

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50099843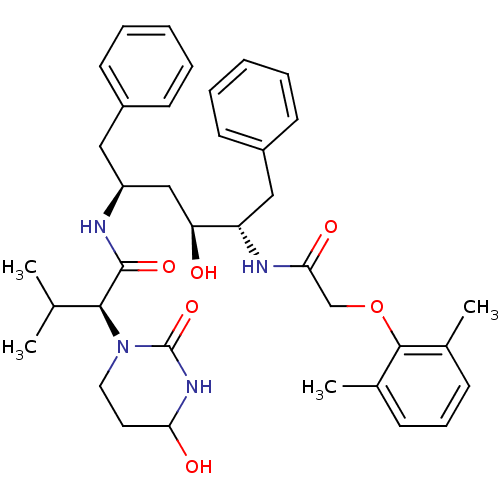 ((S)-N-[(S)-4-[2-(2,6-Dimethyl-phenoxy)-acetylamino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 11: 1351-3 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM578 ((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 11: 1351-3 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BX6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50099842 ((S)-N-[(S)-4-[2-(2,6-Dimethyl-phenoxy)-acetylamino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 11: 1351-3 (2001) BindingDB Entry DOI: 10.7270/Q2HX1BX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM194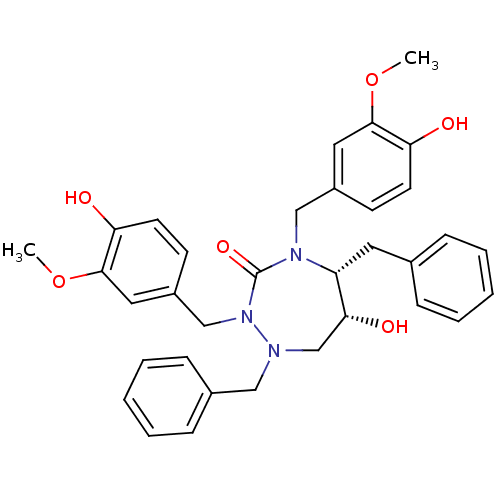 ((5R,6R)-1,5-dibenzyl-6-hydroxy-2,4-bis[(4-hydroxy-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00500 | -65.6 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM195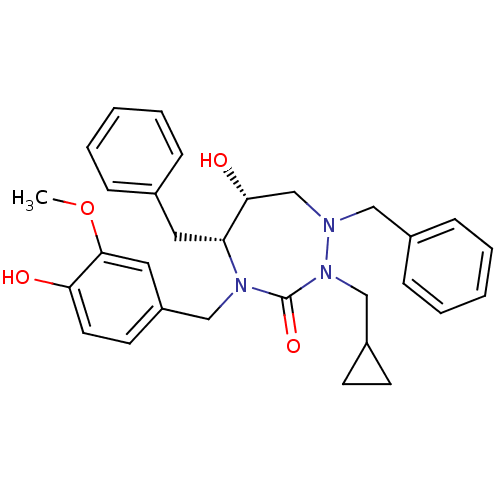 ((5R,6R)-1,5-dibenzyl-2-(cyclopropylmethyl)-6-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0620 | -59.2 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM192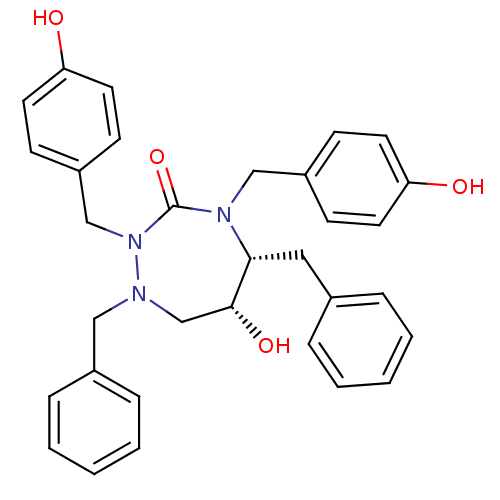 ((5R,6R)-1,5-dibenzyl-6-hydroxy-2,4-bis[(4-hydroxyp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | -58.9 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM189 ((5R,6R)-1,5-dibenzyl-6-hydroxy-2,4-bis({[4-(hydrox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.220 | -56.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM193 ((5R,6R)-1,5-dibenzyl-6-hydroxy-2,4-bis[(3-methoxyp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.220 | -56.