 Found 2181 hits with Last Name = 'pott' and Initial = 'm'
Found 2181 hits with Last Name = 'pott' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342639
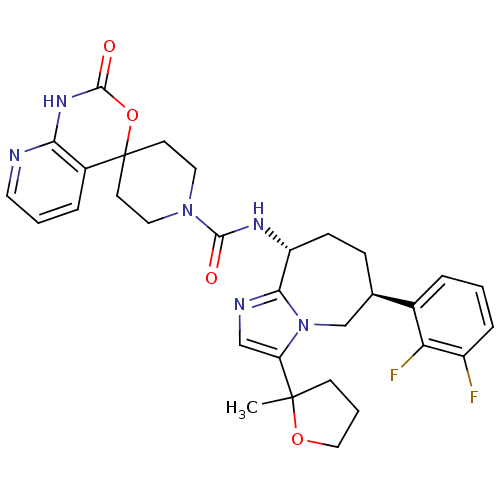
(CHEMBL1770729 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES CC1(CCCO1)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC2(CC1)OC(=O)Nc1ncccc21 |r| Show InChI InChI=1S/C31H34F2N6O4/c1-30(10-4-16-42-30)24-17-35-27-23(9-8-19(18-39(24)27)20-5-2-7-22(32)25(20)33)36-28(40)38-14-11-31(12-15-38)21-6-3-13-34-26(21)37-29(41)43-31/h2-3,5-7,13,17,19,23H,4,8-12,14-16,18H2,1H3,(H,36,40)(H,34,37,41)/t19-,23-,30?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342638
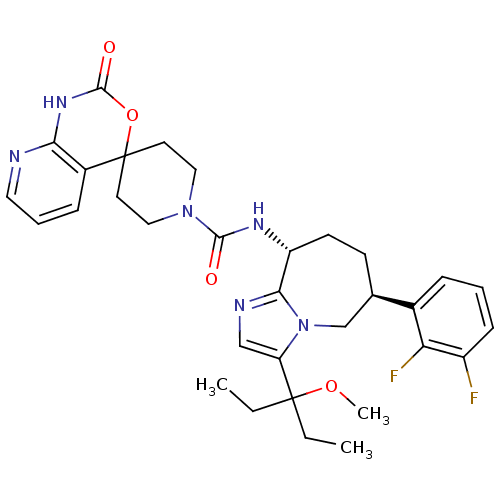
(CHEMBL1770728 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES CCC(CC)(OC)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC2(CC1)OC(=O)Nc1ncccc21 |r| Show InChI InChI=1S/C32H38F2N6O4/c1-4-31(5-2,43-3)25-18-36-28-24(12-11-20(19-40(25)28)21-8-6-10-23(33)26(21)34)37-29(41)39-16-13-32(14-17-39)22-9-7-15-35-27(22)38-30(42)44-32/h6-10,15,18,20,24H,4-5,11-14,16-17,19H2,1-3H3,(H,37,41)(H,35,38,42)/t20-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342625
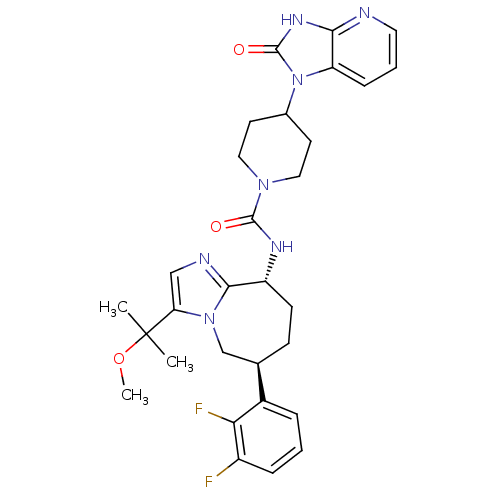
(CHEMBL1770715 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES COC(C)(C)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC(CC1)n1c2cccnc2[nH]c1=O |r| Show InChI InChI=1S/C30H35F2N7O3/c1-30(2,42-3)24-16-34-27-22(10-9-18(17-38(24)27)20-6-4-7-21(31)25(20)32)35-28(40)37-14-11-19(12-15-37)39-23-8-5-13-33-26(23)36-29(39)41/h4-8,13,16,18-19,22H,9-12,14-15,17H2,1-3H3,(H,35,40)(H,33,36,41)/t18-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342636
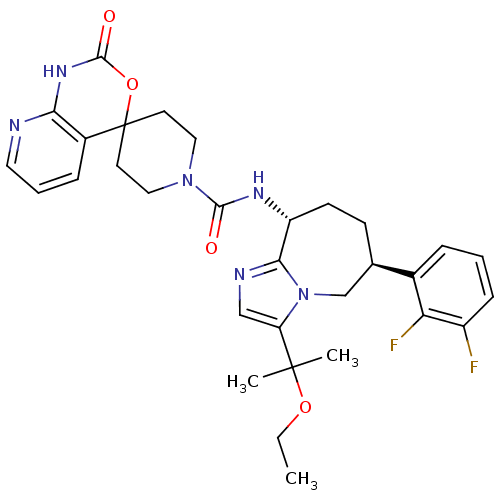
(CHEMBL1770726 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES CCOC(C)(C)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC2(CC1)OC(=O)Nc1ncccc21 |r| Show InChI InChI=1S/C31H36F2N6O4/c1-4-42-30(2,3)24-17-35-27-23(11-10-19(18-39(24)27)20-7-5-9-22(32)25(20)33)36-28(40)38-15-12-31(13-16-38)21-8-6-14-34-26(21)37-29(41)43-31/h5-9,14,17,19,23H,4,10-13,15-16,18H2,1-3H3,(H,36,40)(H,34,37,41)/t19-,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342634
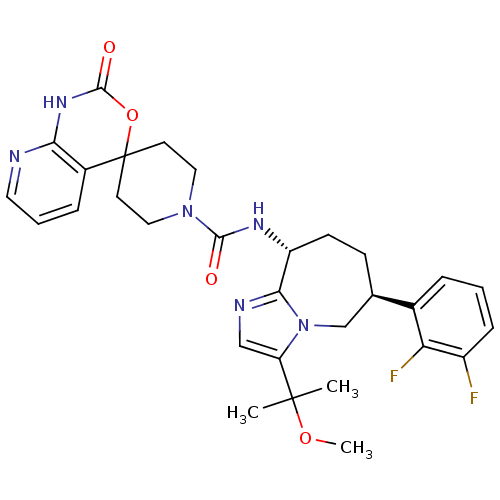
(CHEMBL1770724 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES COC(C)(C)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC2(CC1)OC(=O)Nc1ncccc21 |r| Show InChI InChI=1S/C30H34F2N6O4/c1-29(2,41-3)23-16-34-26-22(10-9-18(17-38(23)26)19-6-4-8-21(31)24(19)32)35-27(39)37-14-11-30(12-15-37)20-7-5-13-33-25(20)36-28(40)42-30/h4-8,13,16,18,22H,9-12,14-15,17H2,1-3H3,(H,35,39)(H,33,36,40)/t18-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342630
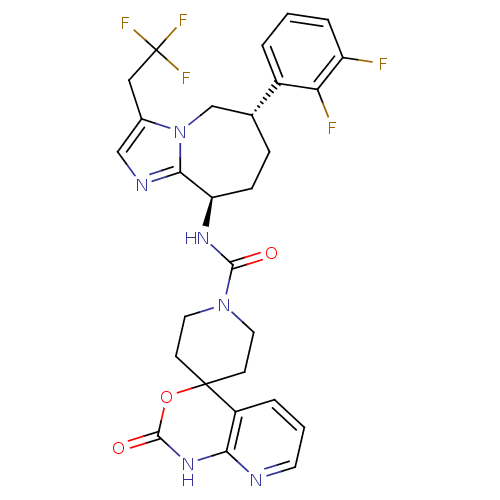
(CHEMBL1770720 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC4(CC3)OC(=O)Nc3ncccc43)c3ncc(CC(F)(F)F)n3C2)c1F |r| Show InChI InChI=1S/C28H27F5N6O3/c29-20-5-1-3-18(22(20)30)16-6-7-21(24-35-14-17(39(24)15-16)13-28(31,32)33)36-25(40)38-11-8-27(9-12-38)19-4-2-10-34-23(19)37-26(41)42-27/h1-5,10,14,16,21H,6-9,11-13,15H2,(H,36,40)(H,34,37,41)/t16-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342633
