 Found 185 hits with Last Name = 'pitts' and Initial = 'ke'
Found 185 hits with Last Name = 'pitts' and Initial = 'ke' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052329

(CHEMBL3318538)Show SMILES Nc1ccc2nc(SC3=C(O)CC(CC3=O)c3ccccc3)[nH]c2c1 |c:8| Show InChI InChI=1S/C19H17N3O2S/c20-13-6-7-14-15(10-13)22-19(21-14)25-18-16(23)8-12(9-17(18)24)11-4-2-1-3-5-11/h1-7,10,12,23H,8-9,20H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by UV endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052300

(CHEMBL3318527)Show InChI InChI=1S/C16H14N2O2S/c19-13-9-12(11-5-2-1-3-6-11)10-14(20)15(13)21-16-17-7-4-8-18-16/h1-8,12,19H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by UV endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052322

(CHEMBL3318535)Show SMILES OC1=C(Sc2nc3ccccc3o2)C(=O)CC(C1)c1ccccc1 |c:1| Show InChI InChI=1S/C19H15NO3S/c21-15-10-13(12-6-2-1-3-7-12)11-16(22)18(15)24-19-20-14-8-4-5-9-17(14)23-19/h1-9,13,21H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by UV endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052304
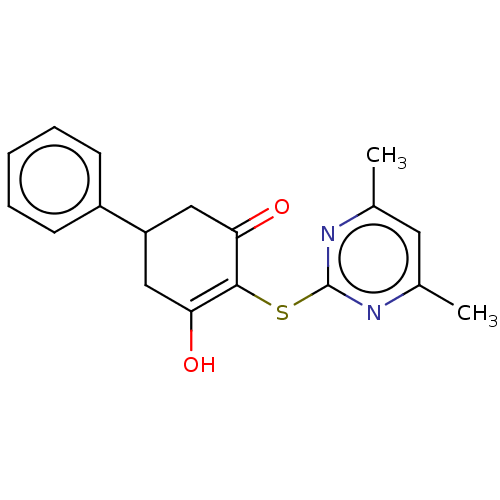
(CHEMBL3318529)Show SMILES Cc1cc(C)nc(SC2=C(O)CC(CC2=O)c2ccccc2)n1 |c:8| Show InChI InChI=1S/C18H18N2O2S/c1-11-8-12(2)20-18(19-11)23-17-15(21)9-14(10-16(17)22)13-6-4-3-5-7-13/h3-8,14,21H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by UV endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052292

(CHEMBL3318524)Show InChI InChI=1S/C15H14N2O2S2/c1-9-16-17-15(20-9)21-14-12(18)7-11(8-13(14)19)10-5-3-2-4-6-10/h2-6,11,18H,7-8H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by UV endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052325
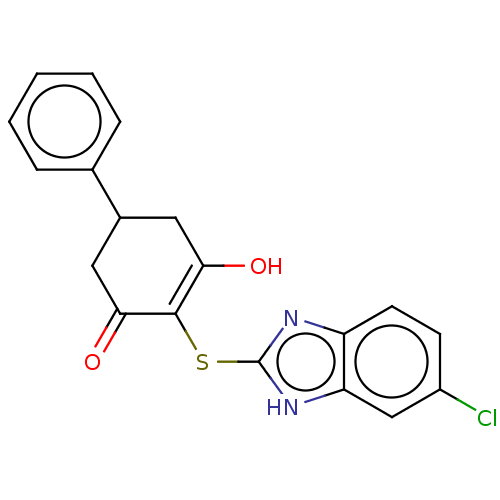
(CHEMBL3318536)Show SMILES OC1=C(Sc2nc3ccc(Cl)cc3[nH]2)C(=O)CC(C1)c1ccccc1 |c:1| Show InChI InChI=1S/C19H15ClN2O2S/c20-13-6-7-14-15(10-13)22-19(21-14)25-18-16(23)8-12(9-17(18)24)11-4-2-1-3-5-11/h1-7,10,12,23H,8-9H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by UV endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052319

