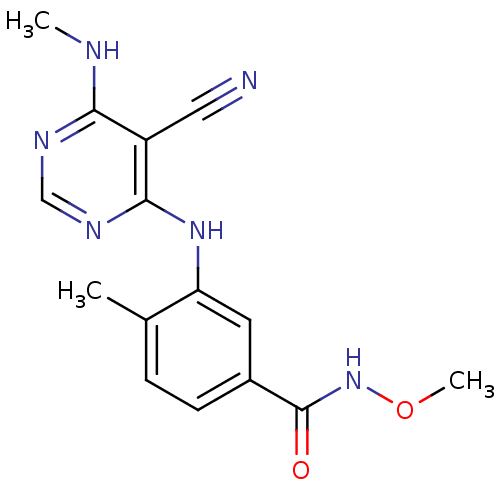 (3-{[5-cyano-6-(methylamino)pyrimidin-4-yl]amino}-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 16 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 48: 6261-70 (2005) Article DOI: 10.1021/jm0503594 BindingDB Entry DOI: 10.7270/Q25X276T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50131131 (2-(6-Methylamino-pyrimidin-4-ylamino)-benzothiazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM50129307 (2-(3-tert-Butyl-ureido)-benzothiazole-6-carboxylic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Fyn protein kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50129307 (2-(3-tert-Butyl-ureido)-benzothiazole-6-carboxylic...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 333 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Src protein tryrosine kinase | Bioorg Med Chem Lett 13: 2587-90 (2003) BindingDB Entry DOI: 10.7270/Q2FX78V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50239718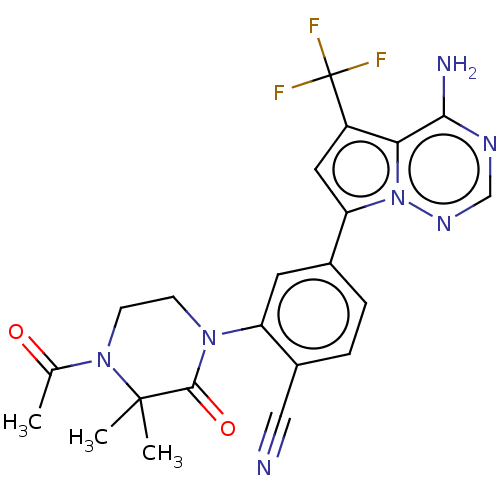 (CHEMBL4064666 | US10214537, Example 639) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine | J Med Chem 60: 5193-5208 (2017) Article DOI: 10.1021/acs.jmedchem.7b00618 BindingDB Entry DOI: 10.7270/Q2WW7KVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13271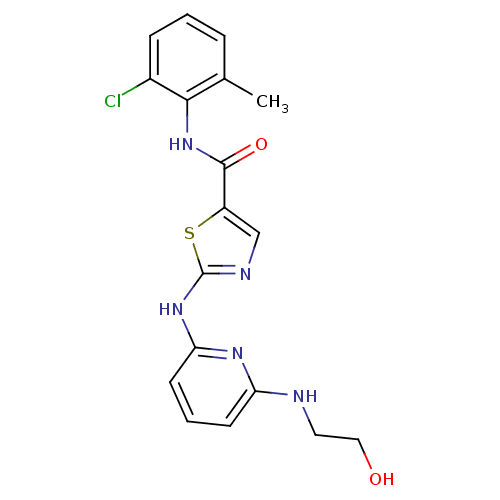 (BMS-354825 2-Heteroarylamino-thiazole Analog 12p |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13277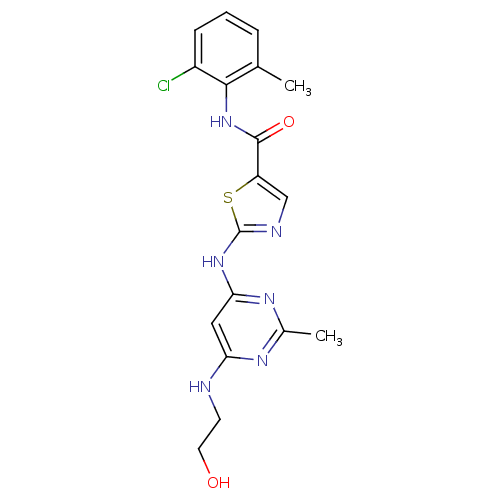 (BMS-354825 2-Heteroarylamino-thiazole Analog 12v |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50492385 (CHEMBL2401994) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human p38alpha expressed in Escherichia coli using MBP as substrate preincubated for 10 mins prior to substrate addition measured after... | Bioorg Med Chem Lett 23: 4120-6 (2013) Article DOI: 10.1016/j.bmcl.2013.05.047 BindingDB Entry DOI: 10.7270/Q2FN193N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13262 (BMS-354825 2-Heteroarylamino-thiazole Analog 12g |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50267827 (CHEMBL4076794) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) using CSKtide as substrate after 30 mins in presence of [gamma33P]ATP by liquid