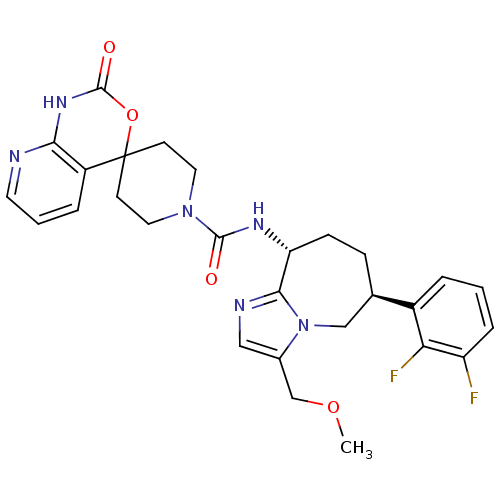
(CHEMBL1770723 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES COCc1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC2(CC1)OC(=O)Nc1ncccc21 |r| Show InChI InChI=1S/C28H30F2N6O4/c1-39-16-18-14-32-25-22(8-7-17(15-36(18)25)19-4-2-6-21(29)23(19)30)33-26(37)35-12-9-28(10-13-35)20-5-3-11-31-24(20)34-27(38)40-28/h2-6,11,14,17,22H,7-10,12-13,15-16H2,1H3,(H,33,37)(H,31,34,38)/t17-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342612
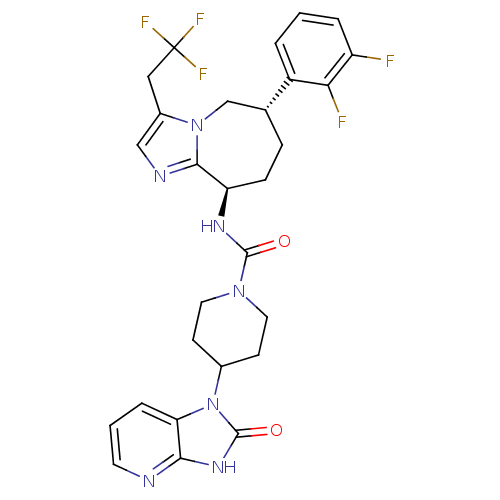
(CHEMBL1770557 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC(CC3)n3c4cccnc4[nH]c3=O)c3ncc(CC(F)(F)F)n3C2)c1F |r| Show InChI InChI=1S/C28H28F5N7O2/c29-20-4-1-3-19(23(20)30)16-6-7-21(25-35-14-18(39(25)15-16)13-28(31,32)33)36-26(41)38-11-8-17(9-12-38)40-22-5-2-10-34-24(22)37-27(40)42/h1-5,10,14,16-17,21H,6-9,11-13,15H2,(H,36,41)(H,34,37,42)/t16-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342637
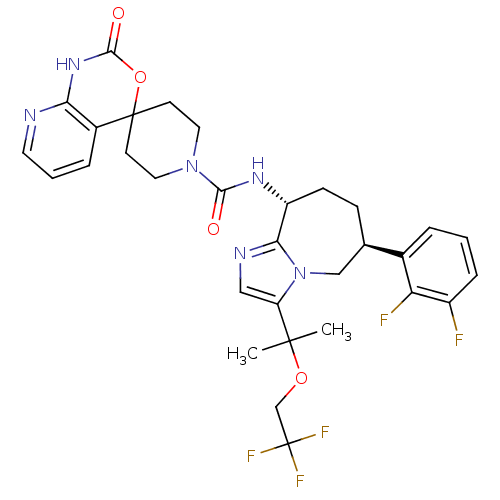
(CHEMBL1770727 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES CC(C)(OCC(F)(F)F)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC2(CC1)OC(=O)Nc1ncccc21 |r| Show InChI InChI=1S/C31H33F5N6O4/c1-29(2,45-17-31(34,35)36)23-15-38-26-22(9-8-18(16-42(23)26)19-5-3-7-21(32)24(19)33)39-27(43)41-13-10-30(11-14-41)20-6-4-12-37-25(20)40-28(44)46-30/h3-7,12,15,18,22H,8-11,13-14,16-17H2,1-2H3,(H,39,43)(H,37,40,44)/t18-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342632
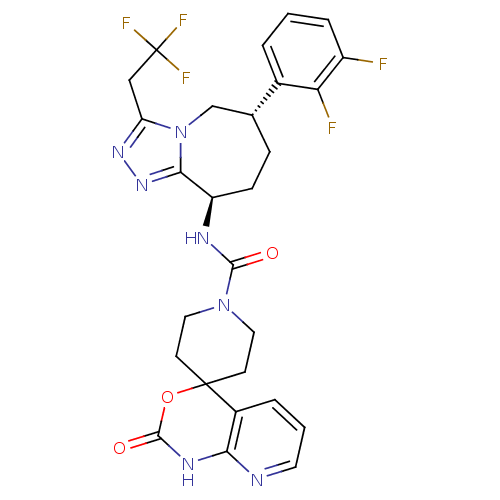
(CHEMBL1770722 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC4(CC3)OC(=O)Nc3ncccc43)c3nnc(CC(F)(F)F)n3C2)c1F |r| Show InChI InChI=1S/C27H26F5N7O3/c28-18-5-1-3-16(21(18)29)15-6-7-19(23-37-36-20(39(23)14-15)13-27(30,31)32)34-24(40)38-11-8-26(9-12-38)17-4-2-10-33-22(17)35-25(41)42-26/h1-5,10,15,19H,6-9,11-14H2,(H,34,40)(H,33,35,41)/t15-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342635
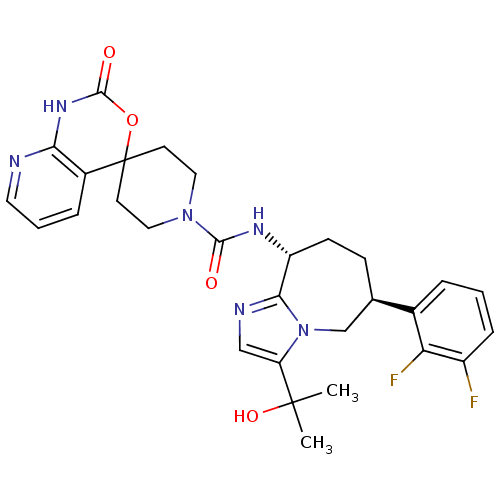
(CHEMBL1770725 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES CC(C)(O)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC2(CC1)OC(=O)Nc1ncccc21 |r| Show InChI InChI=1S/C29H32F2N6O4/c1-28(2,40)22-15-33-25-21(9-8-17(16-37(22)25)18-5-3-7-20(30)23(18)31)34-26(38)36-13-10-29(11-14-36)19-6-4-12-32-24(19)35-27(39)41-29/h3-7,12,15,17,21,40H,8-11,13-14,16H2,1-2H3,(H,34,38)(H,32,35,39)/t17-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496270

(CHEMBL3127679)Show SMILES CC(C)N1CCN(CC1)C(=O)C1CC2(C1)CCN(CC2)C1CCOCC1 Show InChI InChI=1S/C21H37N3O2/c1-17(2)22-9-11-24(12-10-22)20(25)18-15-21(16-18)5-7-23(8-6-21)19-3-13-26-14-4-19/h17-19H,3-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM50412684
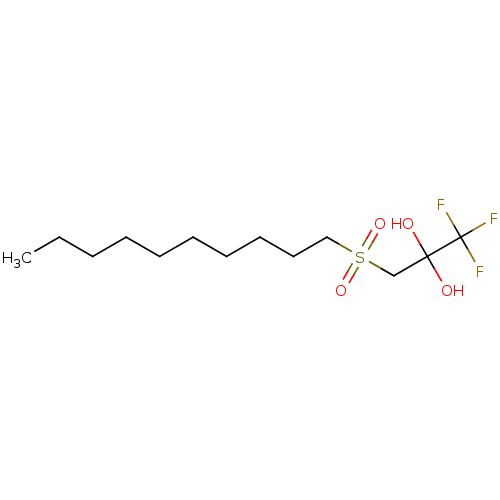