(CHEMBL3318534)Show SMILES OC1=C(Sc2nc3ccccc3s2)C(=O)CC(C1)c1ccccc1 |c:1| Show InChI InChI=1S/C19H15NO2S2/c21-15-10-13(12-6-2-1-3-7-12)11-16(22)18(15)24-19-20-14-8-4-5-9-17(14)23-19/h1-9,13,21H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by UV endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50052407

(CHEMBL3318485)Show SMILES OC1=C(Sc2ccccc2C#N)C(=O)CC(C1)c1c(Br)cccc1Br |c:1| Show InChI InChI=1S/C19H13Br2NO2S/c20-13-5-3-6-14(21)18(13)12-8-15(23)19(16(24)9-12)25-17-7-2-1-4-11(17)10-22/h1-7,12,23H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHB by UV endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052290

(CHEMBL3318522)Show SMILES OC1=C(Sc2ncc[nH]2)C(=O)CC(C1)c1ccccc1 |c:1| Show InChI InChI=1S/C15H14N2O2S/c18-12-8-11(10-4-2-1-3-5-10)9-13(19)14(12)20-15-16-6-7-17-15/h1-7,11,18H,8-9H2,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by UV endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052248

(5-hydroxy-4-((2-nitrophenyl)thio)-1,6-dihydro-[1,1...)Show SMILES OC1=C(Sc2ccccc2[N+]([O-])=O)C(=O)CC(C1)c1ccccc1 |c:1| Show InChI InChI=1S/C18H15NO4S/c20-15-10-13(12-6-2-1-3-7-12)11-16(21)18(15)24-17-9-5-4-8-14(17)19(22)23/h1-9,13,20H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by MS endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052297
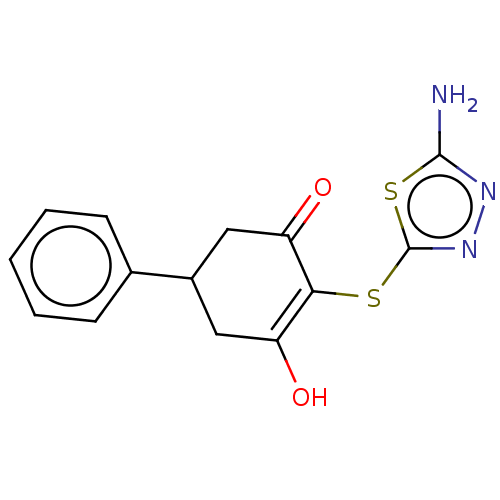
(CHEMBL3318525)Show InChI InChI=1S/C14H13N3O2S2/c15-13-16-17-14(21-13)20-12-10(18)6-9(7-11(12)19)8-4-2-1-3-5-8/h1-5,9,18H,6-7H2,(H2,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by UV endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50052260
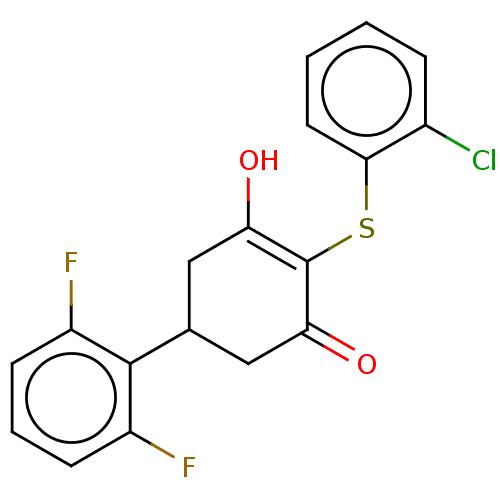
(CHEMBL3318473)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)CC(C1)c1c(F)cccc1F |c:1| Show InChI InChI=1S/C18H13ClF2O2S/c19-11-4-1-2-7-16(11)24-18-14(22)8-10(9-15(18)23)17-12(20)5-3-6-13(17)21/h1-7,10,22H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHB by UV endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052249
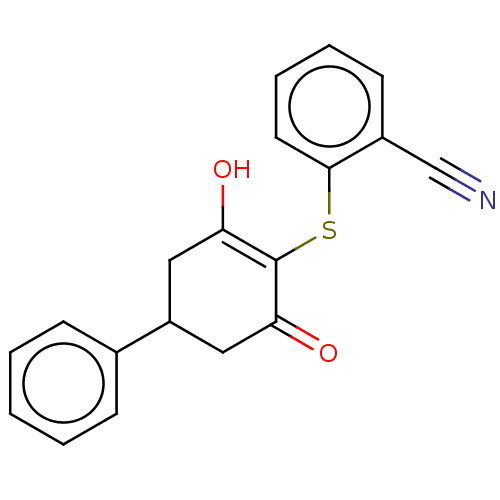
(CHEMBL3318423)Show SMILES OC1=C(Sc2ccccc2C#N)C(=O)CC(C1)c1ccccc1 |c:1| Show InChI InChI=1S/C19H15NO2S/c20-12-14-8-4-5-9-18(14)23-19-16(21)10-15(11-17(19)22)13-6-2-1-3-7-13/h1-9,15,21H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by UV endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052248