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM190 ((5R,6R)-2,4-Bis[4-(hydroxymethyl)benzyl]-1-(3-fura...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.490 | -54.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM191 ((5R,6R)-1,2,4,5-tetrabenzyl-6-hydroxy-1,2,4-triaze...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -50.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | J Med Chem 39: 392-7 (1996) Article DOI: 10.1021/jm9507183 BindingDB Entry DOI: 10.7270/Q2V40SC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM196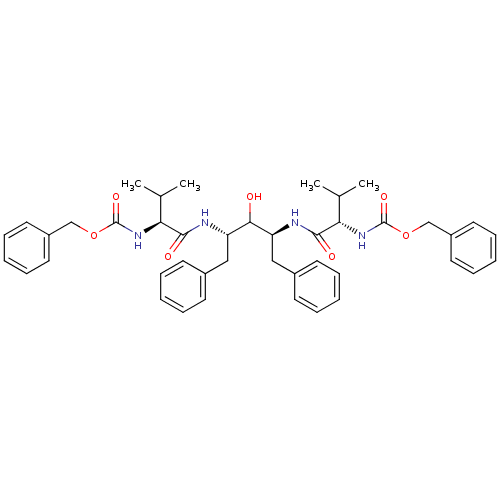 (A-74704 | CHEMBL307193 | benzyl N-[(1S)-1-{[(2S,4S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.5 | -48.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease activity was measured by a continuous fluorometric assay using the internally quenched fluorogenic substrate DABCYL-GABA-Ser-Gln-Tyr-P... | Science 249: 527-33 (1990) Article DOI: 10.1126/science.2200122 BindingDB Entry DOI: 10.7270/Q2QC01NV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339869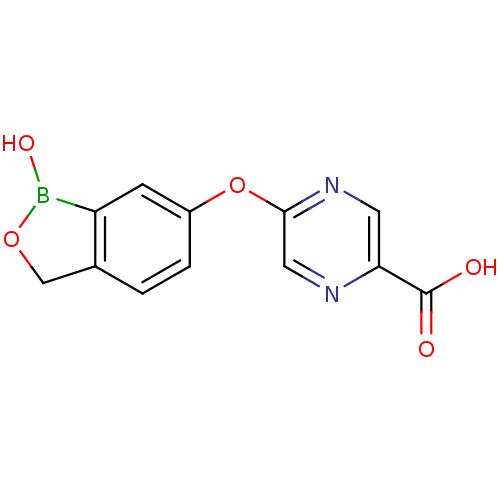 (5-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339868 (4-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339870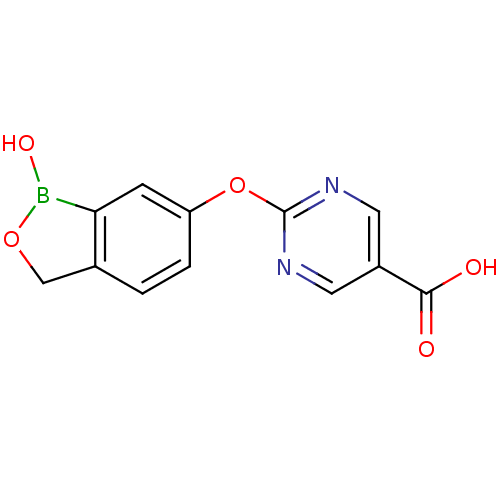 (2-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339846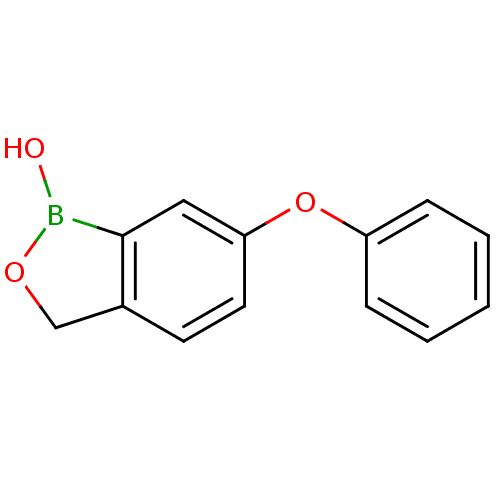 (6-phenoxybenzo[c][1,2]oxaborol-1(3H)-ol | CHEMBL17...