(CHEMBL467451)Show InChI InChI=1S/C13H25F3O4S/c1-2-3-4-5-6-7-8-9-10-21(19,20)11-12(17,18)13(14,15)16/h17-18H,2-11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of human carboxylesterase 1 after 24 hrs |
Bioorg Med Chem 17: 149-64 (2008)
Article DOI: 10.1016/j.bmc.2008.11.008
BindingDB Entry DOI: 10.7270/Q2JH3NFK |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM50468028
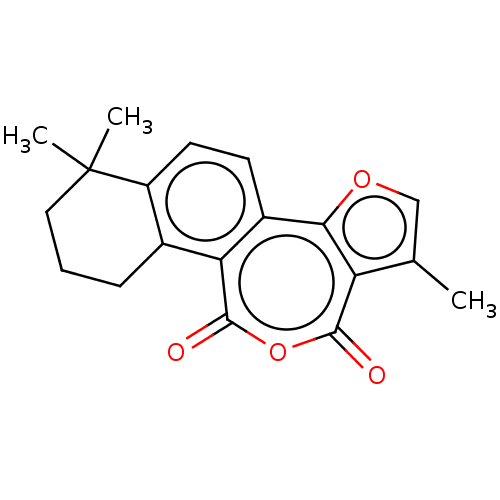
(CHEMBL4290236)Show InChI InChI=1S/C19H18O4/c1-10-9-22-16-12-6-7-13-11(5-4-8-19(13,2)3)15(12)18(21)23-17(20)14(10)16/h6-7,9H,4-5,8H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.328 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human CE1 using o-NPA as substrate |
J Nat Prod 81: 2410-2418 (2018)
Article DOI: 10.1021/acs.jnatprod.8b00378
BindingDB Entry DOI: 10.7270/Q2NC63XX |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342624
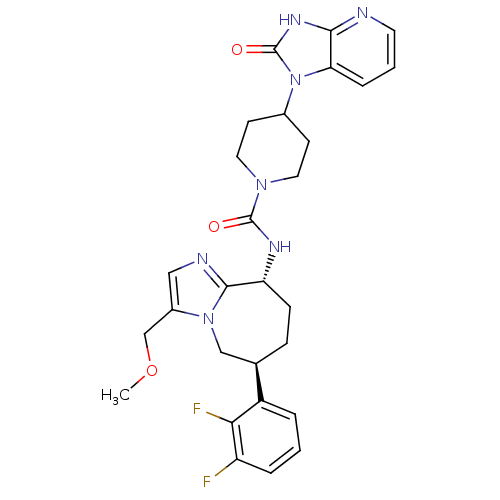
(CHEMBL1770714 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES COCc1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC(CC1)n1c2cccnc2[nH]c1=O |r| Show InChI InChI=1S/C28H31F2N7O3/c1-40-16-19-14-32-26-22(8-7-17(15-36(19)26)20-4-2-5-21(29)24(20)30)33-27(38)35-12-9-18(10-13-35)37-23-6-3-11-31-25(23)34-28(37)39/h2-6,11,14,17-18,22H,7-10,12-13,15-16H2,1H3,(H,33,38)(H,31,34,39)/t17-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM50412686
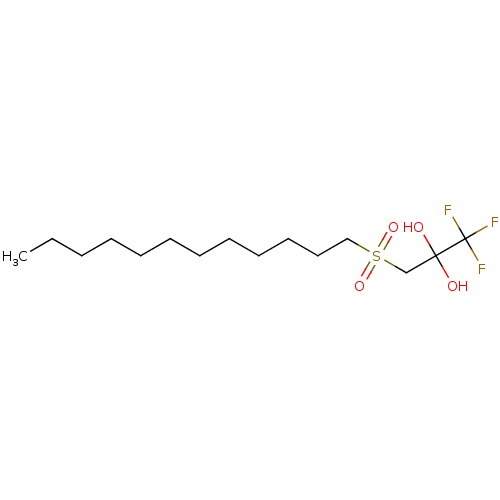
(CHEMBL467450)Show InChI InChI=1S/C15H29F3O4S/c1-2-3-4-5-6-7-8-9-10-11-12-23(21,22)13-14(19,20)15(16,17)18/h19-20H,2-13H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of human carboxylesterase 1 after 24 hrs |
Bioorg Med Chem 17: 149-64 (2008)
Article DOI: 10.1016/j.bmc.2008.11.008
BindingDB Entry DOI: 10.7270/Q2JH3NFK |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM50412687
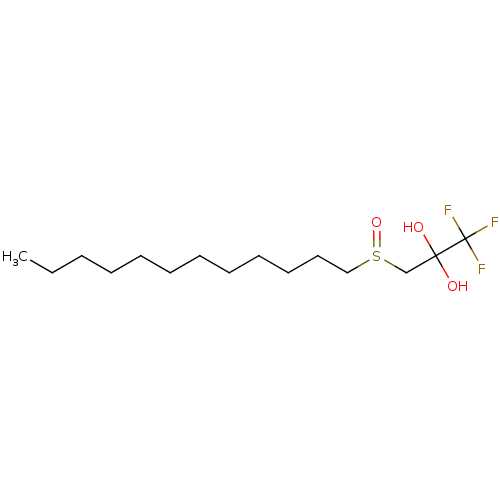
(CHEMBL463556)Show InChI InChI=1S/C15H29F3O3S/c1-2-3-4-5-6-7-8-9-10-11-12-22(21)13-14(19,20)15(16,17)18/h19-20H,2-13H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of human carboxylesterase 1 after 24 hrs |
Bioorg Med Chem 17: 149-64 (2008)
Article DOI: 10.1016/j.bmc.2008.11.008
BindingDB Entry DOI: 10.7270/Q2JH3NFK |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342629
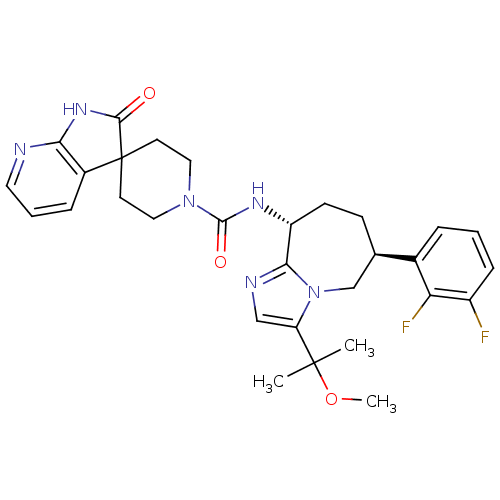
(CHEMBL1770719 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES COC(C)(C)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC2(CC1)C(=O)Nc1ncccc21 |r| Show InChI InChI=1S/C30H34F2N6O3/c1-29(2,41-3)23-16-34-26-22(10-9-18(17-38(23)26)19-6-4-8-21(31)24(19)32)35-28(40)37-14-11-30(12-15-37)20-7-5-13-33-25(20)36-27(30)39/h4-8,13,16,18,22H,9-12,14-15,17H2,1-3H3,(H,35,40)(H,33,36,39)/t18-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM50468026

(CHEMBL4293626)Show SMILES C[C@H]1COc2c1c(=O)oc(=O)c1c3CCCC(C)(C)c3ccc21 |r| Show InChI InChI=1S/C19H20O4/c1-10-9-22-16-12-6-7-13-11(5-4-8-19(13,2)3)15(12)18(21)23-17(20)14(10)16/h6-7,10H,4-5,8-9H2,1-3H3/t10-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human CE1 using o-NPA as substrate |