(5-hydroxy-4-((2-nitrophenyl)thio)-1,6-dihydro-[1,1...)Show SMILES OC1=C(Sc2ccccc2[N+]([O-])=O)C(=O)CC(C1)c1ccccc1 |c:1| Show InChI InChI=1S/C18H15NO4S/c20-15-10-13(12-6-2-1-3-7-12)11-16(21)18(15)24-17-9-5-4-8-14(17)19(22)23/h1-9,13,20H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by UV endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50052400
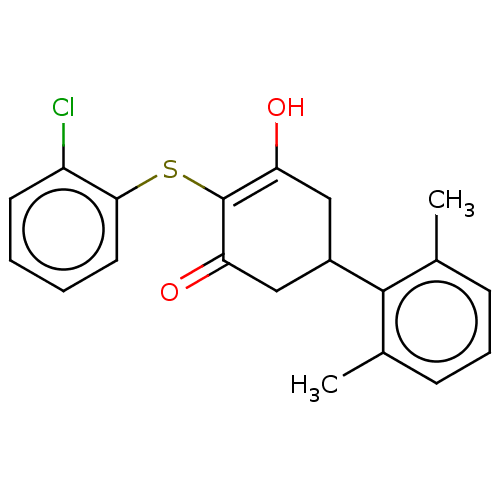
(CHEMBL3318475)Show SMILES Cc1cccc(C)c1C1CC(O)=C(Sc2ccccc2Cl)C(=O)C1 |t:12| Show InChI InChI=1S/C20H19ClO2S/c1-12-6-5-7-13(2)19(12)14-10-16(22)20(17(23)11-14)24-18-9-4-3-8-15(18)21/h3-9,14,22H,10-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHB by UV endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50052247

(CHEMBL3318482)Show SMILES OC1=C(Sc2ccccc2C#N)C(=O)CC(C1)c1c(Cl)cccc1Br |c:1| Show InChI InChI=1S/C19H13BrClNO2S/c20-13-5-3-6-14(21)18(13)12-8-15(23)19(16(24)9-12)25-17-7-2-1-4-11(17)10-22/h1-7,12,23H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHB by UV endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052326
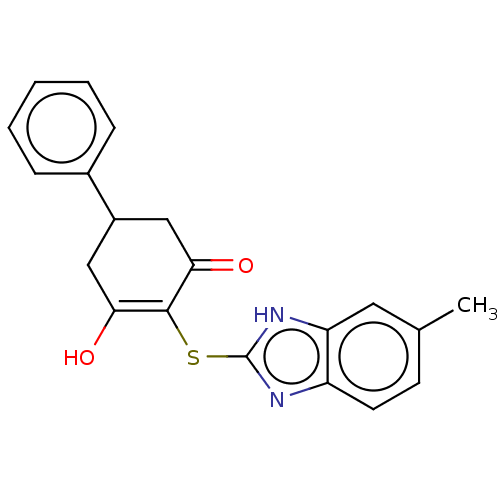
(CHEMBL3318537)Show SMILES Cc1ccc2nc(SC3=C(O)CC(CC3=O)c3ccccc3)[nH]c2c1 |c:8| Show InChI InChI=1S/C20H18N2O2S/c1-12-7-8-15-16(9-12)22-20(21-15)25-19-17(23)10-14(11-18(19)24)13-5-3-2-4-6-13/h2-9,14,23H,10-11H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by UV endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052301

