

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50125265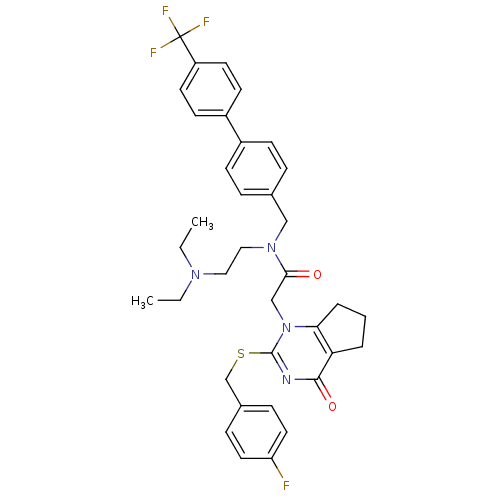 (CHEMBL204021 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of Lp-PLA2 in whole human plasma pre-incubated for 15 mins before 2-thio-PAF substrate addition | J Med Chem 59: 10738-10749 (2016) Article DOI: 10.1021/acs.jmedchem.6b01427 BindingDB Entry DOI: 10.7270/Q27946ND | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50125265 (CHEMBL204021 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human Lp-PLA2 pre-incubated for 30 mins before PED6 fluorogenic substrate | J Med Chem 59: 10738-10749 (2016) Article DOI: 10.1021/acs.jmedchem.6b01427 BindingDB Entry DOI: 10.7270/Q27946ND | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50125265 (CHEMBL204021 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of recombinant human Lp-PLA2 incubated for 20 mins by Thio-PAF assay | J Med Chem 59: 10738-10749 (2016) Article DOI: 10.1021/acs.jmedchem.6b01427 BindingDB Entry DOI: 10.7270/Q27946ND | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50125265 (CHEMBL204021 | N-(2-Diethylamino-ethyl)-2-[2-(4-fl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human recombinant Lp-PLA2 using 2-thio-PAF as substrate after 20 mins by CPM-based