J Nat Prod 81: 2410-2418 (2018)
Article DOI: 10.1021/acs.jnatprod.8b00378
BindingDB Entry DOI: 10.7270/Q2NC63XX |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342631
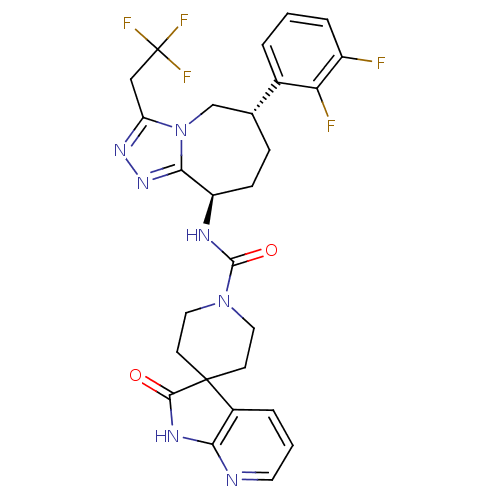
(CHEMBL1770721 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC4(CC3)C(=O)Nc3ncccc43)c3nnc(CC(F)(F)F)n3C2)c1F |r| Show InChI InChI=1S/C27H26F5N7O2/c28-18-5-1-3-16(21(18)29)15-6-7-19(23-37-36-20(39(23)14-15)13-27(30,31)32)34-25(41)38-11-8-26(9-12-38)17-4-2-10-33-22(17)35-24(26)40/h1-5,10,15,19H,6-9,11-14H2,(H,34,41)(H,33,35,40)/t15-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM50412685
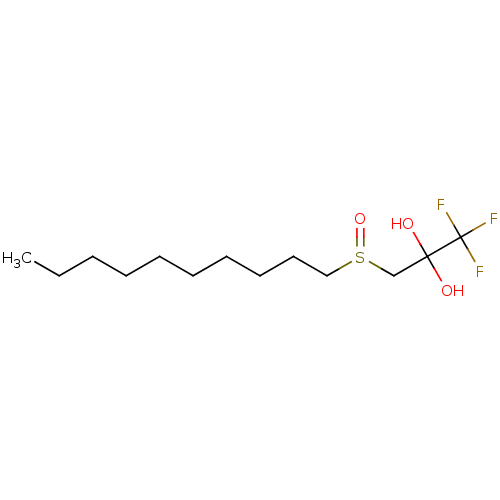
(CHEMBL464002)Show InChI InChI=1S/C13H25F3O3S/c1-2-3-4-5-6-7-8-9-10-20(19)11-12(17,18)13(14,15)16/h17-18H,2-11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of human carboxylesterase 1 after 24 hrs |
Bioorg Med Chem 17: 149-64 (2008)
Article DOI: 10.1016/j.bmc.2008.11.008
BindingDB Entry DOI: 10.7270/Q2JH3NFK |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM50412692
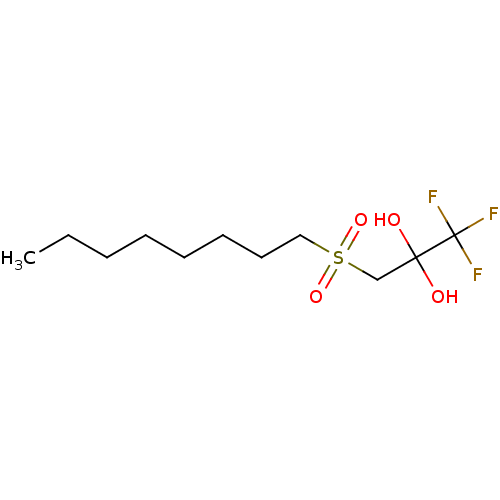
(CHEMBL460807)Show InChI InChI=1S/C11H21F3O4S/c1-2-3-4-5-6-7-8-19(17,18)9-10(15,16)11(12,13)14/h15-16H,2-9H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.603 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of human carboxylesterase 1 after 24 hrs |
Bioorg Med Chem 17: 149-64 (2008)
Article DOI: 10.1016/j.bmc.2008.11.008
BindingDB Entry DOI: 10.7270/Q2JH3NFK |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM50412693
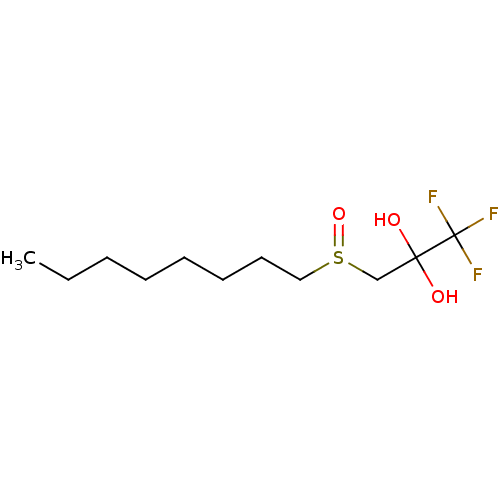
(CHEMBL460806)Show InChI InChI=1S/C11H21F3O3S/c1-2-3-4-5-6-7-8-18(17)9-10(15,16)11(12,13)14/h15-16H,2-9H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.603 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of human carboxylesterase 1 after 24 hrs |
Bioorg Med Chem 17: 149-64 (2008)
Article DOI: 10.1016/j.bmc.2008.11.008
BindingDB Entry DOI: 10.7270/Q2JH3NFK |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342626
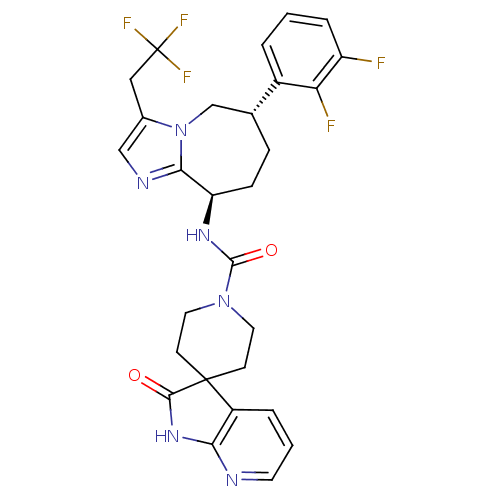
(CHEMBL1770716 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC4(CC3)C(=O)Nc3ncccc43)c3ncc(CC(F)(F)F)n3C2)c1F |r| Show InChI InChI=1S/C28H27F5N6O2/c29-20-5-1-3-18(22(20)30)16-6-7-21(24-35-14-17(39(24)15-16)13-28(31,32)33)36-26(41)38-11-8-27(9-12-38)19-4-2-10-34-23(19)37-25(27)40/h1-5,10,14,16,21H,6-9,11-13,15H2,(H,36,41)(H,34,37,40)/t16-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342628
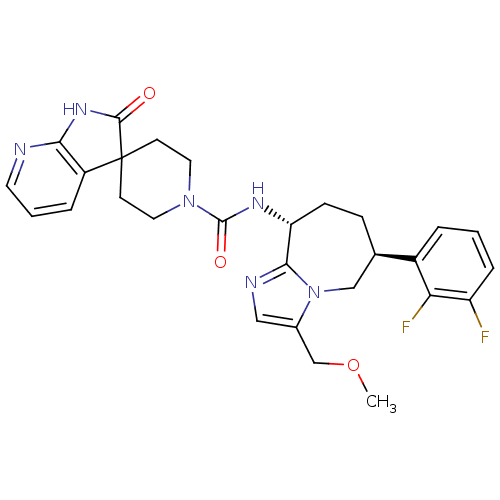
(CHEMBL1770718 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES COCc1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC2(CC1)C(=O)Nc1ncccc21 |r| Show InChI InChI=1S/C28H30F2N6O3/c1-39-16-18-14-32-25-22(8-7-17(15-36(18)25)19-4-2-6-21(29)23(19)30)33-27(38)35-12-9-28(10-13-35)20-5-3-11-31-24(20)34-26(28)37/h2-6,11,14,17,22H,7-10,12-13,15-16H2,1H3,(H,33,38)(H,31,34,37)/t17-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342627