(CHEMBL3318528)Show InChI InChI=1S/C17H16N2O2S/c1-11-7-8-18-17(19-11)22-16-14(20)9-13(10-15(16)21)12-5-3-2-4-6-12/h2-8,13,20H,9-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by UV endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50052405
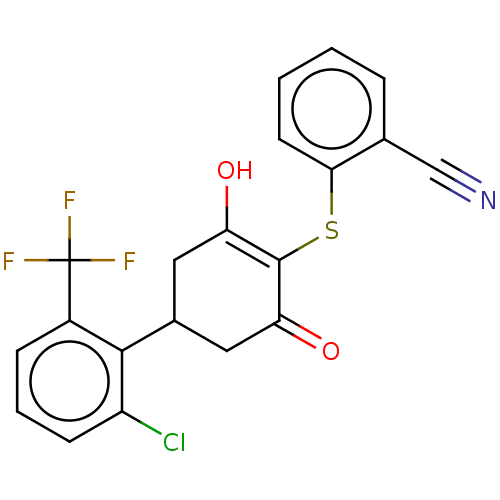
(CHEMBL3318483)Show SMILES OC1=C(Sc2ccccc2C#N)C(=O)CC(C1)c1c(Cl)cccc1C(F)(F)F |c:1| Show InChI InChI=1S/C20H13ClF3NO2S/c21-14-6-3-5-13(20(22,23)24)18(14)12-8-15(26)19(16(27)9-12)28-17-7-2-1-4-11(17)10-25/h1-7,12,26H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHB by UV endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052306
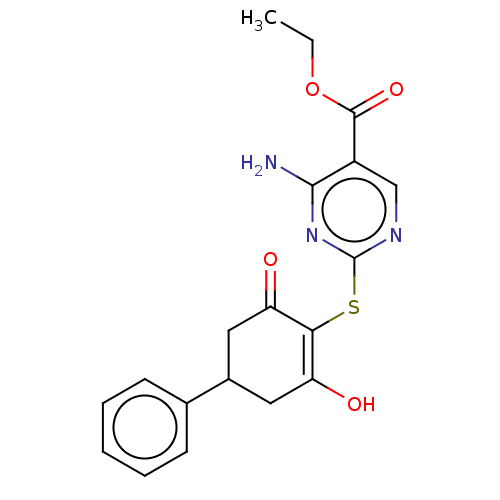
(CHEMBL3318531)Show SMILES CCOC(=O)c1cnc(SC2=C(O)CC(CC2=O)c2ccccc2)nc1N |c:10| Show InChI InChI=1S/C19H19N3O4S/c1-2-26-18(25)13-10-21-19(22-17(13)20)27-16-14(23)8-12(9-15(16)24)11-6-4-3-5-7-11/h3-7,10,12,23H,2,8-9H2,1H3,(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by UV endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50052258