fluorescence assay | J Med Chem 59: 5356-67 (2016) Article DOI: 10.1021/acs.jmedchem.6b00212 BindingDB Entry DOI: 10.7270/Q2Z60R0G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513015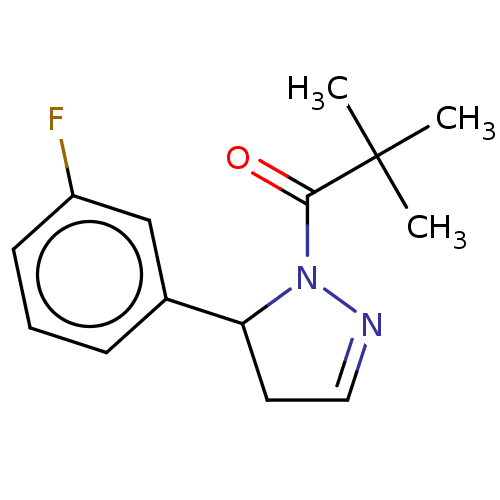 (CHEMBL4537171) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50507336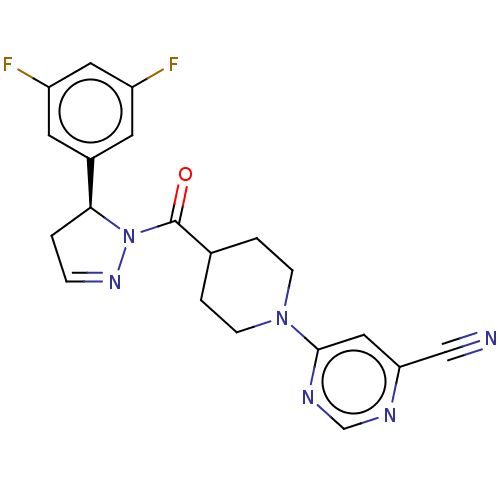 (CHEMBL4514271) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513040 (CHEMBL4593226) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50182422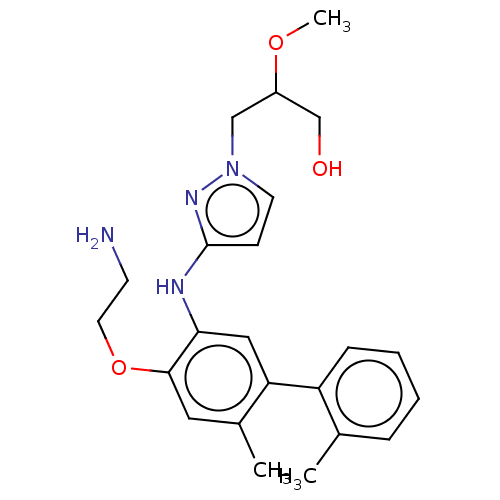 (CHEMBL3818650) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human recombinant Lp-PLA2 using 2-thio-PAF as substrate after 20 mins by CPM-based fluorescence assay | J Med Chem 59: 5356-67 (2016) Article DOI: 10.1021/acs.jmedchem.6b00212 BindingDB Entry DOI: 10.7270/Q2Z60R0G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513004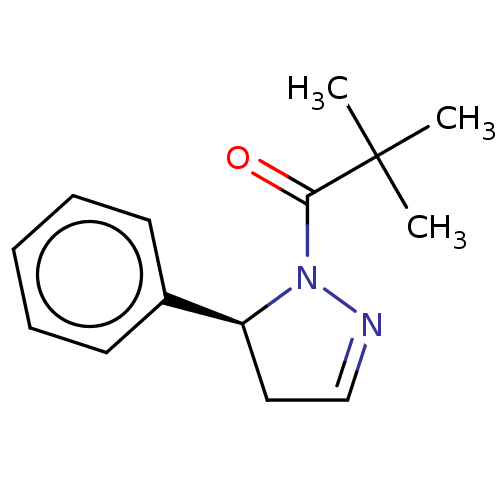 (CHEMBL4521353) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513024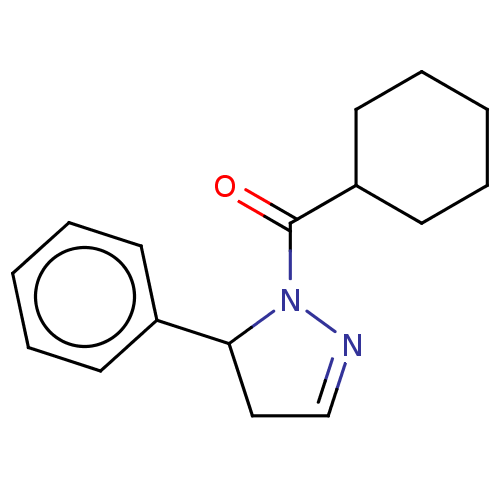 (CHEMBL4471642) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513011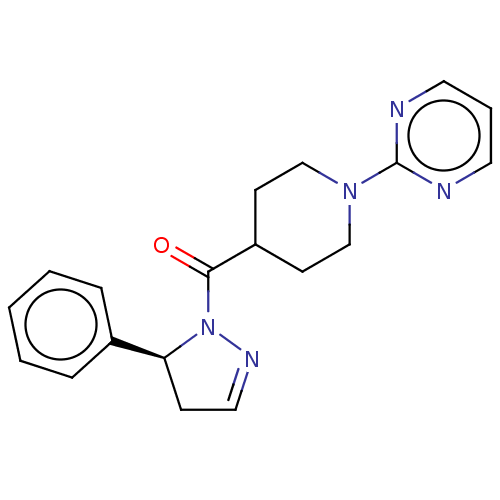 (CHEMBL4455042) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513013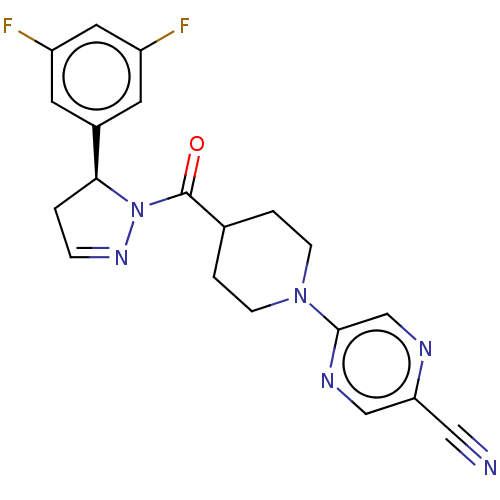 (CHEMBL4450890) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513016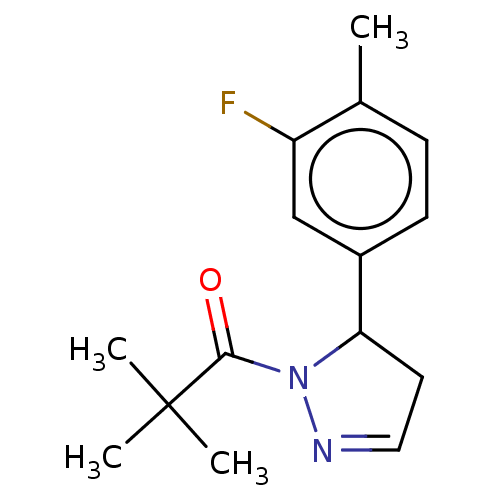 (CHEMBL4514028) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513034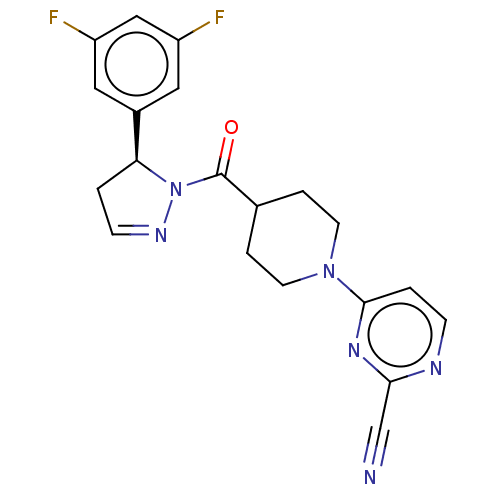 (CHEMBL4541955) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RIP1 in human U937 cells assessed as reduction in TNFalpha/QVD-Oph-induced necroptosis measured after 24 hrs by cell titer-glo luminesc... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50182421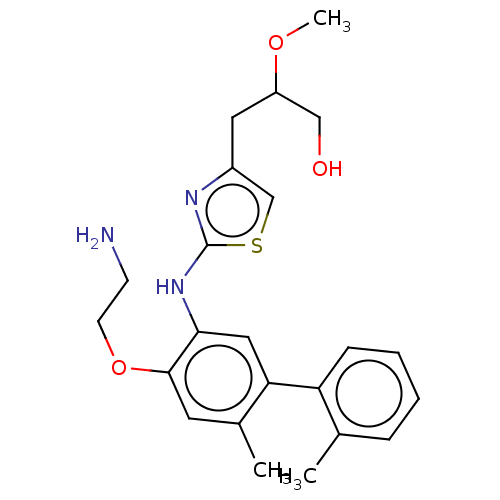 (CHEMBL3817896) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human recombinant Lp-PLA2 using 2-thio-PAF as substrate after 20 mins by CPM-based fluorescence assay | J Med Chem 59: 5356-67 (2016) Article DOI: 10.1021/acs.jmedchem.6b00212 BindingDB Entry DOI: 10.7270/Q2Z60R0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50512998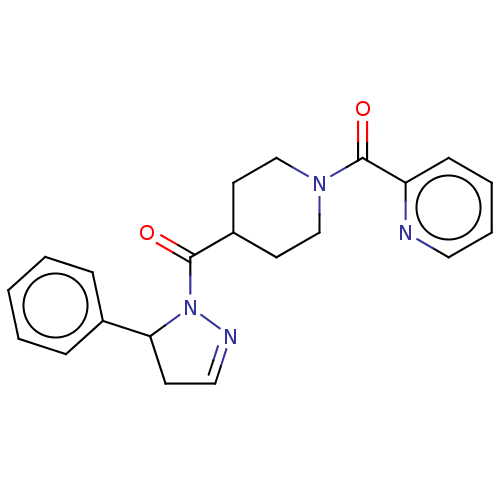 (CHEMBL4575848) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513016 (CHEMBL4514028) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RIP1 in human U937 cells assessed as reduction in TNFalpha/QVD-Oph-induced necroptosis measured after 24 hrs by cell titer-glo luminesc... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513034 (CHEMBL4541955) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50512996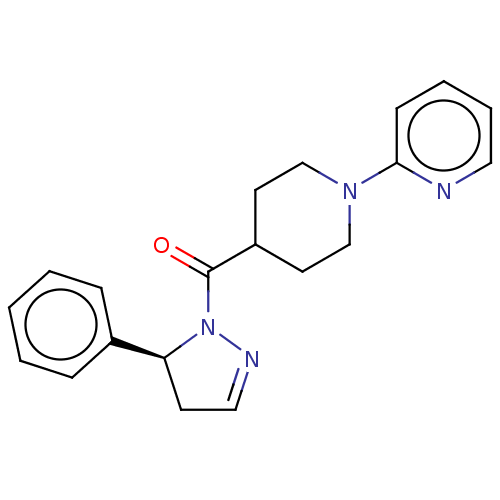 (CHEMBL4454462) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513030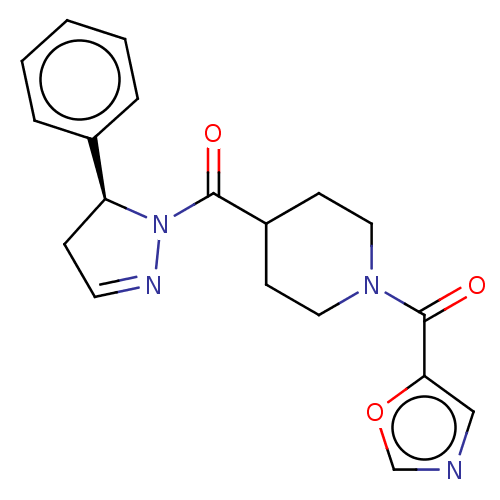 (CHEMBL4548784) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50271572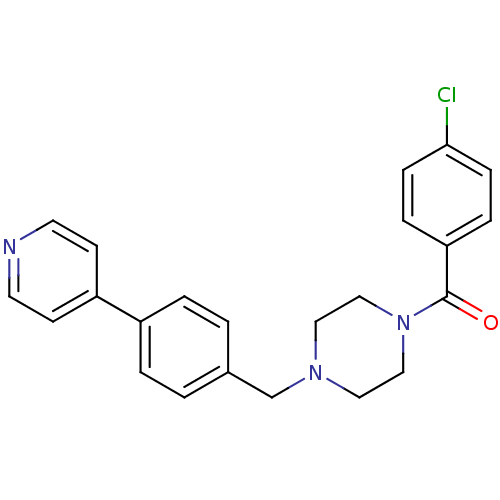 (1-[4-Chlorophenylcarbonyl]-4-[4-(pyridin-4-yl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of 2,3-oxidosqualene-lanosterol cyclase (unknown origin) | Bioorg Med Chem 16: 6218-32 (2008) Article DOI: 10.1016/j.bmc.2008.04.034 BindingDB Entry DOI: 10.7270/Q2M61K14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Mus musculus) | BDBM50512997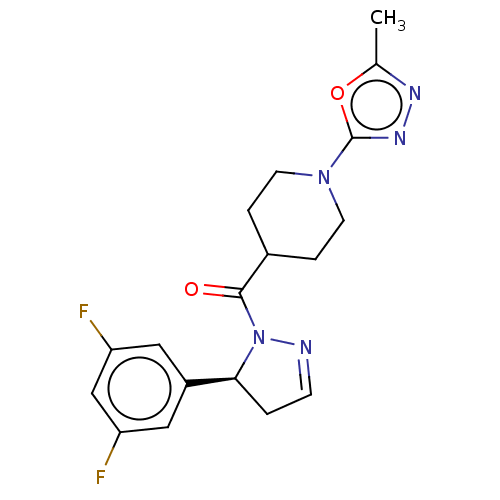 (CHEMBL4545939) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RIP1 in mouse L929 cells assessed as reduction in TNF/zVAD.fmk-induced necrotic death measured after 24 hrs by cell titer-glo luminesce... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50182420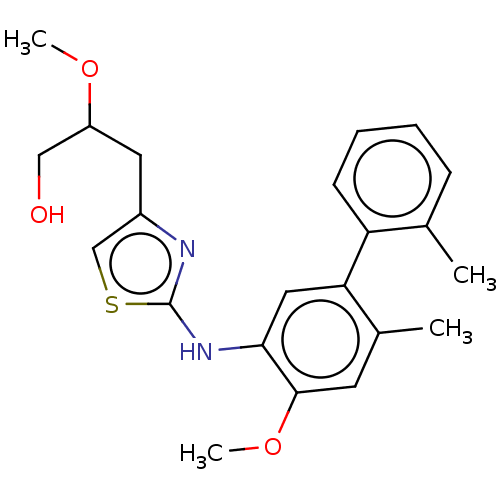 (CHEMBL3819600) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human recombinant Lp-PLA2 using 2-thio-PAF as substrate after 20 mins by CPM-based fluorescence assay | J Med Chem 59: 5356-67 (2016) Article DOI: 10.1021/acs.jmedchem.6b00212 