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339856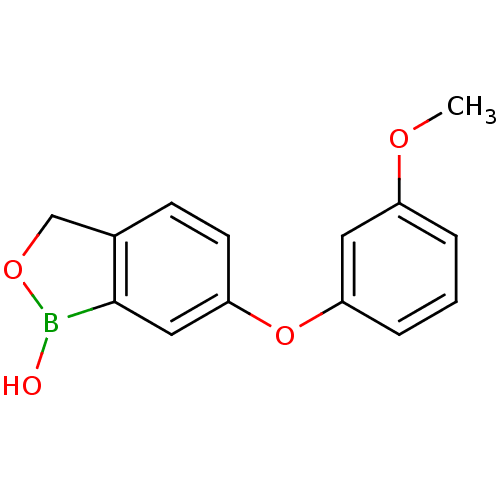 (6-(3-methoxyphenoxy)benzo[c][1,2]oxaborol-1(3H)-ol...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339862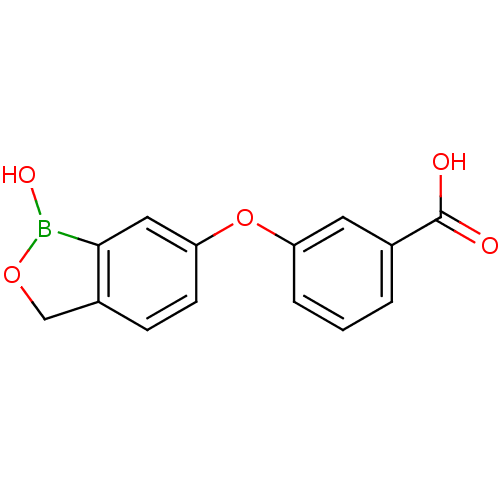 (3-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase, cytoplasmic (Saccharomyces cerevisiae S288c) | BDBM50370987 (TAVABOROLE) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae cytoplasmic leucyl-tRNA synthetase after 20 mins | Science 316: 1759-1761 (2007) Article DOI: 10.1126/science.1142189 BindingDB Entry DOI: 10.7270/Q2P84CQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339860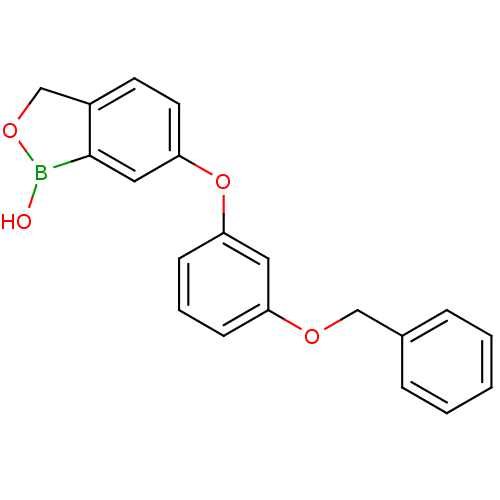 (6-(3-(benzyloxy)phenoxy)benzo[c][1,2]oxaborol-1(3H...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339857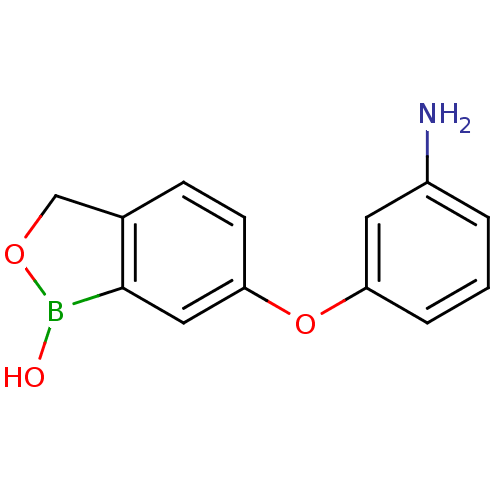 (6-(3-aminophenoxy)benzo[c][1,2]oxaborol-1(3H)-ol |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339859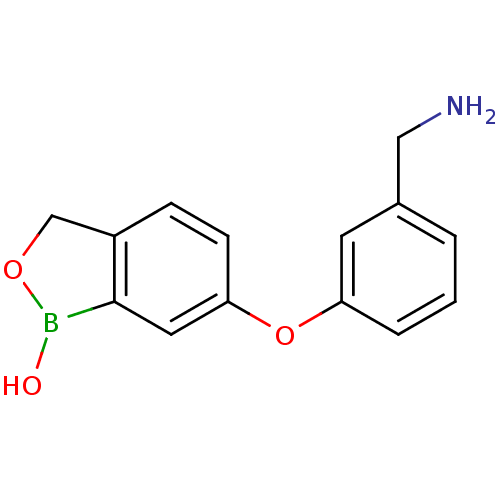 (6-(3-(aminomethyl)phenoxy)benzo[c][1,2]oxaborol-1(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339855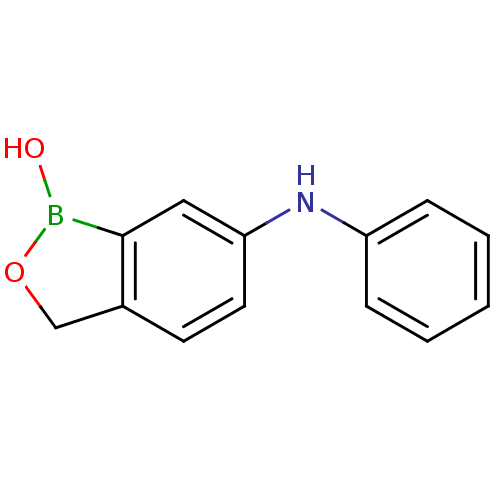 (6-(phenylamino)benzo[c][1,2]oxaborol-1(3H)-ol | CH...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339867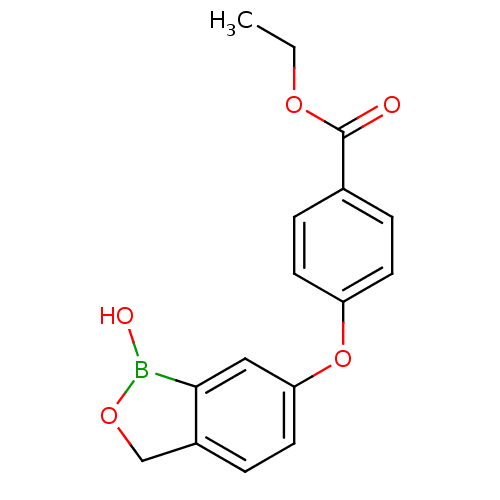 (CHEMBL1761273 | ethyl 4-(1-hydroxy-1,3-dihydrobenz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339863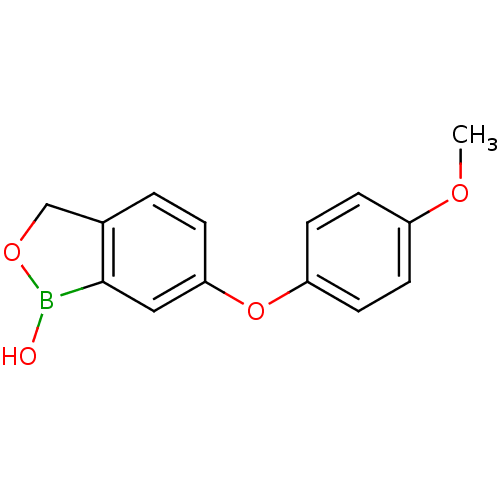 (6-(4-methoxyphenoxy)benzo[c][1,2]oxaborol-1(3H)-ol...