(CHEMBL3318471)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)CC(C1)c1c(Cl)cccc1Br |c:1| Show InChI InChI=1S/C18H13BrCl2O2S/c19-11-4-3-6-13(21)17(11)10-8-14(22)18(15(23)9-10)24-16-7-2-1-5-12(16)20/h1-7,10,22H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHB by UV endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50052291
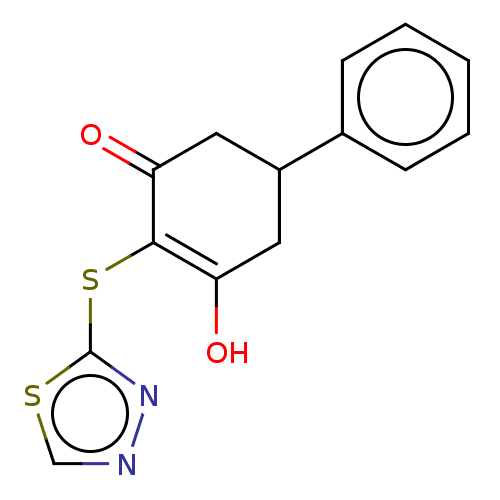
(CHEMBL3318523)Show InChI InChI=1S/C14H12N2O2S2/c17-11-6-10(9-4-2-1-3-5-9)7-12(18)13(11)20-14-16-15-8-19-14/h1-5,8,10,17H,6-7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by UV endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase A chain
(Homo sapiens (Human)) | BDBM50040030
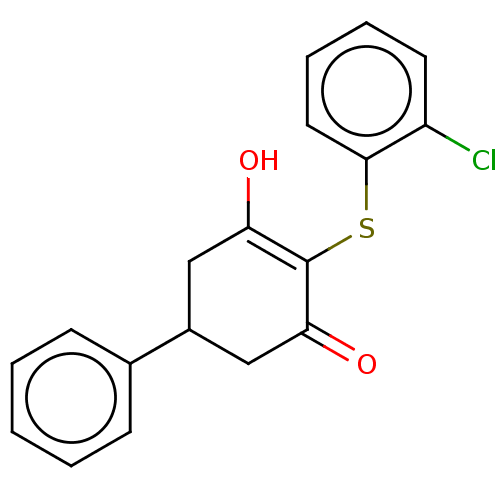
(4-((2-chlorophenyl)thio)-5-hydroxy-1,6-dihydro-[1,...)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)CC(C1)c1ccccc1 |c:1| Show InChI InChI=1S/C18H15ClO2S/c19-14-8-4-5-9-17(14)22-18-15(20)10-13(11-16(18)21)12-6-2-1-3-7-12/h1-9,13,20H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHA by UV endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
L-lactate dehydrogenase B chain
(Homo sapiens (Human)) | BDBM50052246
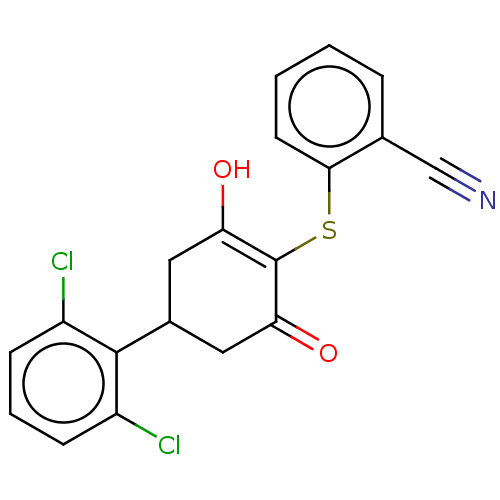
(CHEMBL3318481)Show SMILES OC1=C(Sc2ccccc2C#N)C(=O)CC(C1)c1c(Cl)cccc1Cl |c:1| Show InChI InChI=1S/C19H13Cl2NO2S/c20-13-5-3-6-14(21)18(13)12-8-15(23)19(16(24)9-12)25-17-7-2-1-4-11(17)10-22/h1-7,12,23H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant carboxy-terminal His-tagged LDHB by UV endpoint assay |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50052246

(CHEMBL3318481)Show SMILES OC1=C(Sc2ccccc2C#N)C(=O)CC(C1)c1c(Cl)cccc1Cl |c:1| Show InChI InChI=1S/C19H13Cl2NO2S/c20-13-5-3-6-14(21)18(13)12-8-15(23)19(16(24)9-12)25-17-7-2-1-4-11(17)10-22/h1-7,12,23H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50052247