scintillation counting method | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of lck inase | J Med Chem 47: 6658-61 (2004) Article DOI: 10.1021/jm049486a BindingDB Entry DOI: 10.7270/Q2ZG6RRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50267826 (CHEMBL4096145) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of GST-tagged JAK1 (unknown origin) after 3 hrs by Caliper assay | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM20655 (N-benzyl-4-{[5-(methoxycarbamoyl)-2-methylphenyl]a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | 9.60 | n/a | n/a | 7.5 | 22 |
Novartis Pharmaceuticals | Assay Description The kinase activity was determined by quantitation of the amount of radioactive phosphate transferred to myelin basic protein (MBP) with or without i... | J Med Chem 51: 4-16 (2008) Article DOI: 10.1021/jm7009414 BindingDB Entry DOI: 10.7270/Q23T9FHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13216 (BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50267796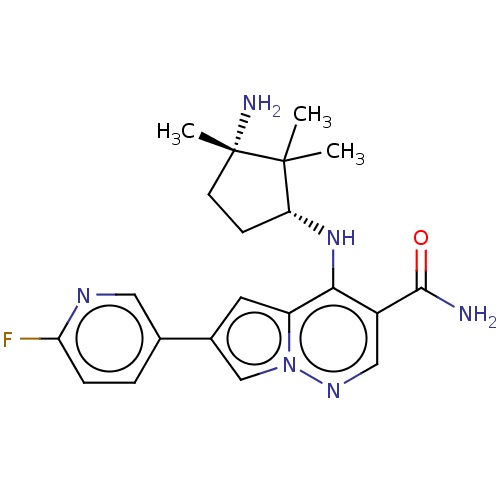 (CHEMBL4098840) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) using CSKtide as substrate after 30 mins in presence of [gamma33P]ATP by liquid scintillation counting method | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50267804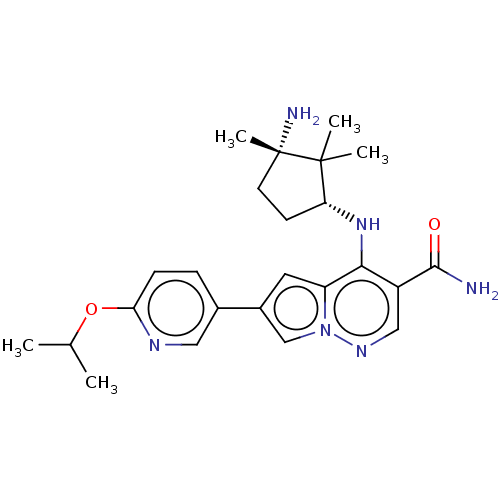 (CHEMBL4070136) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development, Bristol-Myers Squibb Research and Development, P.O. Box 4000, Princeton, NJ 08543, USA. Electronic address: john.hynes@bms.com. Curated by ChEMBL | Assay Description Inhibition of JAK3 (unknown origin) using CSKtide as substrate after 30 mins in presence of [gamma33P]ATP by liquid scintillation counting method | Bioorg Med Chem Lett 27: 3101-3106 (2017) Article DOI: 10.1016/j.bmcl.2017.05.043 BindingDB Entry DOI: 10.7270/Q2G73H7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13273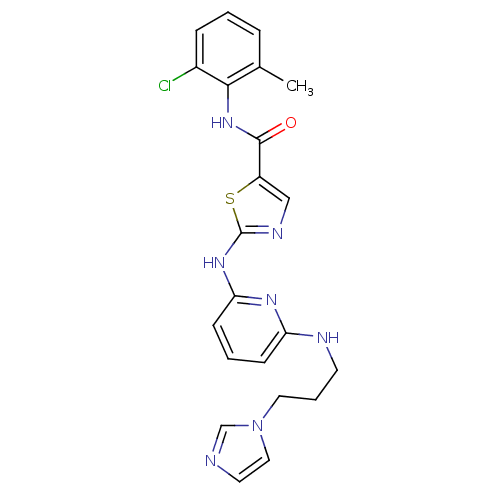 (2-(6-(3-(1H-Imidazol-1-yl)propylamino)pyridin-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab... | J Med Chem 49: 6819-32 (2006) Article DOI: 10.1021/jm060727j BindingDB Entry DOI: 10.7270/Q2QN6501 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1432 total ) | Next | Last >> |