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339850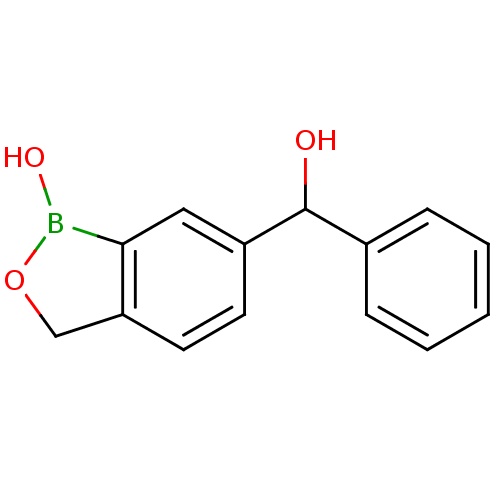 (6-(hydroxy(phenyl)methyl)benzo[c][1,2]oxaborol-1(3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339861 (6-(3-((dimethylamino)methyl)phenoxy)benzo[c][1,2]o...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339858 (6-(3-(hydroxymethyl)phenoxy)benzo[c][1,2]oxaborol-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339864 (6-(4-aminophenoxy)benzo[c][1,2]oxaborol-1(3H)-ol |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339847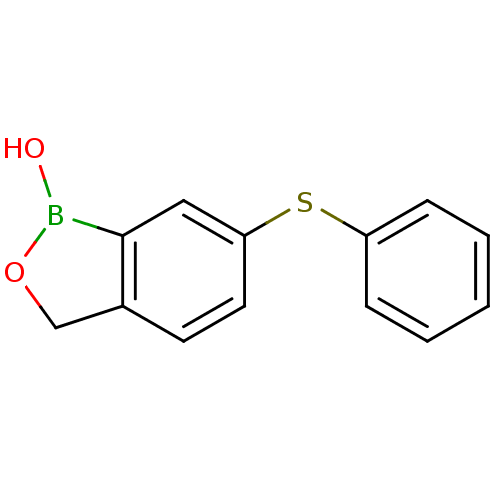 (6-(phenylthio)benzo[c][1,2]oxaborol-1(3H)-ol | CHE...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339865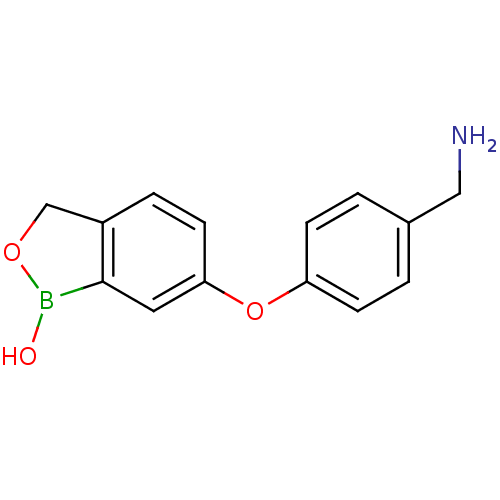 (6-(4-(aminomethyl)phenoxy)benzo[c][1,2]oxaborol-1(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339854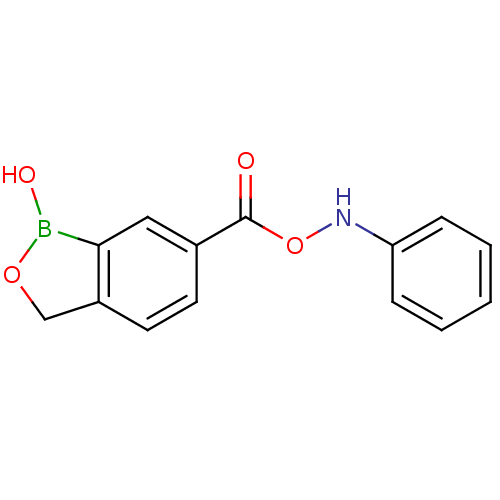 (6-((phenylaminooxy)carbonyl)benzo[c][1,2]oxaborol-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339848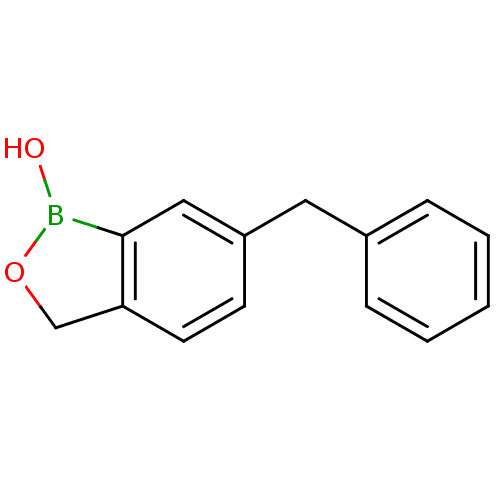 (6-benzylbenzo[c][1,2]oxaborol-1(3H)-ol | CHEMBL176...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339849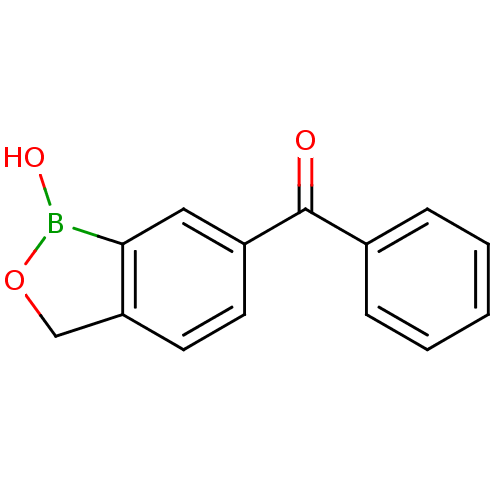 ((1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase, cytoplasmic (Saccharomyces cerevisiae S288c) | BDBM50370987 (TAVABOROLE) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | 3.