 Found 67 hits with Last Name = 'pizzolato' and Initial = 'g'
Found 67 hits with Last Name = 'pizzolato' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50426474
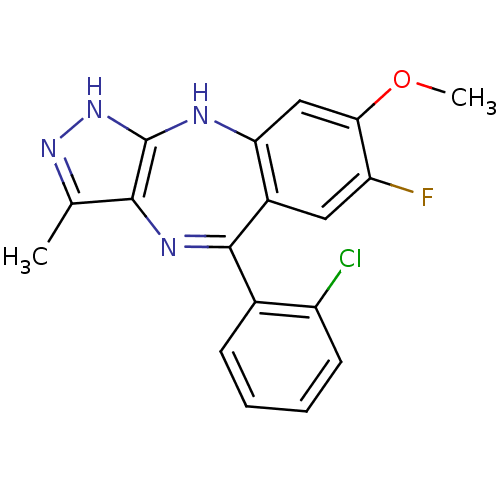
(CHEMBL1980391)Show SMILES COc1cc2Nc3[nH]nc(C)c3N=C(c3ccccc3Cl)c2cc1F |t:13| Show InChI InChI=1S/C18H14ClFN4O/c1-9-16-18(24-23-9)21-14-8-15(25-2)13(20)7-11(14)17(22-16)10-5-3-4-6-12(10)19/h3-8H,1-2H3,(H2,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) |
ACS Med Chem Lett 4: 259-63 (2013)
Article DOI: 10.1021/ml300351e
BindingDB Entry DOI: 10.7270/Q2902541 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50328264

(5-(2-chlorophenyl)-3-isopropyl-7-nitro-2,10-dihydr...)Show SMILES CC(C)c1n[nH]c2Nc3ccc(cc3C(=Nc12)c1ccccc1Cl)[N+]([O-])=O |c:15| Show InChI InChI=1S/C19H16ClN5O2/c1-10(2)16-18-19(24-23-16)21-15-8-7-11(25(26)27)9-13(15)17(22-18)12-5-3-4-6-14(12)20/h3-10H,1-2H3,(H2,21,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2-cyclin E |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50434284
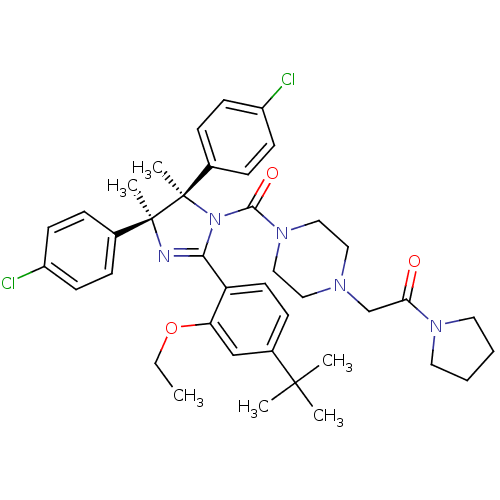
(CHEMBL2386349)Show SMILES CCOc1cc(ccc1C1=N[C@@](C)(c2ccc(Cl)cc2)[C@](C)(N1C(=O)N1CCN(CC(=O)N2CCCC2)CC1)c1ccc(Cl)cc1)C(C)(C)C |r,t:10| Show InChI InChI=1S/C40H49Cl2N5O3/c1-7-50-34-26-30(38(2,3)4)14-19-33(34)36-43-39(5,28-10-15-31(41)16-11-28)40(6,29-12-17-32(42)18-13-29)47(36)37(49)46-24-22-44(23-25-46)27-35(48)45-20-8-9-21-45/h10-19,26H,7-9,20-25,27H2,1-6H3/t39-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal human recombinant MDM2 assessed as inhibition of protein interaction with p53 by HTRF assay |
ACS Med Chem Lett 4: 466-9 (2013)
Article DOI: 10.1021/ml4000657
BindingDB Entry DOI: 10.7270/Q2JS9RTS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50328256
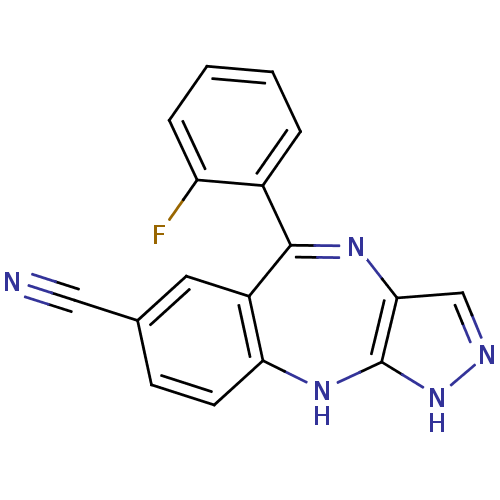
(5-(2-fluorophenyl)-2,10-dihydrobenzo[e]pyrazolo[4,...)Show SMILES Fc1ccccc1C1=Nc2cn[nH]c2Nc2ccc(cc12)C#N |t:8| Show InChI InChI=1S/C17H10FN5/c18-13-4-2-1-3-11(13)16-12-7-10(8-19)5-6-14(12)22-17-15(21-16)9-20-23-17/h1-7,9H,(H2,20,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2-cyclin E |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50328279
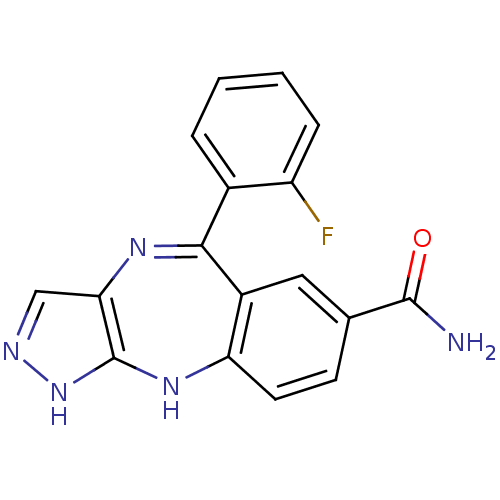
(5-(2-fluorophenyl)-2,10-dihydrobenzo[e]pyrazolo[4,...)Show SMILES NC(=O)c1ccc2Nc3[nH]ncc3N=C(c3ccccc3F)c2c1 |t:14| Show InChI InChI=1S/C17H12FN5O/c18-12-4-2-1-3-10(12)15-11-7-9(16(19)24)5-6-13(11)22-17-14(21-15)8-20-23-17/h1-8H,(H2,19,24)(H2,20,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2-cyclin E |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50434287