(CHEMBL3318482)Show SMILES OC1=C(Sc2ccccc2C#N)C(=O)CC(C1)c1c(Cl)cccc1Br |c:1| Show InChI InChI=1S/C19H13BrClNO2S/c20-13-5-3-6-14(21)18(13)12-8-15(23)19(16(24)9-12)25-17-7-2-1-4-11(17)10-22/h1-7,12,23H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50052401
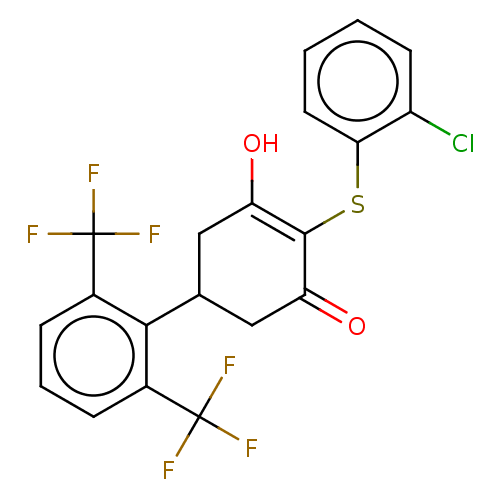
(CHEMBL3318477)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)CC(C1)c1c(cccc1C(F)(F)F)C(F)(F)F |c:1| Show InChI InChI=1S/C20H13ClF6O2S/c21-13-6-1-2-7-16(13)30-18-14(28)8-10(9-15(18)29)17-11(19(22,23)24)4-3-5-12(17)20(25,26)27/h1-7,10,28H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50052259
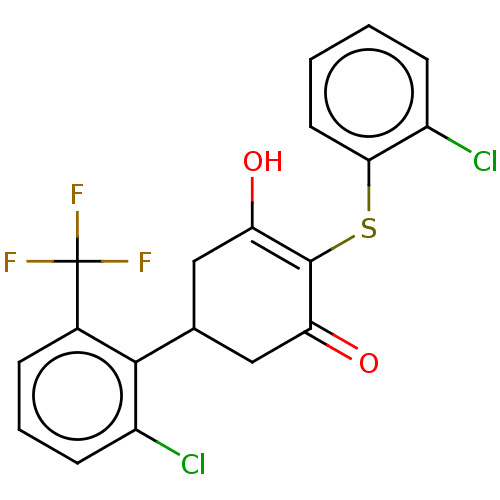
(CHEMBL3318472)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)CC(C1)c1c(Cl)cccc1C(F)(F)F |c:1| Show InChI InChI=1S/C19H13Cl2F3O2S/c20-12-5-1-2-7-16(12)27-18-14(25)8-10(9-15(18)26)17-11(19(22,23)24)4-3-6-13(17)21/h1-7,10,25H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50052254
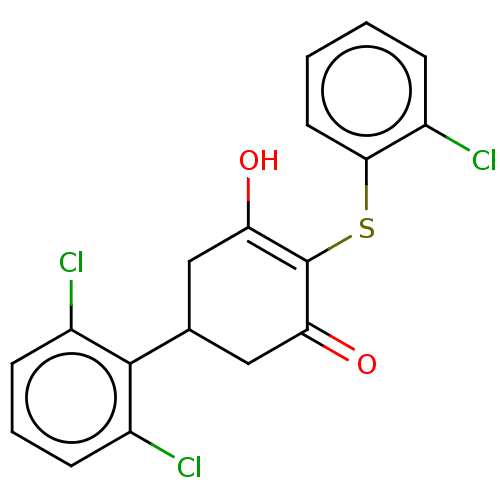
(2',6'-dichloro-4-((2-chlorophenyl)thio)-5-...)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)CC(C1)c1c(Cl)cccc1Cl |c:1| Show InChI InChI=1S/C18H13Cl3O2S/c19-11-4-1-2-7-16(11)24-18-14(22)8-10(9-15(18)23)17-12(20)5-3-6-13(17)21/h1-7,10,22H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50052249

(CHEMBL3318423)Show SMILES OC1=C(Sc2ccccc2C#N)C(=O)CC(C1)c1ccccc1 |c:1| Show InChI InChI=1S/C19H15NO2S/c20-12-14-8-4-5-9-18(14)23-19-16(21)10-15(11-17(19)22)13-6-2-1-3-7-13/h1-9,15,21H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50040030