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae cytoplasmic leucyl-tRNA synthetase after 2 mins | Science 316: 1759-1761 (2007) Article DOI: 10.1126/science.1142189 BindingDB Entry DOI: 10.7270/Q2P84CQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339866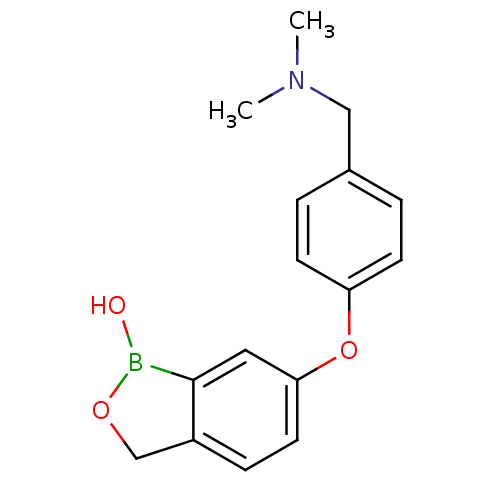 (6-(4-((dimethylamino)methyl)phenoxy)benzo[c][1,2]o...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339853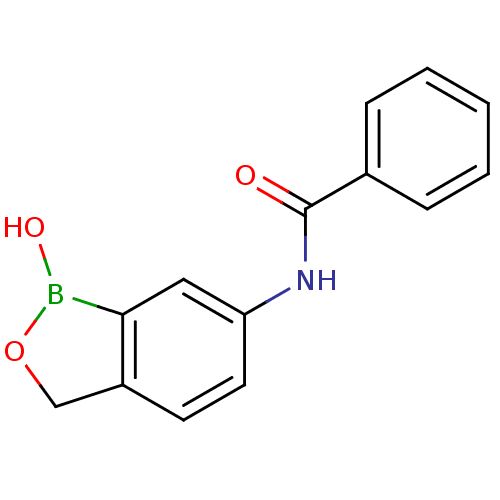 (CHEMBL1761258 | N-(1-hydroxy-1,3-dihydrobenzo[c][1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 4.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339851 (6-(phenylsulfinyl)benzo[c][1,2]oxaborol-1(3H)-ol |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50339852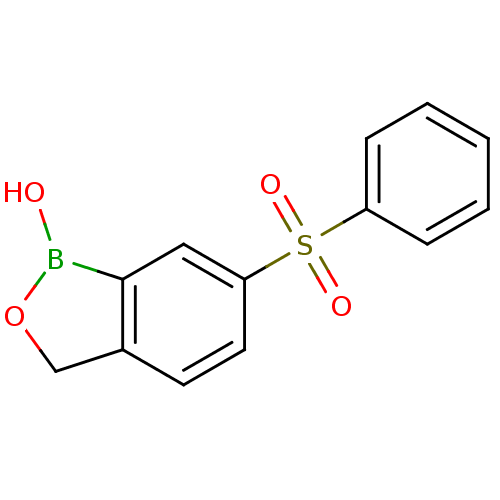 (6-(phenylsulfonyl)benzo[c][1,2]oxaborol-1(3H)-ol |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Enterobacter cloacae P99 beta-lactamase AmpC P99 using nitrocefin as substrate after 30 mins by spectrophotometric analysis | Bioorg Med Chem Lett 21: 2533-6 (2011) Article DOI: 10.1016/j.bmcl.2011.02.024 BindingDB Entry DOI: 10.7270/Q2G44QK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE4B2 of cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM589753 (US11559538, Example 16 | US11559538, Example 208 |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Cytokine inhibitory activity is determined by measuring the effect of test agent on the release of the cytokines IL-4, IL-13 and IFNγ from human... | Citation and Details BindingDB Entry DOI: 10.7270/Q2542SHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-dependent protein kinase catalytic subunit alpha (Homo sapiens (Human)) | BDBM258601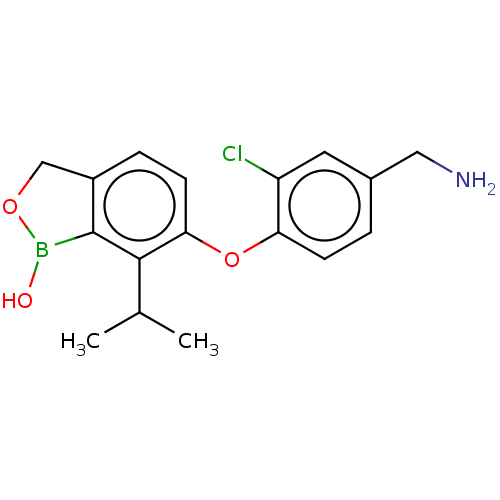 (US9493490, 6-(4-(aminomethyl)-2-chlorophenoxy)-7-i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0550 | n/a | n/a | n/a | n/a | n/a | 25 |