(CHEMBL2386346)Show SMILES CCOc1cc(ccc1C1=N[C@@](C)(c2ccc(Cl)cc2)[C@](C)(N1C(=O)N1CCN(CCCS(C)(=O)=O)CC1)c1ccc(Cl)cc1)C(C)(C)C |r,t:10| Show InChI InChI=1S/C38H48Cl2N4O4S/c1-8-48-33-26-29(36(2,3)4)14-19-32(33)34-41-37(5,27-10-15-30(39)16-11-27)38(6,28-12-17-31(40)18-13-28)44(34)35(45)43-23-21-42(22-24-43)20-9-25-49(7,46)47/h10-19,26H,8-9,20-25H2,1-7H3/t37-,38+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal human recombinant MDM2 assessed as inhibition of protein interaction with p53 by HTRF assay |
ACS Med Chem Lett 4: 466-9 (2013)
Article DOI: 10.1021/ml4000657
BindingDB Entry DOI: 10.7270/Q2JS9RTS |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50434283
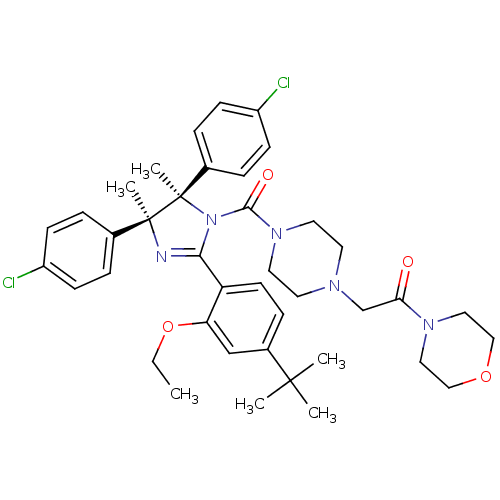
(CHEMBL2386350)Show SMILES CCOc1cc(ccc1C1=N[C@@](C)(c2ccc(Cl)cc2)[C@](C)(N1C(=O)N1CCN(CC(=O)N2CCOCC2)CC1)c1ccc(Cl)cc1)C(C)(C)C |r,t:10| Show InChI InChI=1S/C40H49Cl2N5O4/c1-7-51-34-26-30(38(2,3)4)12-17-33(34)36-43-39(5,28-8-13-31(41)14-9-28)40(6,29-10-15-32(42)16-11-29)47(36)37(49)46-20-18-44(19-21-46)27-35(48)45-22-24-50-25-23-45/h8-17,26H,7,18-25,27H2,1-6H3/t39-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal human recombinant MDM2 assessed as inhibition of protein interaction with p53 by HTRF assay |
ACS Med Chem Lett 4: 466-9 (2013)
Article DOI: 10.1021/ml4000657
BindingDB Entry DOI: 10.7270/Q2JS9RTS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50328267
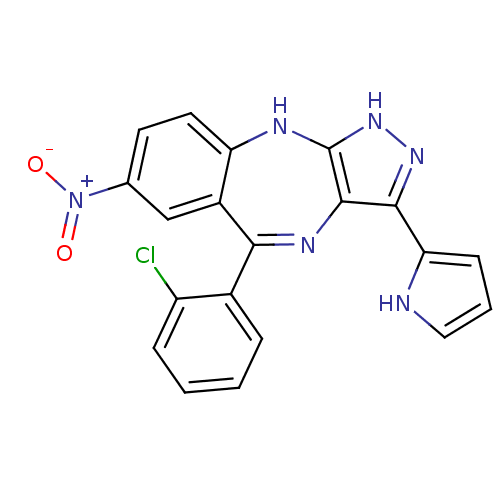
(5-(2-chlorophenyl)-7-nitro-3-(1H-pyrrol-2-yl)-2,10...)Show SMILES [O-][N+](=O)c1ccc2Nc3[nH]nc(-c4ccc[nH]4)c3N=C(c3ccccc3Cl)c2c1 |t:20| Show InChI InChI=1S/C20H13ClN6O2/c21-14-5-2-1-4-12(14)17-13-10-11(27(28)29)7-8-15(13)23-20-19(24-17)18(25-26-20)16-6-3-9-22-16/h1-10,22H,(H2,23,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2-cyclin E |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50434289

(CHEMBL2386344)Show SMILES CCOc1cc(ccc1C1=N[C@@](C)(c2ccc(Cl)cc2)[C@](C)(N1C(=O)N1CCN(CCC(F)(F)F)CC1)c1ccc(Cl)cc1)C(C)(C)C |r,t:10| Show InChI InChI=1S/C37H43Cl2F3N4O2/c1-7-48-31-24-27(34(2,3)4)12-17-30(31)32-43-35(5,25-8-13-28(38)14-9-25)36(6,26-10-15-29(39)16-11-26)46(32)33(47)45-22-20-44(21-23-45)19-18-37(40,41)42/h8-17,24H,7,18-23H2,1-6H3/t35-,36+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal human recombinant MDM2 assessed as inhibition of protein interaction with p53 by HTRF assay |
ACS Med Chem Lett 4: 466-9 (2013)
Article DOI: 10.1021/ml4000657
BindingDB Entry DOI: 10.7270/Q2JS9RTS |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50434290

(CHEMBL2386343)Show SMILES CCOc1cc(ccc1C1=N[C@@](C)(c2ccc(Cl)cc2)[C@](C)(N1C(=O)N1CCN(CC1)C(C)=O)c1ccc(Cl)cc1)C(C)(C)C |r,t:10| Show InChI InChI=1S/C36H42Cl2N4O3/c1-8-45-31-23-27(34(3,4)5)13-18-30(31)32-39-35(6,25-9-14-28(37)15-10-25)36(7,26-11-16-29(38)17-12-26)42(32)33(44)41-21-19-40(20-22-41)24(2)43/h9-18,23H,8,19-22H2,1-7H3/t35-,36+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal human recombinant MDM2 assessed as inhibition of protein interaction with p53 by HTRF assay |
ACS Med Chem Lett 4: 466-9 (2013)
Article DOI: 10.1021/ml4000657
BindingDB Entry DOI: 10.7270/Q2JS9RTS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50328280
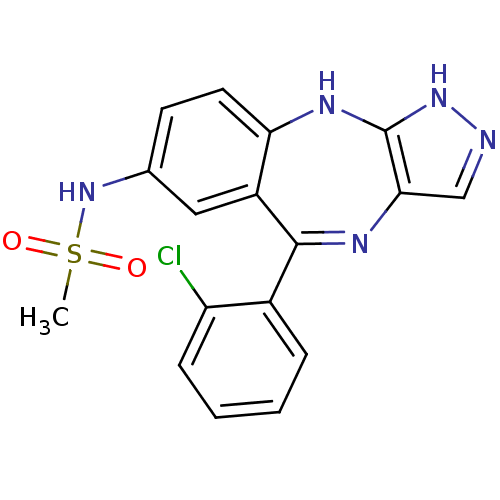
(CHEMBL1258022 | N-(5-(2-chlorophenyl)-2,10-dihydro...)Show SMILES CS(=O)(=O)Nc1ccc2Nc3[nH]ncc3N=C(c3ccccc3Cl)c2c1 |t:16| Show InChI InChI=1S/C17H14ClN5O2S/c1-26(24,25)23-10-6-7-14-12(8-10)16(11-4-2-3-5-13(11)18)20-15-9-19-22-17(15)21-14/h2-9,23H,1H3,(H2,19,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2-cyclin E |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk2
(Homo sapiens (Human)) | BDBM50426474