(4-((2-chlorophenyl)thio)-5-hydroxy-1,6-dihydro-[1,...)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)CC(C1)c1ccccc1 |c:1| Show InChI InChI=1S/C18H15ClO2S/c19-14-8-4-5-9-17(14)22-18-15(20)10-13(11-16(18)21)12-6-2-1-3-7-12/h1-9,13,20H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50052247

(CHEMBL3318482)Show SMILES OC1=C(Sc2ccccc2C#N)C(=O)CC(C1)c1c(Cl)cccc1Br |c:1| Show InChI InChI=1S/C19H13BrClNO2S/c20-13-5-3-6-14(21)18(13)12-8-15(23)19(16(24)9-12)25-17-7-2-1-4-11(17)10-22/h1-7,12,23H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50052246

(CHEMBL3318481)Show SMILES OC1=C(Sc2ccccc2C#N)C(=O)CC(C1)c1c(Cl)cccc1Cl |c:1| Show InChI InChI=1S/C19H13Cl2NO2S/c20-13-5-3-6-14(21)18(13)12-8-15(23)19(16(24)9-12)25-17-7-2-1-4-11(17)10-22/h1-7,12,23H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50040030

(4-((2-chlorophenyl)thio)-5-hydroxy-1,6-dihydro-[1,...)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)CC(C1)c1ccccc1 |c:1| Show InChI InChI=1S/C18H15ClO2S/c19-14-8-4-5-9-17(14)22-18-15(20)10-13(11-16(18)21)12-6-2-1-3-7-12/h1-9,13,20H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50052249

(CHEMBL3318423)Show SMILES OC1=C(Sc2ccccc2C#N)C(=O)CC(C1)c1ccccc1 |c:1| Show InChI InChI=1S/C19H15NO2S/c20-12-14-8-4-5-9-18(14)23-19-16(21)10-15(11-17(19)22)13-6-2-1-3-7-13/h1-9,15,21H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50052254

(2',6'-dichloro-4-((2-chlorophenyl)thio)-5-...)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)CC(C1)c1c(Cl)cccc1Cl |c:1| Show InChI InChI=1S/C18H13Cl3O2S/c19-11-4-1-2-7-16(11)24-18-14(22)8-10(9-15(18)23)17-12(20)5-3-6-13(17)21/h1-7,10,22H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50052259

(CHEMBL3318472)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)CC(C1)c1c(Cl)cccc1C(F)(F)F |c:1| Show InChI InChI=1S/C19H13Cl2F3O2S/c20-12-5-1-2-7-16(12)27-18-14(25)8-10(9-15(18)26)17-11(19(22,23)24)4-3-6-13(17)21/h1-7,10,25H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50052401

(CHEMBL3318477)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)CC(C1)c1c(cccc1C(F)(F)F)C(F)(F)F |c:1| Show InChI InChI=1S/C20H13ClF6O2S/c21-13-6-1-2-7-16(13)30-18-14(28)8-10(9-15(18)29)17-11(19(22,23)24)4-3-5-12(17)20(25,26)27/h1-7,10,28H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50052246

(CHEMBL3318481)Show SMILES OC1=C(Sc2ccccc2C#N)C(=O)CC(C1)c1c(Cl)cccc1Cl |c:1| Show InChI InChI=1S/C19H13Cl2NO2S/c20-13-5-3-6-14(21)18(13)12-8-15(23)19(16(24)9-12)25-17-7-2-1-4-11(17)10-22/h1-7,12,23H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50052247

(CHEMBL3318482)Show SMILES OC1=C(Sc2ccccc2C#N)C(=O)CC(C1)c1c(Cl)cccc1Br |c:1| Show InChI InChI=1S/C19H13BrClNO2S/c20-13-5-3-6-14(21)18(13)12-8-15(23)19(16(24)9-12)25-17-7-2-1-4-11(17)10-22/h1-7,12,23H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50040030

