

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50544198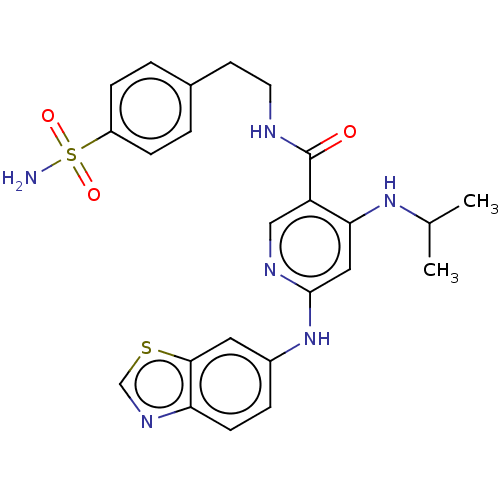 (CHEMBL4636136) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Biocon Bristol Myers Squibb Research Center Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | ACS Med Chem Lett 11: 1402-1409 (2020) Article DOI: 10.1021/acsmedchemlett.0c00082 BindingDB Entry DOI: 10.7270/Q2542S4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438455 (US10618903, Example 83 | US10618903, Example 84 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438631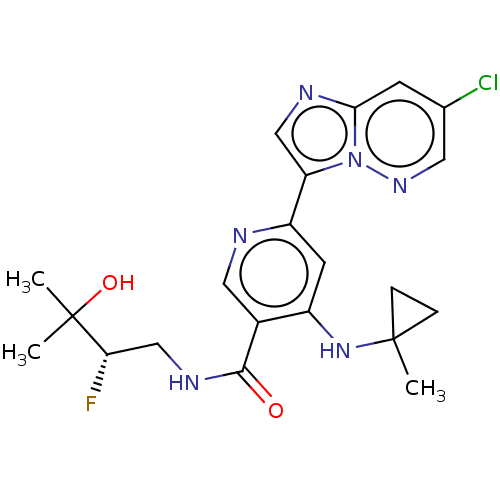 (US10618903, Example 198 | US10618903, Example 207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438665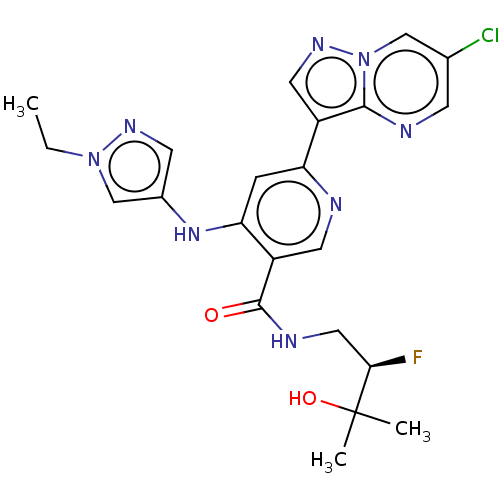 (US10618903, Example 232) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438672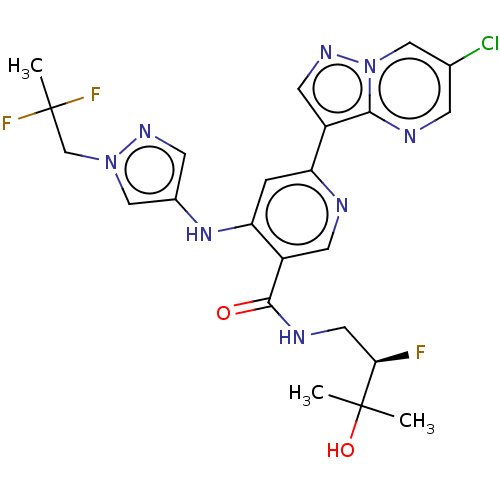 (US10618903, Example 239) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438679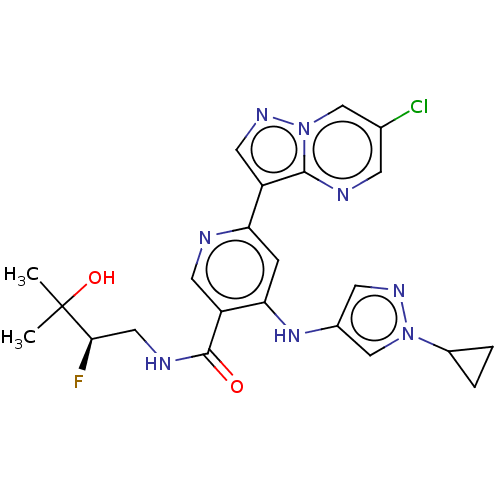 (US10618903, Example 246) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438683 (US10618903, Example 250) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438684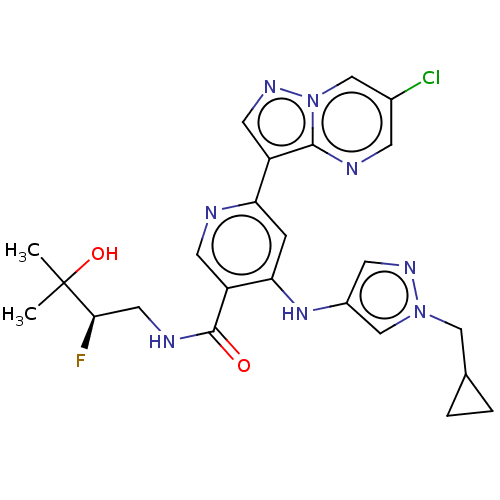 (US10618903, Example 251) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438686 (US10618903, Example 253) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438695 (US10618903, Example 262) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438708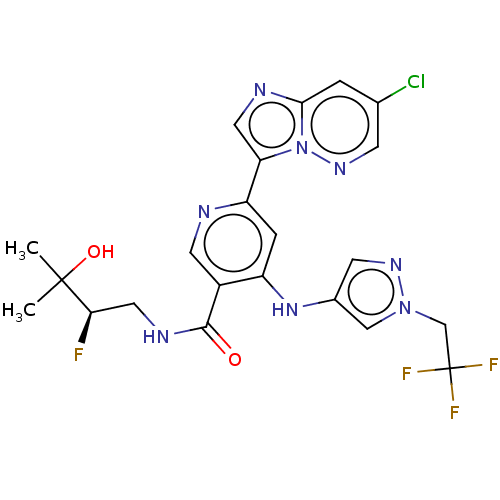 (US10618903, Example 275) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438711 (US10618903, Example 278) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438705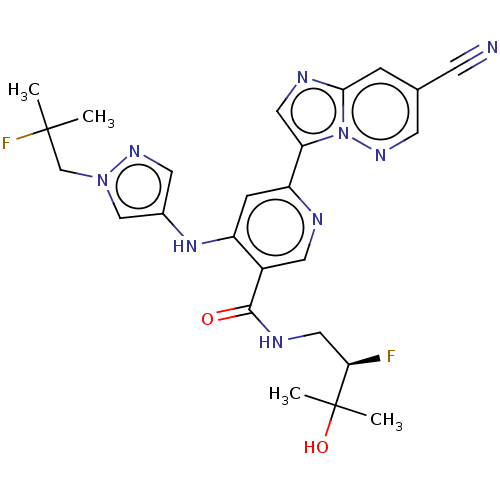 (US10618903, Example 272 | US10618903, Example 279) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438420 (US10618903, Example 48) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438443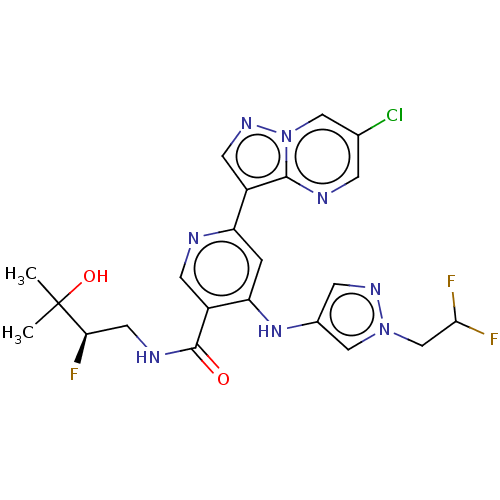 (US10618903, Example 71) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438484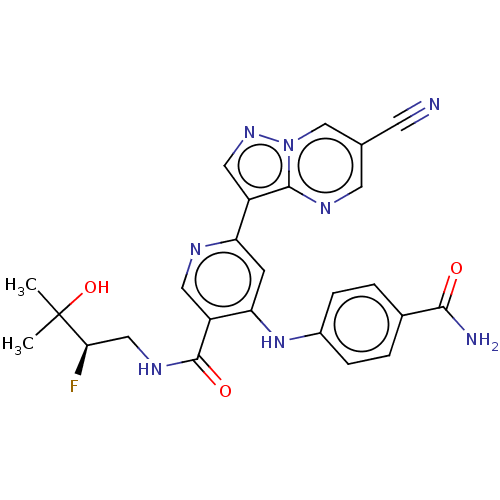 (US10618903, Example 112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438664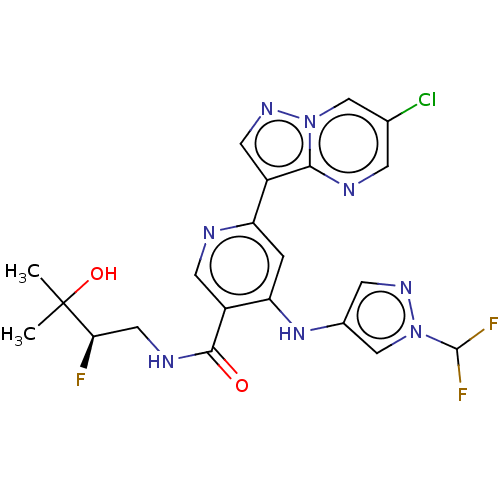 (US10618903, Example 231) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438716 (US10618903, Example 283) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438717 (US10618903, Example 284) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438719 (US10618903, Example 286) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438515 (US10618903, Example 143) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438517 (US10618903, Example 145) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438518 (US10618903, Example 146) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438447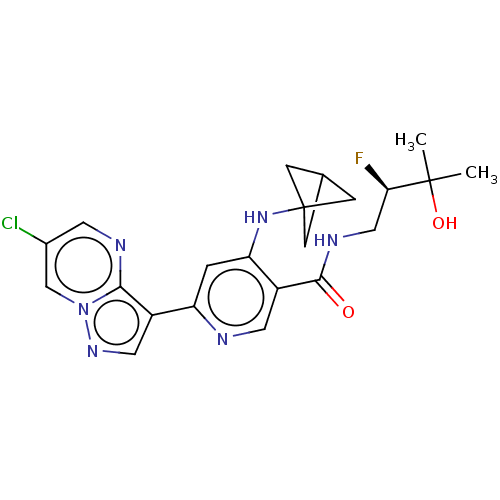 (US10618903, Example 75 | US10618903, Example 76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438455 (US10618903, Example 83 | US10618903, Example 84 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438455 (US10618903, Example 83 | US10618903, Example 84 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438379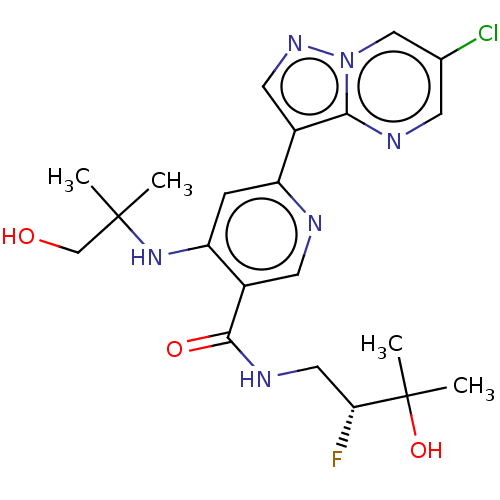 ((R)-6-(6-chloropyrazolo[1,5-a]pyrimidin-3-yl)-N-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438459 (US10618903, Example 127 | US10618903, Example 87) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438468 (US10618903, Example 96) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438635 (US10618903, Example 202) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438637 (US10618903, Example 204) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438631 (US10618903, Example 198 | US10618903, Example 207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438644 (US10618903, Example 211) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438646 (US10618903, Example 213) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438650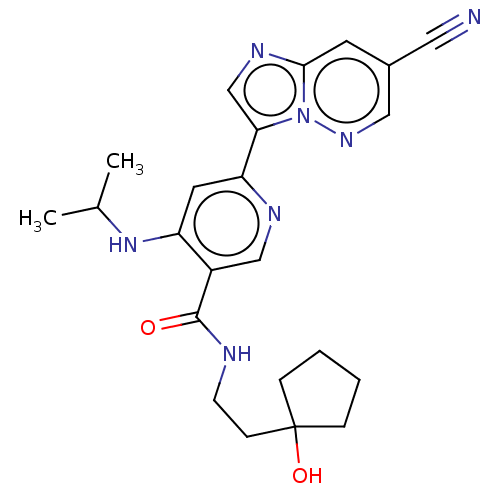 (US10618903, Example 217) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438658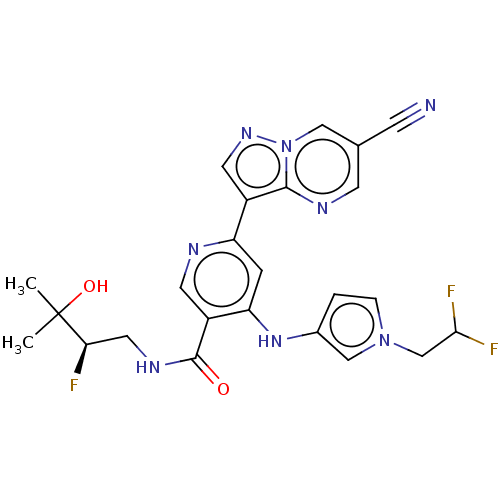 (US10618903, Example 225) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438660 (US10618903, Example 227) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438661 (US10618903, Example 228) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438668 (US10618903, Example 235) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438659 (US10618903, Example 226 | US10618903, Example 237) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438671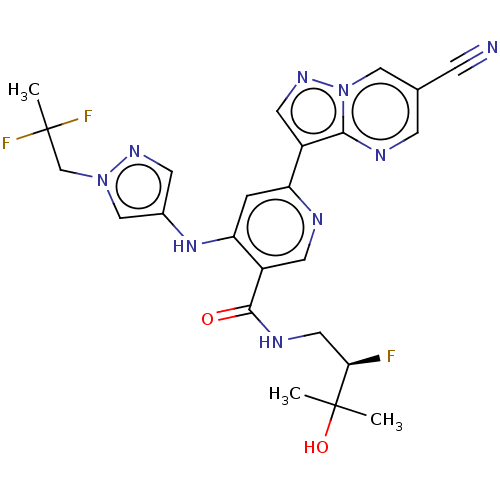 (US10618903, Example 238) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438673 (US10618903, Example 240) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438669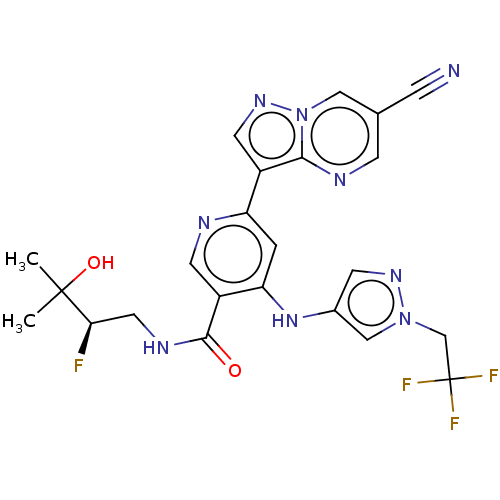 (US10618903, Example 236 | US10618903, Example 241) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438675 (US10618903, Example 242) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438680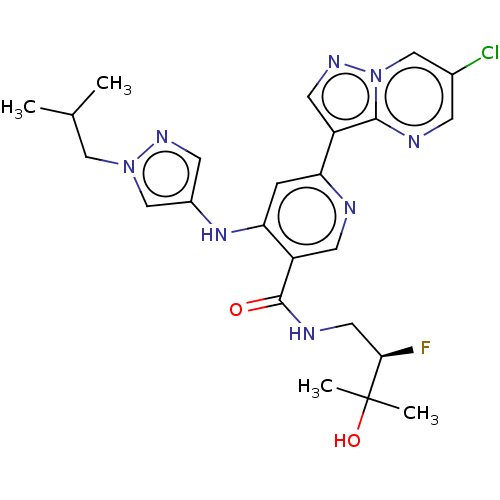 (US10618903, Example 247) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438681 (US10618903, Example 248) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438682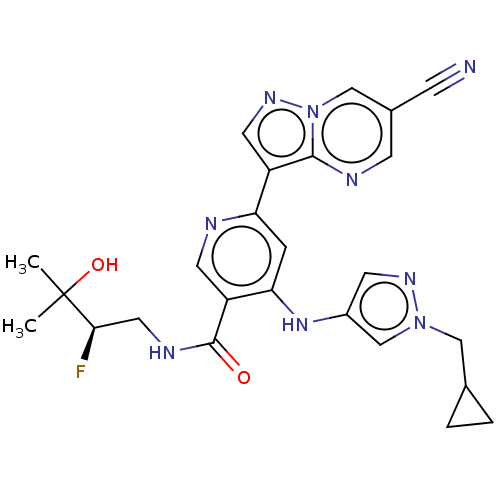 (US10618903, Example 249) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438687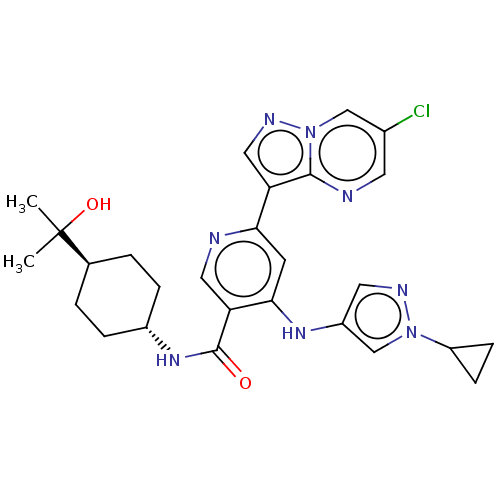 (US10618903, Example 254) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438691 ((R)-6-(7-cyanoimidazo[1,2-b]pyridazin-3-yl)-N-(2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM438692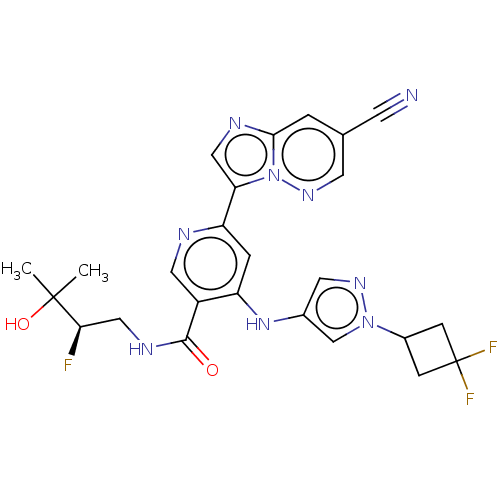 ((R)-6-(7-cyanoimidazo[1,2-b]pyridazin-3-yl)-4-((1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description The assays were performed in U-bottom 384-well plates. The final assay volume was 30 μL prepared from 15 μL additions of enzyme and substra... | US Patent US10618903 (2020) BindingDB Entry DOI: 10.7270/Q2251N7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 377 total ) | Next | Last >> |