(CHEMBL1980391)Show SMILES COc1cc2Nc3[nH]nc(C)c3N=C(c3ccccc3Cl)c2cc1F |t:13| Show InChI InChI=1S/C18H14ClFN4O/c1-9-16-18(24-23-9)21-14-8-15(25-2)13(20)7-11(14)17(22-16)10-5-3-4-6-12(10)19/h3-8H,1-2H3,(H2,21,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CHK2 (unknown origin) |
ACS Med Chem Lett 4: 259-63 (2013)
Article DOI: 10.1021/ml300351e
BindingDB Entry DOI: 10.7270/Q2902541 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50434288
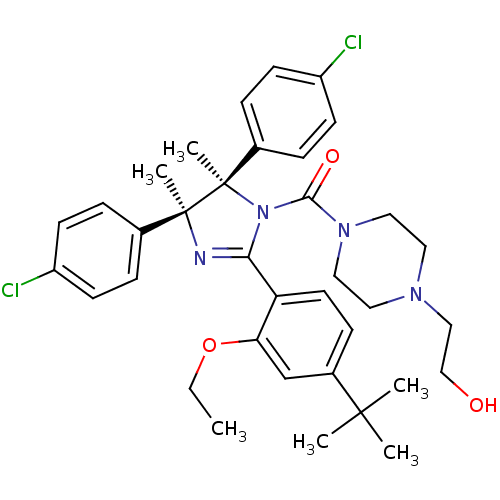
(CHEMBL2386345)Show SMILES CCOc1cc(ccc1C1=N[C@@](C)(c2ccc(Cl)cc2)[C@](C)(N1C(=O)N1CCN(CCO)CC1)c1ccc(Cl)cc1)C(C)(C)C |r,t:10| Show InChI InChI=1S/C36H44Cl2N4O3/c1-7-45-31-24-27(34(2,3)4)12-17-30(31)32-39-35(5,25-8-13-28(37)14-9-25)36(6,26-10-15-29(38)16-11-26)42(32)33(44)41-20-18-40(19-21-41)22-23-43/h8-17,24,43H,7,18-23H2,1-6H3/t35-,36+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal human recombinant MDM2 assessed as inhibition of protein interaction with p53 by HTRF assay |
ACS Med Chem Lett 4: 466-9 (2013)
Article DOI: 10.1021/ml4000657
BindingDB Entry DOI: 10.7270/Q2JS9RTS |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50426474

(CHEMBL1980391)Show SMILES COc1cc2Nc3[nH]nc(C)c3N=C(c3ccccc3Cl)c2cc1F |t:13| Show InChI InChI=1S/C18H14ClFN4O/c1-9-16-18(24-23-9)21-14-8-15(25-2)13(20)7-11(14)17(22-16)10-5-3-4-6-12(10)19/h3-8H,1-2H3,(H2,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 (unknown origin) |
ACS Med Chem Lett 4: 259-63 (2013)
Article DOI: 10.1021/ml300351e
BindingDB Entry DOI: 10.7270/Q2902541 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50434292

(CHEMBL2386169)Show SMILES CCOc1cc(ccc1C1=N[C@@](C)(c2ccc(Cl)cc2)[C@](C)(N1C(=O)N1CCNCC1)c1ccc(Cl)cc1)C(C)(C)C |r,t:10| Show InChI InChI=1S/C34H40Cl2N4O2/c1-7-42-29-22-25(32(2,3)4)12-17-28(29)30-38-33(5,23-8-13-26(35)14-9-23)34(6,24-10-15-27(36)16-11-24)40(30)31(41)39-20-18-37-19-21-39/h8-17,22,37H,7,18-21H2,1-6H3/t33-,34+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal human recombinant MDM2 assessed as inhibition of protein interaction with p53 by HTRF assay |
ACS Med Chem Lett 4: 466-9 (2013)
Article DOI: 10.1021/ml4000657
BindingDB Entry DOI: 10.7270/Q2JS9RTS |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50434285
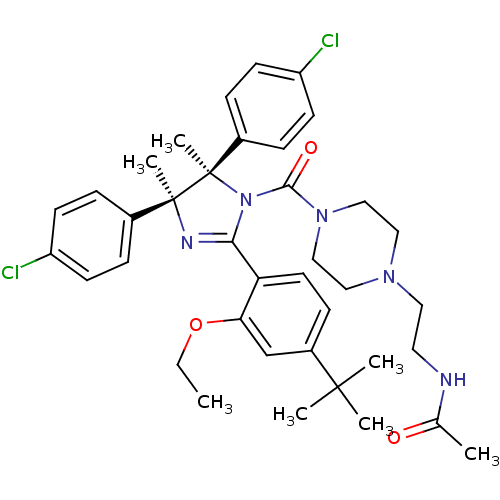
(CHEMBL2386348)Show SMILES CCOc1cc(ccc1C1=N[C@@](C)(c2ccc(Cl)cc2)[C@](C)(N1C(=O)N1CCN(CCNC(C)=O)CC1)c1ccc(Cl)cc1)C(C)(C)C |r,t:10| Show InChI InChI=1S/C38H47Cl2N5O3/c1-8-48-33-25-29(36(3,4)5)13-18-32(33)34-42-37(6,27-9-14-30(39)15-10-27)38(7,28-11-16-31(40)17-12-28)45(34)35(47)44-23-21-43(22-24-44)20-19-41-26(2)46/h9-18,25H,8,19-24H2,1-7H3,(H,41,46)/t37-,38+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal human recombinant MDM2 assessed as inhibition of protein interaction with p53 by HTRF assay |
ACS Med Chem Lett 4: 466-9 (2013)
Article DOI: 10.1021/ml4000657
BindingDB Entry DOI: 10.7270/Q2JS9RTS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50328258