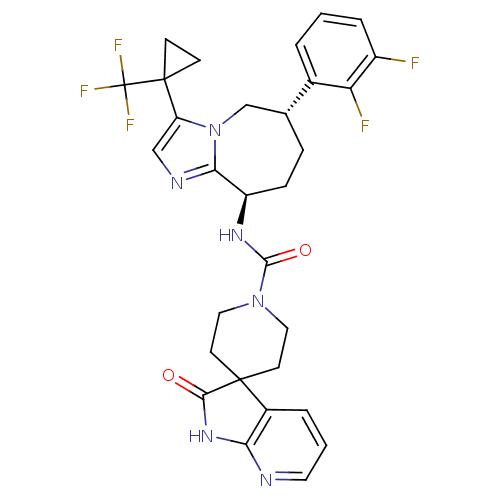
(CHEMBL1770717 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC4(CC3)C(=O)Nc3ncccc43)c3ncc(n3C2)C2(CC2)C(F)(F)F)c1F |r| Show InChI InChI=1S/C30H29F5N6O2/c31-20-5-1-3-18(23(20)32)17-6-7-21(25-37-15-22(41(25)16-17)29(8-9-29)30(33,34)35)38-27(43)40-13-10-28(11-14-40)19-4-2-12-36-24(19)39-26(28)42/h1-5,12,15,17,21H,6-11,13-14,16H2,(H,38,43)(H,36,39,42)/t17-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342623
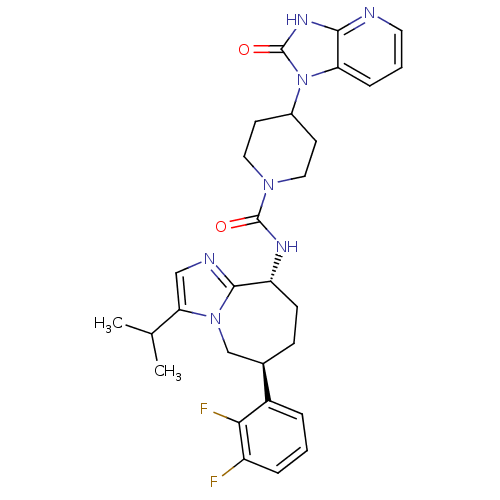
(CHEMBL1770568 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES CC(C)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC(CC1)n1c2cccnc2[nH]c1=O |r| Show InChI InChI=1S/C29H33F2N7O2/c1-17(2)24-15-33-27-22(9-8-18(16-37(24)27)20-5-3-6-21(30)25(20)31)34-28(39)36-13-10-19(11-14-36)38-23-7-4-12-32-26(23)35-29(38)40/h3-7,12,15,17-19,22H,8-11,13-14,16H2,1-2H3,(H,34,39)(H,32,35,40)/t18-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM50412691
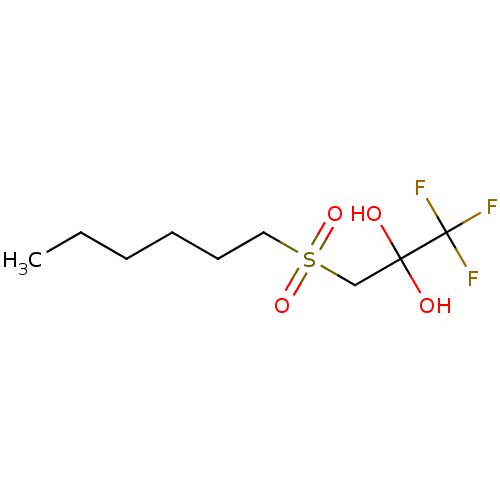
(CHEMBL449775)Show InChI InChI=1S/C9H17F3O4S/c1-2-3-4-5-6-17(15,16)7-8(13,14)9(10,11)12/h13-14H,2-7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of human carboxylesterase 1 after 24 hrs |
Bioorg Med Chem 17: 149-64 (2008)
Article DOI: 10.1016/j.bmc.2008.11.008
BindingDB Entry DOI: 10.7270/Q2JH3NFK |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50224431
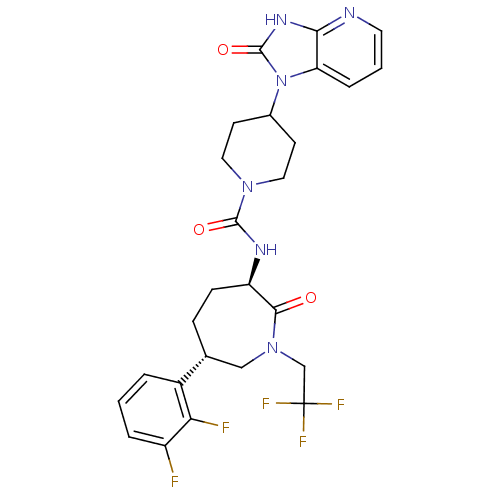
(CHEMBL236593 | MK-0974 | N-[(3R,6S)-6-(2,3-difluor...)Show SMILES Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC(CC3)n3c4cccnc4[nH]c3=O)C(=O)N(CC(F)(F)F)C2)c1F Show InChI InChI=1S/C26H27F5N6O3/c27-18-4-1-3-17(21(18)28)15-6-7-19(23(38)36(13-15)14-26(29,30)31)33-24(39)35-11-8-16(9-12-35)37-20-5-2-10-32-22(20)34-25(37)40/h1-5,10,15-16,19H,6-9,11-14H2,(H,33,39)(H,32,34,40)/t15-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]human CGRP from human CLR expressed in HEK 293 cells coexpressing human RAMP1 after 3 hrs by scintillation counting |
Bioorg Med Chem Lett 19: 6368-72 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.066
BindingDB Entry DOI: 10.7270/Q2PC32FD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50224431

(CHEMBL236593 | MK-0974 | N-[(3R,6S)-6-(2,3-difluor...)Show SMILES Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC(CC3)n3c4cccnc4[nH]c3=O)C(=O)N(CC(F)(F)F)C2)c1F Show InChI InChI=1S/C26H27F5N6O3/c27-18-4-1-3-17(21(18)28)15-6-7-19(23(38)36(13-15)14-26(29,30)31)33-24(39)35-11-8-16(9-12-35)37-20-5-2-10-32-22(20)34-25(37)40/h1-5,10,15-16,19H,6-9,11-14H2,(H,33,39)(H,32,34,40)/t15-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496289

(CHEMBL3124968)Show SMILES CC(C)N1CCN(CC1)C(=O)[C@H]1CC11CCN(CC1)C1CCOCC1 |r| Show InChI InChI=1S/C20H35N3O2/c1-16(2)21-9-11-23(12-10-21)19(24)18-15-20(18)5-7-22(8-6-20)17-3-13-25-14-4-17/h16-18H,3-15H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM50350329
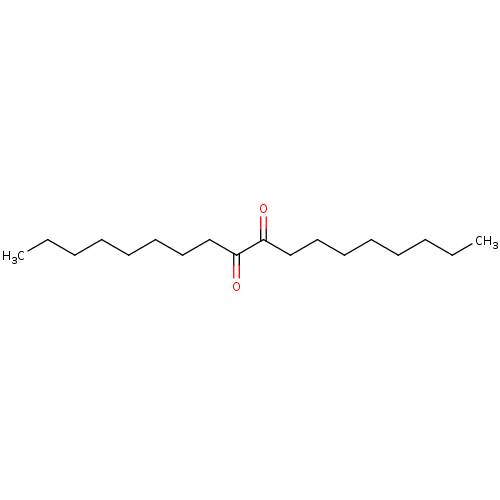