Anacor Pharmaceuticals, Inc. US Patent | Assay Description Phosphorylation of activity of ROCK1 and ROCK2 and those of other kinases, AKT1, GRK2, PKA, PKCa and RSK1 were performed by Reaction Biology (Malvern... | US Patent US9493490 (2016) BindingDB Entry DOI: 10.7270/Q2R2109J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE4B2 of cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM589753 (US11559538, Example 16 | US11559538, Example 208 |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Cytokine inhibitory activity is determined by measuring the effect of test agent on the release of the cytokines IL-4, IL-13 and IFNγ from human... | Citation and Details BindingDB Entry DOI: 10.7270/Q2542SHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-1 (Homo sapiens (Human)) | BDBM258601 (US9493490, 6-(4-(aminomethyl)-2-chlorophenoxy)-7-i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.195 | n/a | n/a | n/a | n/a | n/a | 25 |
Anacor Pharmaceuticals, Inc. US Patent | Assay Description Phosphorylation of activity of ROCK1 and ROCK2 and those of other kinases, AKT1, GRK2, PKA, PKCa and RSK1 were performed by Reaction Biology (Malvern... | US Patent US9493490 (2016) BindingDB Entry DOI: 10.7270/Q2R2109J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE4B2 of cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM589753 (US11559538, Example 16 | US11559538, Example 208 |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Cytokine inhibitory activity is determined by measuring the effect of test agent on the release of the cytokines IL-4, IL-13 and IFNγ from human... | Citation and Details BindingDB Entry DOI: 10.7270/Q2542SHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE4B2 of cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM589895 (US11559538, Example 147 | US11559538, Example 148) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Cytokine inhibitory activity is determined by measuring the effect of test agent on the release of the cytokines IL-4, IL-13 and IFNγ from human... | Citation and Details BindingDB Entry DOI: 10.7270/Q2542SHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE4B2 of cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM589886 (US11559538, Example 138 | US11559538, Example 139 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Cytokine inhibitory activity is determined by measuring the effect of test agent on the release of the cytokines IL-4, IL-13 and IFNγ from human... | Citation and Details BindingDB Entry DOI: 10.7270/Q2542SHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE4B2 of cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM589803 (US11559538, Example 60 | US11559538, Example 61 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Cytokine inhibitory activity is determined by measuring the effect of test agent