(CHEMBL1257912 | N-(5-(2-chlorophenyl)-2,10-dihydro...)Show SMILES CC(=O)Nc1ccc2Nc3[nH]ncc3N=C(c3ccccc3Cl)c2c1 |t:15| Show InChI InChI=1S/C18H14ClN5O/c1-10(25)21-11-6-7-15-13(8-11)17(12-4-2-3-5-14(12)19)22-16-9-20-24-18(16)23-15/h2-9H,1H3,(H,21,25)(H2,20,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50426473
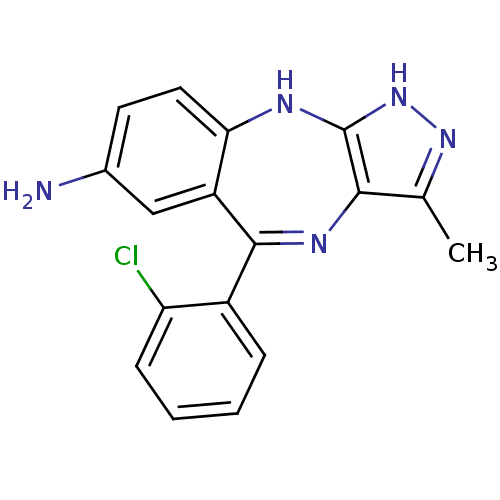
(CHEMBL2322701)Show SMILES Cc1n[nH]c2Nc3ccc(N)cc3C(=Nc12)c1ccccc1Cl |c:14| Show InChI InChI=1S/C17H14ClN5/c1-9-15-17(23-22-9)20-14-7-6-10(19)8-12(14)16(21-15)11-4-2-3-5-13(11)18/h2-8H,19H2,1H3,(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of KDR (unknown origin) |
ACS Med Chem Lett 4: 259-63 (2013)
Article DOI: 10.1021/ml300351e
BindingDB Entry DOI: 10.7270/Q2902541 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50328257
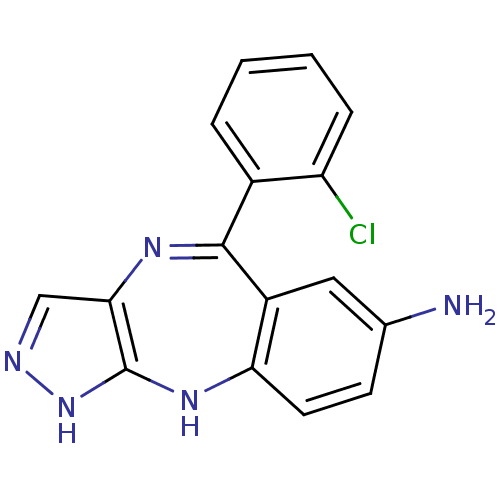
(5-(2-chlorophenyl)-2,10-dihydrobenzo[e]pyrazolo[4,...)Show SMILES Nc1ccc2Nc3[nH]ncc3N=C(c3ccccc3Cl)c2c1 |t:12| Show InChI InChI=1S/C16H12ClN5/c17-12-4-2-1-3-10(12)15-11-7-9(18)5-6-13(11)21-16-14(20-15)8-19-22-16/h1-8H,18H2,(H2,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50328255
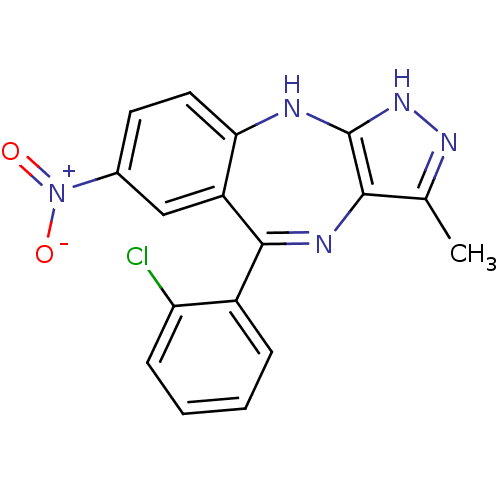
(5-(2-chlorophenyl)-3-methyl-7-nitro-2,10-dihydrobe...)Show SMILES Cc1n[nH]c2Nc3ccc(cc3C(=Nc12)c1ccccc1Cl)[N+]([O-])=O |c:13| Show InChI InChI=1S/C17H12ClN5O2/c1-9-15-17(22-21-9)19-14-7-6-10(23(24)25)8-12(14)16(20-15)11-4-2-3-5-13(11)18/h2-8H,1H3,(H2,19,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2-cyclin E |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50328258

(CHEMBL1257912 | N-(5-(2-chlorophenyl)-2,10-dihydro...)Show SMILES CC(=O)Nc1ccc2Nc3[nH]ncc3N=C(c3ccccc3Cl)c2c1 |t:15| Show InChI InChI=1S/C18H14ClN5O/c1-10(25)21-11-6-7-15-13(8-11)17(12-4-2-3-5-14(12)19)22-16-9-20-24-18(16)23-15/h2-9H,1H3,(H,21,25)(H2,20,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2-cyclin E |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50328256

(5-(2-fluorophenyl)-2,10-dihydrobenzo[e]pyrazolo[4,...)Show SMILES Fc1ccccc1C1=Nc2cn[nH]c2Nc2ccc(cc12)C#N |t:8| Show InChI InChI=1S/C17H10FN5/c18-13-4-2-1-3-11(13)16-12-7-10(8-19)5-6-14(12)22-17-15(21-16)9-20-23-17/h1-7,9H,(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50328274
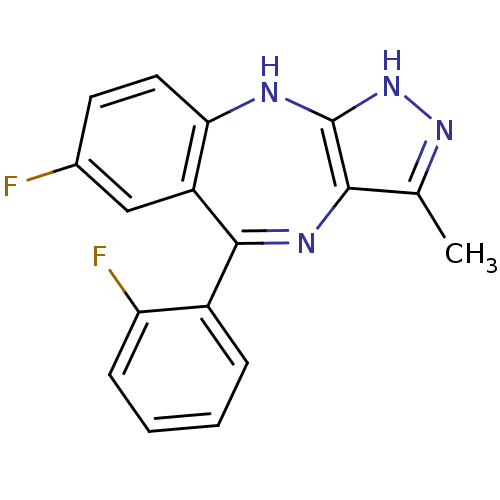
(7-fluoro-5-(2-fluorophenyl)-3-methyl-2,10-dihydrob...)Show SMILES Cc1n[nH]c2Nc3ccc(F)cc3C(=Nc12)c1ccccc1F |c:14| Show InChI InChI=1S/C17H12F2N4/c1-9-15-17(23-22-9)20-14-7-6-10(18)8-12(14)16(21-15)11-4-2-3-5-13(11)19/h2-8H,1H3,(H2,20,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2-cyclin E |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50434286