(CHEMBL1812859)Show InChI InChI=1S/C18H34O2/c1-3-5-7-9-11-13-15-17(19)18(20)16-14-12-10-8-6-4-2/h3-16H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human liver carboxylesterase1 using o-nitrophenyl acetate as substrate after 5 mins by spectrophotometry |
Bioorg Med Chem 19: 4635-43 (2011)
Article DOI: 10.1016/j.bmc.2011.06.012
BindingDB Entry DOI: 10.7270/Q2PR7WB9 |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM50412690
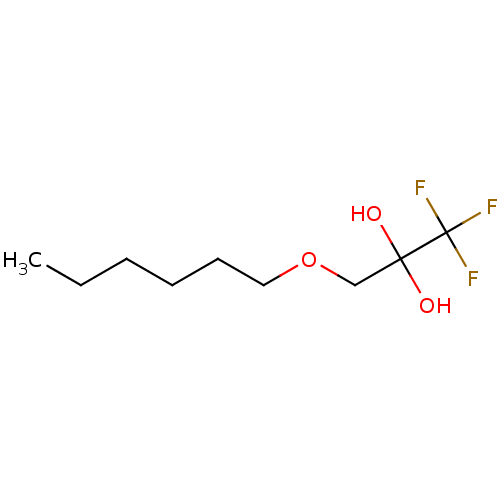
(CHEMBL449475)Show InChI InChI=1S/C9H17F3O3/c1-2-3-4-5-6-15-7-8(13,14)9(10,11)12/h13-14H,2-7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of human carboxylesterase 1 after 24 hrs |
Bioorg Med Chem 17: 149-64 (2008)
Article DOI: 10.1016/j.bmc.2008.11.008
BindingDB Entry DOI: 10.7270/Q2JH3NFK |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342611

(CHEMBL1770556 | N-((6S,9R)-3-cyclopropyl-6-(2,3-di...)Show SMILES Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC(CC3)n3c4cccnc4[nH]c3=O)c3nnc(C4CC4)n3C2)c1F |r| Show InChI InChI=1S/C28H30F2N8O2/c29-20-4-1-3-19(23(20)30)17-8-9-21(26-35-34-25(16-6-7-16)37(26)15-17)32-27(39)36-13-10-18(11-14-36)38-22-5-2-12-31-24(22)33-28(38)40/h1-5,12,16-18,21H,6-11,13-15H2,(H,32,39)(H,31,33,40)/t17-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50302881
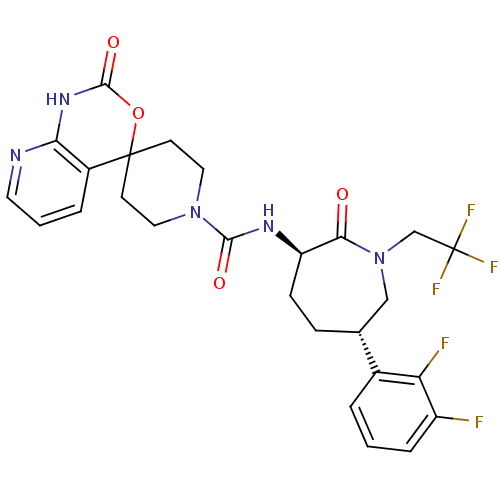
(CHEMBL570746 | N-((3R,6S)-6-(2,3-difluorophenyl)-2...)Show SMILES Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC4(CC3)OC(=O)Nc3ncccc43)C(=O)N(CC(F)(F)F)C2)c1F |r| Show InChI InChI=1S/C26H26F5N5O4/c27-18-5-1-3-16(20(18)28)15-6-7-19(22(37)36(13-15)14-26(29,30)31)33-23(38)35-11-8-25(9-12-35)17-4-2-10-32-21(17)34-24(39)40-25/h1-5,10,15,19H,6-9,11-14H2,(H,33,38)(H,32,34,39)/t15-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]human CGRP from human CLR expressed in HEK 293 cells coexpressing human RAMP1 after 3 hrs by scintillation counting |
Bioorg Med Chem Lett 19: 6368-72 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.066
BindingDB Entry DOI: 10.7270/Q2PC32FD |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342621
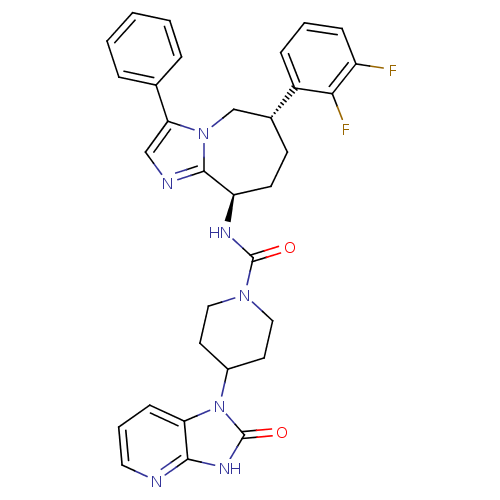
(CHEMBL1770566 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC(CC3)n3c4cccnc4[nH]c3=O)c3ncc(-c4ccccc4)n3C2)c1F |r| Show InChI InChI=1S/C32H31F2N7O2/c33-24-9-4-8-23(28(24)34)21-11-12-25(30-36-18-27(40(30)19-21)20-6-2-1-3-7-20)37-31(42)39-16-13-22(14-17-39)41-26-10-5-15-35-29(26)38-32(41)43/h1-10,15,18,21-22,25H,11-14,16-17,19H2,(H,37,42)(H,35,38,43)/t21-,25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342622
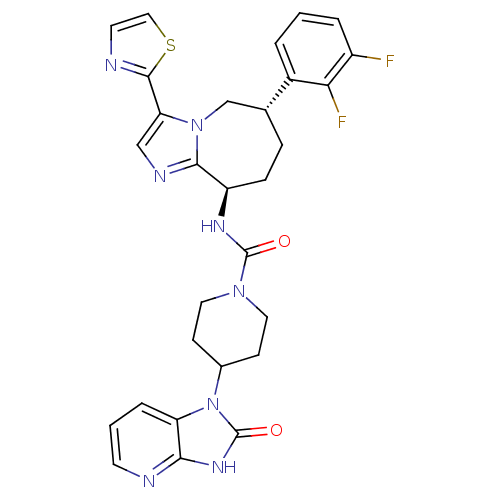
(CHEMBL1770567 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC(CC3)n3c4cccnc4[nH]c3=O)c3ncc(-c4nccs4)n3C2)c1F |r| Show InChI InChI=1S/C29H28F2N8O2S/c30-20-4-1-3-19(24(20)31)17-6-7-21(26-34-15-23(38(26)16-17)27-33-11-14-42-27)35-28(40)37-12-8-18(9-13-37)39-22-5-2-10-32-25(22)36-29(39)41/h1-5,10-11,14-15,17-18,21H,6-9,12-13,16H2,(H,35,40)(H,32,36,41)/t17-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496273