on the release of the cytokines IL-4, IL-13 and IFNγ from human... | Citation and Details BindingDB Entry DOI: 10.7270/Q2542SHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE4B2 of cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM589754 (US11559538, Example 17 | US11559538, Example 50) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Cytokine inhibitory activity is determined by measuring the effect of test agent on the release of the cytokines IL-4, IL-13 and IFNγ from human... | Citation and Details BindingDB Entry DOI: 10.7270/Q2542SHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-4 (Human) | BDBM589948 (US11559538, Example 195 | US11559538, Example 196) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2542SHJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50022647 (2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of purified human renin (pH 6.0) | J Med Chem 31: 2277-88 (1989) BindingDB Entry DOI: 10.7270/Q2PG1QQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE4B2 of cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM589830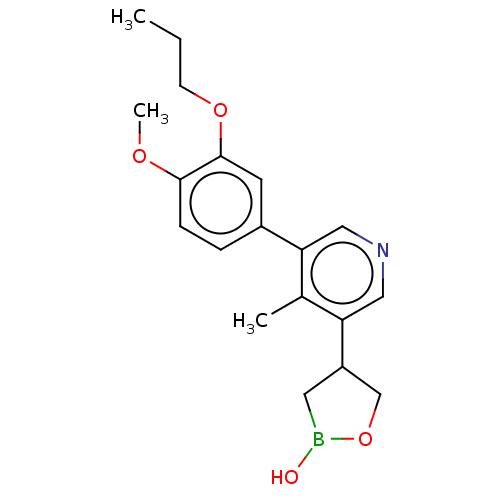 (US11559538, Example 84 | US11559538, Example 85) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Cytokine inhibitory activity is determined by measuring the effect of test agent on the release of the cytokines IL-4, IL-13 and IFNγ from human... | Citation and Details BindingDB Entry DOI: 10.7270/Q2542SHJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1473 total ) | Next | Last >> |