(CHEMBL2386347)Show SMILES CCOc1cc(ccc1C1=N[C@@](C)(c2ccc(Cl)cc2)[C@](C)(N1C(=O)N1CCN(CCNS(C)(=O)=O)CC1)c1ccc(Cl)cc1)C(C)(C)C |r,t:10| Show InChI InChI=1S/C37H47Cl2N5O4S/c1-8-48-32-25-28(35(2,3)4)13-18-31(32)33-41-36(5,26-9-14-29(38)15-10-26)37(6,27-11-16-30(39)17-12-27)44(33)34(45)43-23-21-42(22-24-43)20-19-40-49(7,46)47/h9-18,25,40H,8,19-24H2,1-7H3/t36-,37+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal human recombinant MDM2 assessed as inhibition of protein interaction with p53 by HTRF assay |
ACS Med Chem Lett 4: 466-9 (2013)
Article DOI: 10.1021/ml4000657
BindingDB Entry DOI: 10.7270/Q2JS9RTS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50328281
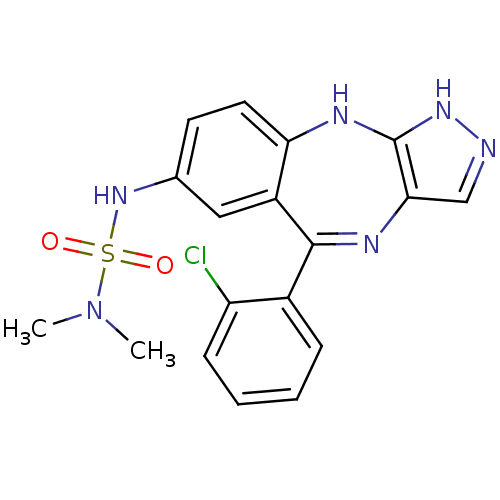
(CHEMBL1258141 | N'-[5-(2-chlorophenyl)-2,10-dihydr...)Show SMILES CN(C)S(=O)(=O)Nc1ccc2Nc3[nH]ncc3N=C(c3ccccc3Cl)c2c1 |t:18| Show InChI InChI=1S/C18H17ClN6O2S/c1-25(2)28(26,27)24-11-7-8-15-13(9-11)17(12-5-3-4-6-14(12)19)21-16-10-20-23-18(16)22-15/h3-10,24H,1-2H3,(H2,20,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2-cyclin E |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50426473

(CHEMBL2322701)Show SMILES Cc1n[nH]c2Nc3ccc(N)cc3C(=Nc12)c1ccccc1Cl |c:14| Show InChI InChI=1S/C17H14ClN5/c1-9-15-17(23-22-9)20-14-7-6-10(19)8-12(14)16(21-15)11-4-2-3-5-13(11)18/h2-8H,19H2,1H3,(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 (unknown origin) |
ACS Med Chem Lett 4: 259-63 (2013)
Article DOI: 10.1021/ml300351e
BindingDB Entry DOI: 10.7270/Q2902541 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50328269
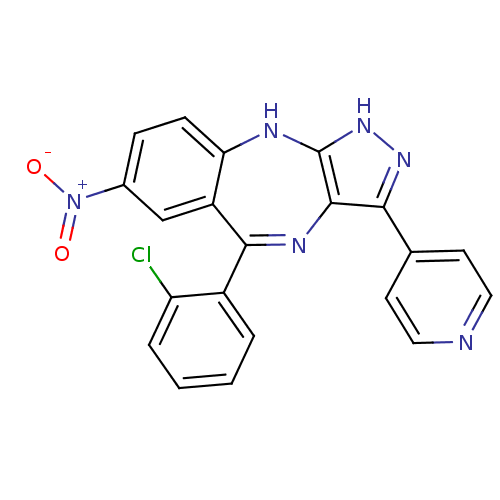
(5-(2-chlorophenyl)-7-nitro-3-(pyridin-4-yl)-2,10-d...)Show SMILES [O-][N+](=O)c1ccc2Nc3[nH]nc(c3N=C(c3ccccc3Cl)c2c1)-c1ccncc1 |t:14| Show InChI InChI=1S/C21H13ClN6O2/c22-16-4-2-1-3-14(16)19-15-11-13(28(29)30)5-6-17(15)24-21-20(25-19)18(26-27-21)12-7-9-23-10-8-12/h1-11H,(H2,24,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2-cyclin E |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50434280
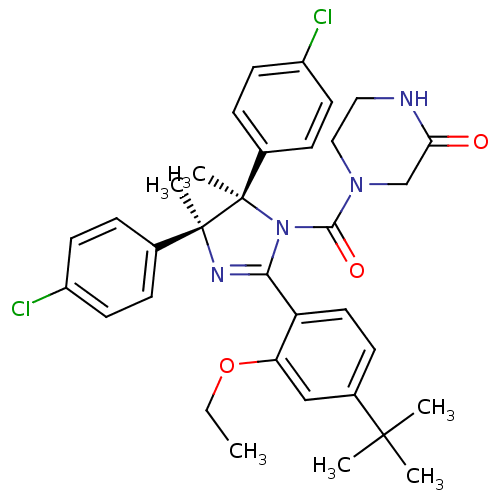
(CHEMBL2386168)Show SMILES CCOc1cc(ccc1C1=N[C@@](C)(c2ccc(Cl)cc2)[C@](C)(N1C(=O)N1CCNC(=O)C1)c1ccc(Cl)cc1)C(C)(C)C |r,t:10| Show InChI InChI=1S/C34H38Cl2N4O3/c1-7-43-28-20-24(32(2,3)4)12-17-27(28)30-38-33(5,22-8-13-25(35)14-9-22)34(6,23-10-15-26(36)16-11-23)40(30)31(42)39-19-18-37-29(41)21-39/h8-17,20H,7,18-19,21H2,1-6H3,(H,37,41)/t33-,34+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal human recombinant MDM2 assessed as inhibition of protein interaction with p53 by HTRF assay |
ACS Med Chem Lett 4: 466-9 (2013)
Article DOI: 10.1021/ml4000657
BindingDB Entry DOI: 10.7270/Q2JS9RTS |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50426474