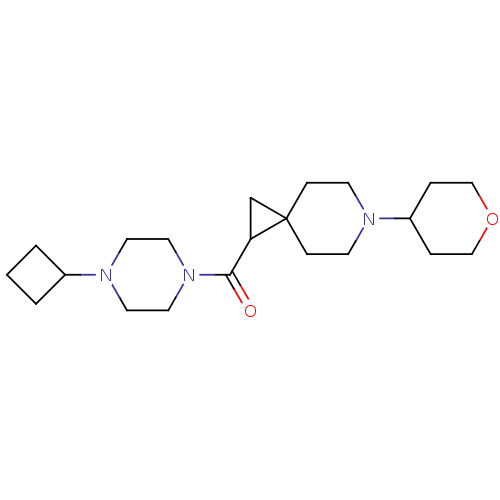
(CHEMBL3127700)Show SMILES O=C(C1CC11CCN(CC1)C1CCOCC1)N1CCN(CC1)C1CCC1 Show InChI InChI=1S/C21H35N3O2/c25-20(24-12-10-23(11-13-24)17-2-1-3-17)19-16-21(19)6-8-22(9-7-21)18-4-14-26-15-5-18/h17-19H,1-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496272
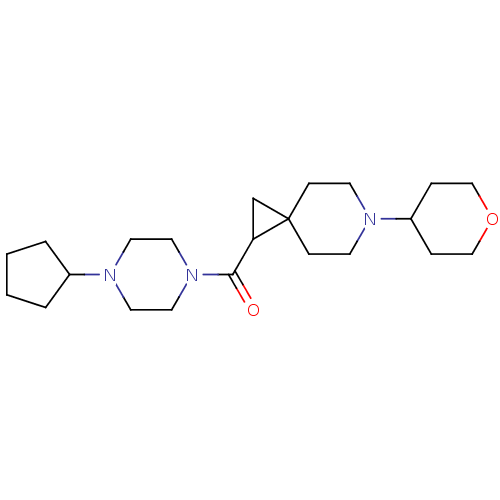
(CHEMBL3127701)Show SMILES O=C(C1CC11CCN(CC1)C1CCOCC1)N1CCN(CC1)C1CCCC1 Show InChI InChI=1S/C22H37N3O2/c26-21(25-13-11-24(12-14-25)18-3-1-2-4-18)20-17-22(20)7-9-23(10-8-22)19-5-15-27-16-6-19/h18-20H,1-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM22859
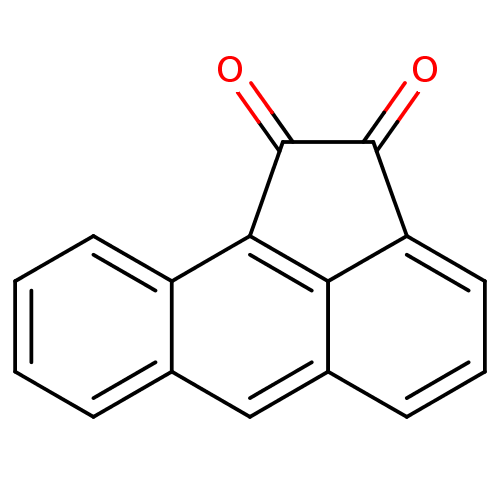
(1,2-Dione-Based Compound, 16 | 1,2-dihydroaceanthr...)Show InChI InChI=1S/C16H8O2/c17-15-12-7-3-5-10-8-9-4-1-2-6-11(9)14(13(10)12)16(15)18/h1-8H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital
| Assay Description
CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... |
J Med Chem 50: 5727-34 (2007)
Article DOI: 10.1021/jm0706867
BindingDB Entry DOI: 10.7270/Q2Q52MWQ |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50302883

(CHEMBL571203 | N-((3R,6S)-6-(2,3-difluorophenyl)-2...)Show SMILES Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC4(CC3)C(=O)Nc3ncccc43)C(=O)N(CC(F)(F)F)C2)c1F |r| Show InChI InChI=1S/C26H26F5N5O3/c27-18-5-1-3-16(20(18)28)15-6-7-19(22(37)36(13-15)14-26(29,30)31)33-24(39)35-11-8-25(9-12-35)17-4-2-10-32-21(17)34-23(25)38/h1-5,10,15,19H,6-9,11-14H2,(H,33,39)(H,32,34,38)/t15-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]human CGRP from human CLR expressed in HEK 293 cells coexpressing human RAMP1 after 3 hrs by scintillation counting |
Bioorg Med Chem Lett 19: 6368-72 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.066
BindingDB Entry DOI: 10.7270/Q2PC32FD |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM50350326
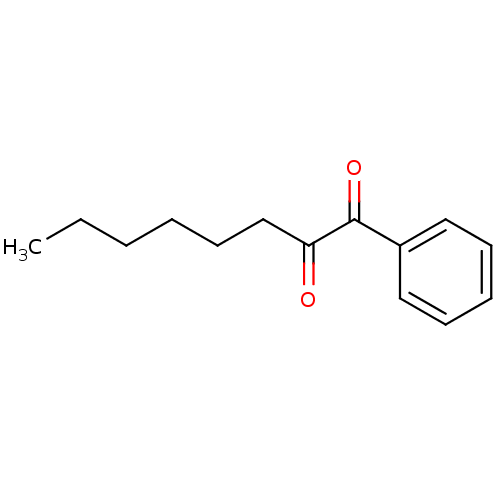
(CHEMBL1812864)Show InChI InChI=1S/C14H18O2/c1-2-3-4-8-11-13(15)14(16)12-9-6-5-7-10-12/h5-7,9-10H,2-4,8,11H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human liver carboxylesterase1 using o-nitrophenyl acetate as substrate after 5 mins by spectrophotometry |
Bioorg Med Chem 19: 4635-43 (2011)
Article DOI: 10.1016/j.bmc.2011.06.012
BindingDB Entry DOI: 10.7270/Q2PR7WB9 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342617
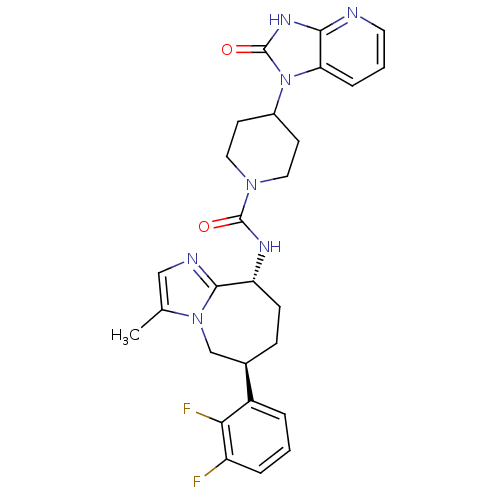
(CHEMBL1770562 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES Cc1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC(CC1)n1c2cccnc2[nH]c1=O |r| Show InChI InChI=1S/C27H29F2N7O2/c1-16-14-31-25-21(8-7-17(15-35(16)25)19-4-2-5-20(28)23(19)29)32-26(37)34-12-9-18(10-13-34)36-22-6-3-11-30-24(22)33-27(36)38/h2-6,11,14,17-18,21H,7-10,12-13,15H2,1H3,(H,32,37)(H,30,33,38)/t17-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM50371959
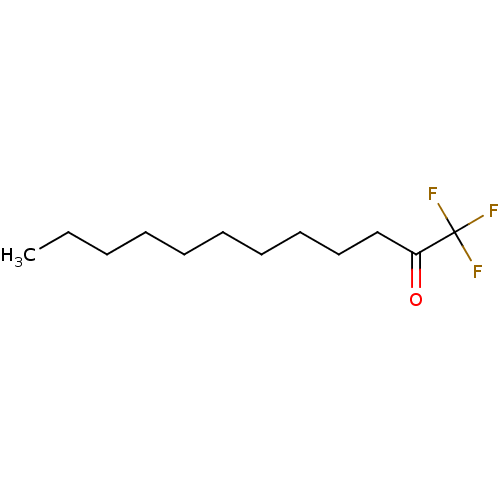