(4-((2-chlorophenyl)thio)-5-hydroxy-1,6-dihydro-[1,...)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)CC(C1)c1ccccc1 |c:1| Show InChI InChI=1S/C18H15ClO2S/c19-14-8-4-5-9-17(14)22-18-15(20)10-13(11-16(18)21)12-6-2-1-3-7-12/h1-9,13,20H,10-11H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50052249

(CHEMBL3318423)Show SMILES OC1=C(Sc2ccccc2C#N)C(=O)CC(C1)c1ccccc1 |c:1| Show InChI InChI=1S/C19H15NO2S/c20-12-14-8-4-5-9-18(14)23-19-16(21)10-15(11-17(19)22)13-6-2-1-3-7-13/h1-9,15,21H,10-11H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50052254

(2',6'-dichloro-4-((2-chlorophenyl)thio)-5-...)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)CC(C1)c1c(Cl)cccc1Cl |c:1| Show InChI InChI=1S/C18H13Cl3O2S/c19-11-4-1-2-7-16(11)24-18-14(22)8-10(9-15(18)23)17-12(20)5-3-6-13(17)21/h1-7,10,22H,8-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50052259

(CHEMBL3318472)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)CC(C1)c1c(Cl)cccc1C(F)(F)F |c:1| Show InChI InChI=1S/C19H13Cl2F3O2S/c20-12-5-1-2-7-16(12)27-18-14(25)8-10(9-15(18)26)17-11(19(22,23)24)4-3-6-13(17)21/h1-7,10,25H,8-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50052401

(CHEMBL3318477)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)CC(C1)c1c(cccc1C(F)(F)F)C(F)(F)F |c:1| Show InChI InChI=1S/C20H13ClF6O2S/c21-13-6-1-2-7-16(13)30-18-14(28)8-10(9-15(18)29)17-11(19(22,23)24)4-3-5-12(17)20(25,26)27/h1-7,10,28H,8-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50052246

(CHEMBL3318481)Show SMILES OC1=C(Sc2ccccc2C#N)C(=O)CC(C1)c1c(Cl)cccc1Cl |c:1| Show InChI InChI=1S/C19H13Cl2NO2S/c20-13-5-3-6-14(21)18(13)12-8-15(23)19(16(24)9-12)25-17-7-2-1-4-11(17)10-22/h1-7,12,23H,8-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50052247

(CHEMBL3318482)Show SMILES OC1=C(Sc2ccccc2C#N)C(=O)CC(C1)c1c(Cl)cccc1Br |c:1| Show InChI InChI=1S/C19H13BrClNO2S/c20-13-5-3-6-14(21)18(13)12-8-15(23)19(16(24)9-12)25-17-7-2-1-4-11(17)10-22/h1-7,12,23H,8-9H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50040030

(4-((2-chlorophenyl)thio)-5-hydroxy-1,6-dihydro-[1,...)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)CC(C1)c1ccccc1 |c:1| Show InChI InChI=1S/C18H15ClO2S/c19-14-8-4-5-9-17(14)22-18-15(20)10-13(11-16(18)21)12-6-2-1-3-7-12/h1-9,13,20H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50052249

(CHEMBL3318423)Show SMILES OC1=C(Sc2ccccc2C#N)C(=O)CC(C1)c1ccccc1 |c:1| Show InChI InChI=1S/C19H15NO2S/c20-12-14-8-4-5-9-18(14)23-19-16(21)10-15(11-17(19)22)13-6-2-1-3-7-13/h1-9,15,21H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50052254

(2',6'-dichloro-4-((2-chlorophenyl)thio)-5-...)Show SMILES OC1=C(Sc2ccccc2Cl)C(=O)CC(C1)c1c(Cl)cccc1Cl |c:1| Show InChI InChI=1S/C18H13Cl3O2S/c19-11-4-1-2-7-16(11)24-18-14(22)8-10(9-15(18)23)17-12(20)5-3-6-13(17)21/h1-7,10,22H,8-9H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 24: 3764-71 (2014)
Article DOI: 10.1016/j.bmcl.2014.06.076
BindingDB Entry DOI: 10.7270/Q2445P46 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data