(CHEMBL1980391)Show SMILES COc1cc2Nc3[nH]nc(C)c3N=C(c3ccccc3Cl)c2cc1F |t:13| Show InChI InChI=1S/C18H14ClFN4O/c1-9-16-18(24-23-9)21-14-8-15(25-2)13(20)7-11(14)17(22-16)10-5-3-4-6-12(10)19/h3-8H,1-2H3,(H2,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of aurora-A (unknown origin) |
ACS Med Chem Lett 4: 259-63 (2013)
Article DOI: 10.1021/ml300351e
BindingDB Entry DOI: 10.7270/Q2902541 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50328272

(5-(2-fluorophenyl)-3-methyl-2,10-dihydrobenzo[e]py...)Show SMILES Cc1n[nH]c2Nc3ccccc3C(=Nc12)c1ccccc1F |c:13| Show InChI InChI=1S/C17H13FN4/c1-10-15-17(22-21-10)19-14-9-5-3-7-12(14)16(20-15)11-6-2-4-8-13(11)18/h2-9H,1H3,(H2,19,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2-cyclin E |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50328260
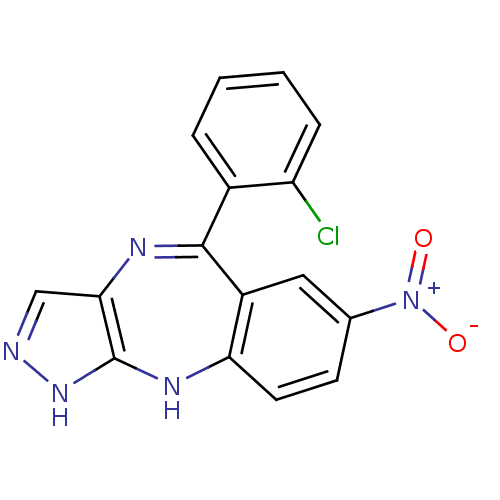
(5-(2-chlorophenyl)-7-nitro-2,10-dihydrobenzo[e]pyr...)Show SMILES [O-][N+](=O)c1ccc2Nc3[nH]ncc3N=C(c3ccccc3Cl)c2c1 |t:14| Show InChI InChI=1S/C16H10ClN5O2/c17-12-4-2-1-3-10(12)15-11-7-9(22(23)24)5-6-13(11)20-16-14(19-15)8-18-21-16/h1-8H,(H2,18,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2-cyclin E |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50328255

(5-(2-chlorophenyl)-3-methyl-7-nitro-2,10-dihydrobe...)Show SMILES Cc1n[nH]c2Nc3ccc(cc3C(=Nc12)c1ccccc1Cl)[N+]([O-])=O |c:13| Show InChI InChI=1S/C17H12ClN5O2/c1-9-15-17(22-21-9)19-14-7-6-10(23(24)25)8-12(14)16(20-15)11-4-2-3-5-13(11)18/h2-8H,1H3,(H2,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50328257

(5-(2-chlorophenyl)-2,10-dihydrobenzo[e]pyrazolo[4,...)Show SMILES Nc1ccc2Nc3[nH]ncc3N=C(c3ccccc3Cl)c2c1 |t:12| Show InChI InChI=1S/C16H12ClN5/c17-12-4-2-1-3-10(12)15-11-7-9(18)5-6-13(11)21-16-14(20-15)8-19-22-16/h1-8H,18H2,(H2,19,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2-cyclin E |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50426473

(CHEMBL2322701)Show SMILES Cc1n[nH]c2Nc3ccc(N)cc3C(=Nc12)c1ccccc1Cl |c:14| Show InChI InChI=1S/C17H14ClN5/c1-9-15-17(23-22-9)20-14-7-6-10(19)8-12(14)16(21-15)11-4-2-3-5-13(11)18/h2-8H,19H2,1H3,(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
ACS Med Chem Lett 4: 259-63 (2013)
Article DOI: 10.1021/ml300351e
BindingDB Entry DOI: 10.7270/Q2902541 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50229787

((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...)Show SMILES COc1ccc(C2=N[C@H]([C@H](N2C(=O)N2CCNC(=O)C2)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(OC(C)C)c1 |t:6| Show InChI InChI=1S/C30H30Cl2N4O4/c1-18(2)40-25-16-23(39-3)12-13-24(25)29-34-27(19-4-8-21(31)9-5-19)28(20-6-10-22(32)11-7-20)36(29)30(38)35-15-14-33-26(37)17-35/h4-13,16,18,27-28H,14-15,17H2,1-3H3,(H,33,37)/t27-,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal human recombinant MDM2 assessed as inhibition of protein interaction with p53 by HTRF assay |
ACS Med Chem Lett 4: 466-9 (2013)
Article DOI: 10.1021/ml4000657
BindingDB Entry DOI: 10.7270/Q2JS9RTS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50328273
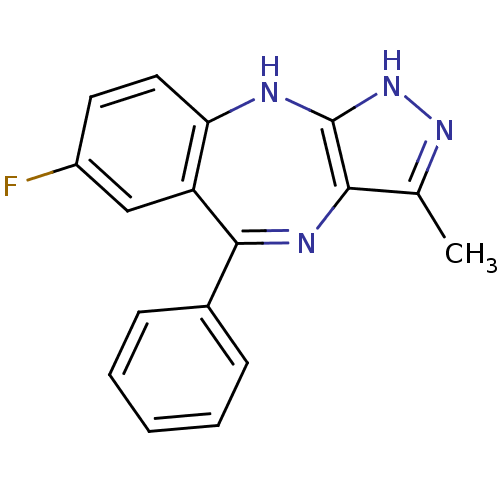
(7-fluoro-3-methyl-5-phenyl-2,10-dihydrobenzo[e]pyr...)Show SMILES Cc1n[nH]c2Nc3ccc(F)cc3C(=Nc12)c1ccccc1 |c:14| Show InChI InChI=1S/C17H13FN4/c1-10-15-17(22-21-10)19-14-8-7-12(18)9-13(14)16(20-15)11-5-3-2-4-6-11/h2-9H,1H3,(H2,19,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2-cyclin E |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50328271
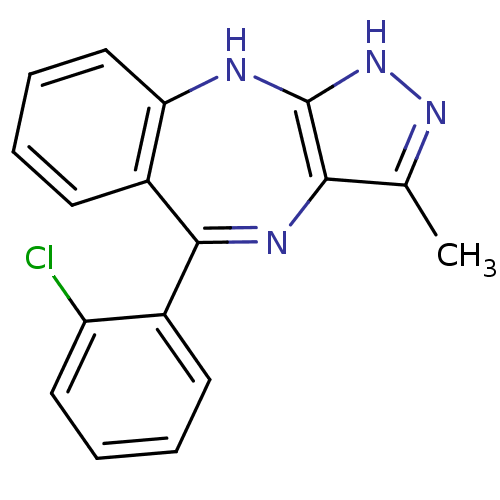
(5-(2-chlorophenyl)-3-methyl-2,10-dihydrobenzo[e]py...)Show SMILES Cc1n[nH]c2Nc3ccccc3C(=Nc12)c1ccccc1Cl |c:13| Show InChI InChI=1S/C17H13ClN4/c1-10-15-17(22-21-10)19-14-9-5-3-7-12(14)16(20-15)11-6-2-4-8-13(11)18/h2-9H,1H3,(H2,19,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2-cyclin E |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50328270