(CHEMBL86668)Show InChI InChI=1S/C12H21F3O/c1-2-3-4-5-6-7-8-9-10-11(16)12(13,14)15/h2-10H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Inhibition of human carboxylesterase 1 after 24 hrs |
Bioorg Med Chem 17: 149-64 (2008)
Article DOI: 10.1016/j.bmc.2008.11.008
BindingDB Entry DOI: 10.7270/Q2JH3NFK |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM50468029

(CHEMBL4282357)Show InChI InChI=1S/C18H12O4/c1-9-4-3-5-12-11(9)6-7-13-15(12)18(20)22-17(19)14-10(2)8-21-16(13)14/h3-8H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human CE1 using o-NPA as substrate |
J Nat Prod 81: 2410-2418 (2018)
Article DOI: 10.1021/acs.jnatprod.8b00378
BindingDB Entry DOI: 10.7270/Q2NC63XX |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM22857
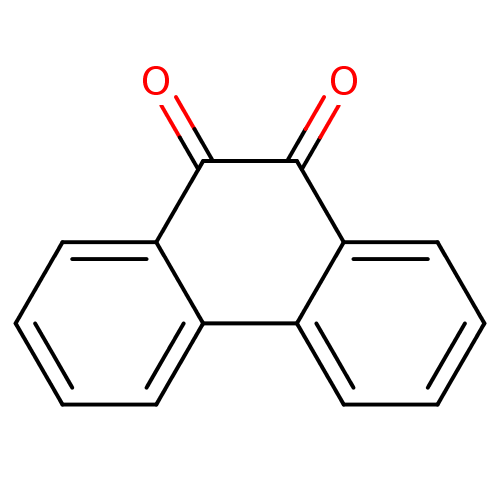
(1,2-Dione-Based Compound, 14 | 9,10-dihydrophenant...)Show InChI InChI=1S/C14H8O2/c15-13-11-7-3-1-5-9(11)10-6-2-4-8-12(10)14(13)16/h1-8H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital
| Assay Description
CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... |
J Med Chem 50: 5727-34 (2007)
Article DOI: 10.1021/jm0706867
BindingDB Entry DOI: 10.7270/Q2Q52MWQ |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342618
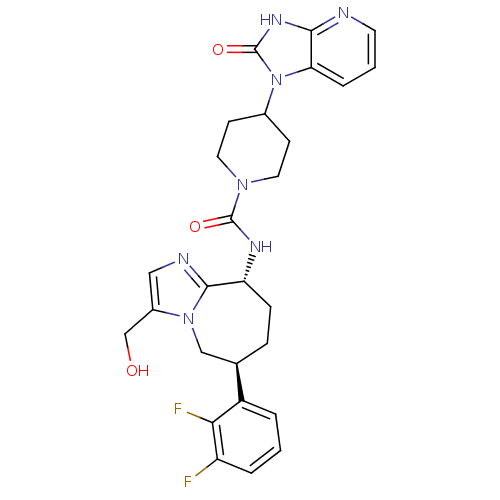
(CHEMBL1770563 | N-((6S,9R)-6-(2,3-difluorophenyl)-...)Show SMILES OCc1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC(CC1)n1c2cccnc2[nH]c1=O |r| Show InChI InChI=1S/C27H29F2N7O3/c28-20-4-1-3-19(23(20)29)16-6-7-21(25-31-13-18(15-37)35(25)14-16)32-26(38)34-11-8-17(9-12-34)36-22-5-2-10-30-24(22)33-27(36)39/h1-5,10,13,16-17,21,37H,6-9,11-12,14-15H2,(H,32,38)(H,30,33,39)/t16-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM22857

(1,2-Dione-Based Compound, 14 | 9,10-dihydrophenant...)Show InChI InChI=1S/C14H8O2/c15-13-11-7-3-1-5-9(11)10-6-2-4-8-12(10)14(13)16/h1-8H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human CE1 using o-NPA as substrate by spectrophotometric assay |
J Nat Prod 76: 36-44 (2013)
Article DOI: 10.1021/np300628a
BindingDB Entry DOI: 10.7270/Q2VX0HWJ |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50342620
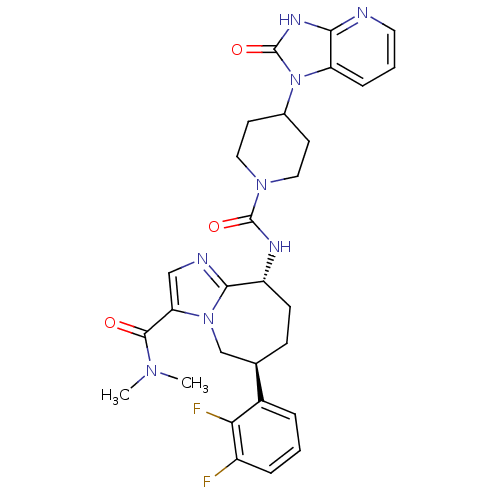
((6S,9R)-6-(2,3-difluorophenyl)-N,N-dimethyl-9-(4-(...)Show SMILES CN(C)C(=O)c1cnc2[C@@H](CC[C@H](Cn12)c1cccc(F)c1F)NC(=O)N1CCC(CC1)n1c2cccnc2[nH]c1=O |r| Show InChI InChI=1S/C29H32F2N8O3/c1-36(2)27(40)23-15-33-26-21(9-8-17(16-38(23)26)19-5-3-6-20(30)24(19)31)34-28(41)37-13-10-18(11-14-37)39-22-7-4-12-32-25(22)35-29(39)42/h3-7,12,15,17-18,21H,8-11,13-14,16H2,1-2H3,(H,34,41)(H,32,35,42)/t17-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CGRP from human recombinant CGRP receptor |
Bioorg Med Chem Lett 21: 2683-6 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.054
BindingDB Entry DOI: 10.7270/Q2VM4CK8 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496281

(CHEMBL3127698)Show InChI InChI=1S/C20H35N3O2/c1-16(2)21-9-11-23(12-10-21)19(24)18-15-20(18)5-7-22(8-6-20)17-3-13-25-14-4-17/h16-18H,3-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data