(3-methyl-5-phenyl-2,10-dihydrobenzo[e]pyrazolo[4,3...)Show InChI InChI=1S/C17H14N4/c1-11-15-17(21-20-11)18-14-10-6-5-9-13(14)16(19-15)12-7-3-2-4-8-12/h2-10H,1H3,(H2,18,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2-cyclin E |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50328257

(5-(2-chlorophenyl)-2,10-dihydrobenzo[e]pyrazolo[4,...)Show SMILES Nc1ccc2Nc3[nH]ncc3N=C(c3ccccc3Cl)c2c1 |t:12| Show InChI InChI=1S/C16H12ClN5/c17-12-4-2-1-3-10(12)15-11-7-9(18)5-6-13(11)21-16-14(20-15)8-19-22-16/h1-8H,18H2,(H2,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FAK |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50328258

(CHEMBL1257912 | N-(5-(2-chlorophenyl)-2,10-dihydro...)Show SMILES CC(=O)Nc1ccc2Nc3[nH]ncc3N=C(c3ccccc3Cl)c2c1 |t:15| Show InChI InChI=1S/C18H14ClN5O/c1-10(25)21-11-6-7-15-13(8-11)17(12-4-2-3-5-14(12)19)22-16-9-20-24-18(16)23-15/h2-9H,1H3,(H,21,25)(H2,20,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of FAK |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50328277
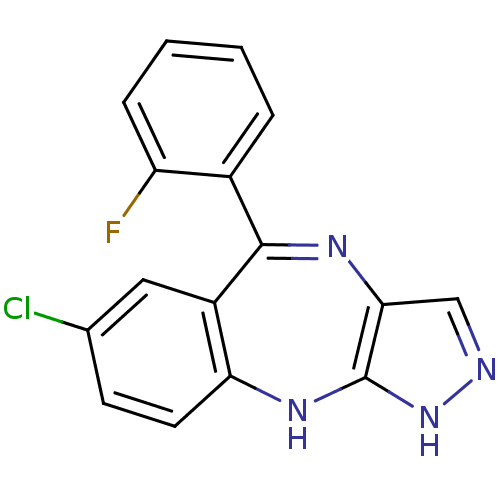
(7-chloro-5-(2-fluorophenyl)-2,10-dihydrobenzo[e]py...)Show SMILES Fc1ccccc1C1=Nc2cn[nH]c2Nc2ccc(Cl)cc12 |t:8| Show InChI InChI=1S/C16H10ClFN4/c17-9-5-6-13-11(7-9)15(10-3-1-2-4-12(10)18)20-14-8-19-22-16(14)21-13/h1-8H,(H2,19,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2-cyclin E |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50434291

(CHEMBL2386170)Show SMILES CCOc1cc(ccc1C1=N[C@@](C)(c2ccc(Cl)cc2)[C@](C)(N1C(=O)N1CCCCC1)c1ccc(Cl)cc1)C(C)(C)C |r,t:10| Show InChI InChI=1S/C35H41Cl2N3O2/c1-7-42-30-23-26(33(2,3)4)15-20-29(30)31-38-34(5,24-11-16-27(36)17-12-24)35(6,25-13-18-28(37)19-14-25)40(31)32(41)39-21-9-8-10-22-39/h11-20,23H,7-10,21-22H2,1-6H3/t34-,35+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 209 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal human recombinant MDM2 assessed as inhibition of protein interaction with p53 by HTRF assay |
ACS Med Chem Lett 4: 466-9 (2013)
Article DOI: 10.1021/ml4000657
BindingDB Entry DOI: 10.7270/Q2JS9RTS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50328265
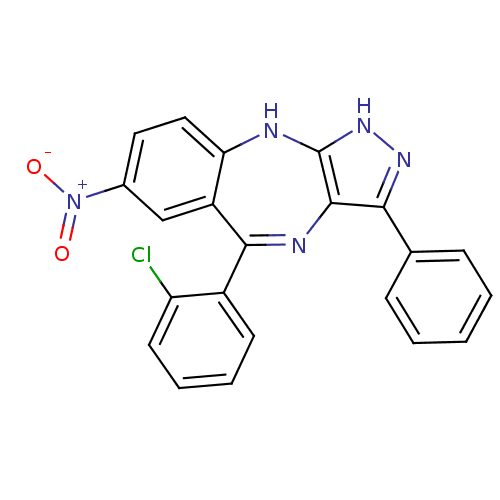
(5-(2-chlorophenyl)-7-nitro-3-phenyl-2,10-dihydrobe...)Show SMILES [O-][N+](=O)c1ccc2Nc3[nH]nc(c3N=C(c3ccccc3Cl)c2c1)-c1ccccc1 |t:14| Show InChI InChI=1S/C22H14ClN5O2/c23-17-9-5-4-8-15(17)20-16-12-14(28(29)30)10-11-18(16)24-22-21(25-20)19(26-27-22)13-6-2-1-3-7-13/h1-12H,(H2,24,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2-cyclin E |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50434281

(CHEMBL2386167)Show SMILES CCOc1cc(ccc1C1=N[C@](C)([C@H](N1C(=O)N1CCN(CCCS(C)(=O)=O)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |r,t:10| Show InChI InChI=1S/C37H46Cl2N4O4S/c1-7-47-32-25-28(36(2,3)4)13-18-31(32)34-40-37(5,27-11-16-30(39)17-12-27)33(26-9-14-29(38)15-10-26)43(34)35(44)42-22-20-41(21-23-42)19-8-24-48(6,45)46/h9-18,25,33H,7-8,19-24H2,1-6H3/t33-,37+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 232 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal human recombinant MDM2 assessed as inhibition of protein interaction with p53 by HTRF assay |
ACS Med Chem Lett 4: 466-9 (2013)
Article DOI: 10.1021/ml4000657
BindingDB Entry DOI: 10.7270/Q2JS9RTS |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50328262
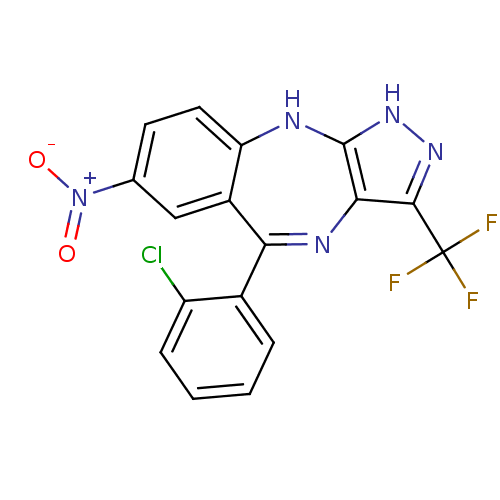
(5-(2-chlorophenyl)-7-nitro-3-(trifluoromethyl)-2,1...)Show SMILES [O-][N+](=O)c1ccc2Nc3[nH]nc(c3N=C(c3ccccc3Cl)c2c1)C(F)(F)F |t:14| Show InChI InChI=1S/C17H9ClF3N5O2/c18-11-4-2-1-3-9(11)13-10-7-8(26(27)28)5-6-12(10)22-16-14(23-13)15(24-25-16)17(19,20)21/h1-7H,(H2,22,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 232 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2-cyclin E |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50328261
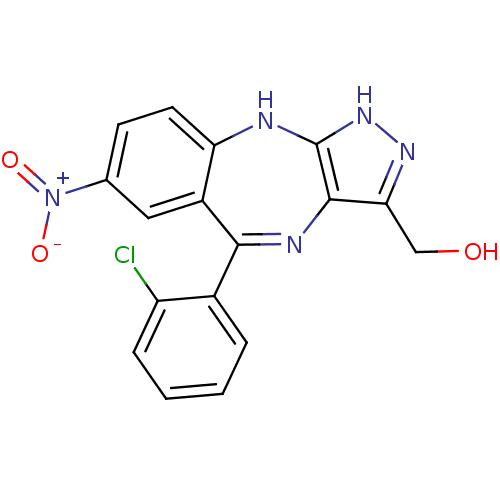
((5-(2-chlorophenyl)-7-nitro-2,10-dihydrobenzo[e]py...)Show SMILES OCc1n[nH]c2Nc3ccc(cc3C(=Nc12)c1ccccc1Cl)[N+]([O-])=O |c:14| Show InChI InChI=1S/C17H12ClN5O3/c18-12-4-2-1-3-10(12)15-11-7-9(23(25)26)5-6-13(11)19-17-16(20-15)14(8-24)21-22-17/h1-7,24H,8H2,(H2,19,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2-cyclin E |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50328263
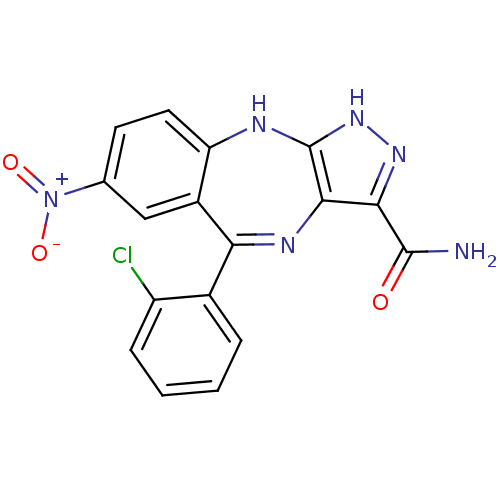
(5-(2-chlorophenyl)-7-nitro-2,10-dihydrobenzo[e]pyr...)Show SMILES NC(=O)c1n[nH]c2Nc3ccc(cc3C(=Nc12)c1ccccc1Cl)[N+]([O-])=O |c:15| Show InChI InChI=1S/C17H11ClN6O3/c18-11-4-2-1-3-9(11)13-10-7-8(24(26)27)5-6-12(10)20-17-15(21-13)14(16(19)25)22-23-17/h1-7H,(H2,19,25)(H2,20,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 258 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2-cyclin E |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50328268

(5-(2-chlorophenyl)-7-nitro-3-(pyridin-3-yl)-2,10-d...)Show SMILES [O-][N+](=O)c1ccc2Nc3[nH]nc(c3N=C(c3ccccc3Cl)c2c1)-c1cccnc1 |t:14| Show InChI InChI=1S/C21H13ClN6O2/c22-16-6-2-1-5-14(16)19-15-10-13(28(29)30)7-8-17(15)24-21-20(25-19)18(26-27-21)12-4-3-9-23-11-12/h1-11H,(H2,24,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2-cyclin E |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM50426473

(CHEMBL2322701)Show SMILES Cc1n[nH]c2Nc3ccc(N)cc3C(=Nc12)c1ccccc1Cl |c:14| Show InChI InChI=1S/C17H14ClN5/c1-9-15-17(23-22-9)20-14-7-6-10(19)8-12(14)16(21-15)11-4-2-3-5-13(11)18/h2-8H,19H2,1H3,(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 303 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK4 (unknown origin) |
ACS Med Chem Lett 4: 259-63 (2013)
Article DOI: 10.1021/ml300351e
BindingDB Entry DOI: 10.7270/Q2902541 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM50328278
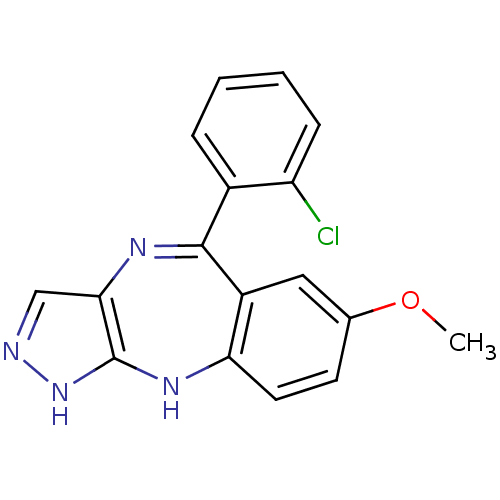
(5-(2-chlorophenyl)-7-methoxy-2,10-dihydrobenzo[e]p...)Show SMILES COc1ccc2Nc3[nH]ncc3N=C(c3ccccc3Cl)c2c1 |t:13| Show InChI InChI=1S/C17H13ClN4O/c1-23-10-6-7-14-12(8-10)16(11-4-2-3-5-13(11)18)20-15-9-19-22-17(15)21-14/h2-9H,1H3,(H2,19,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2-cyclin E |
Bioorg Med Chem Lett 20: 5984-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.079
BindingDB Entry DOI: 10.7270/Q21C1X42 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data