BindingDB Entry DOI: 10.7270/Q2Z60R0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513004 (CHEMBL4521353) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RIP1 in human U937 cells assessed as reduction in TNFalpha/QVD-Oph-induced necroptosis measured after 24 hrs by cell titer-glo luminesc... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513007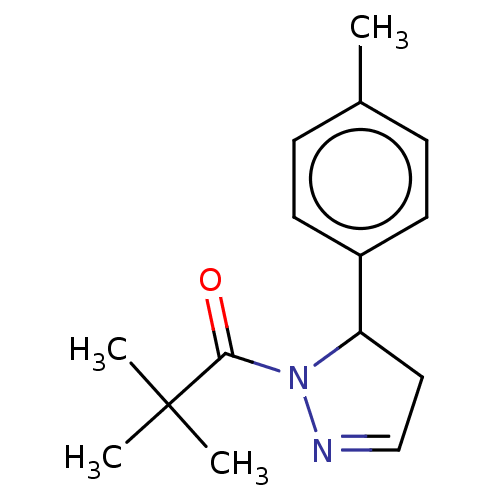 (CHEMBL4436215) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RIP1 in human U937 cells assessed as reduction in TNFalpha/QVD-Oph-induced necroptosis measured after 24 hrs by cell titer-glo luminesc... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50507336 (CHEMBL4514271) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RIP1 in human U937 cells assessed as reduction in TNFalpha/QVD-Oph-induced necroptosis measured after 24 hrs by cell titer-glo luminesc... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513015 (CHEMBL4537171) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RIP1 in human U937 cells assessed as reduction in TNFalpha/QVD-Oph-induced necroptosis measured after 24 hrs by cell titer-glo luminesc... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513000 (CHEMBL4446915) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513054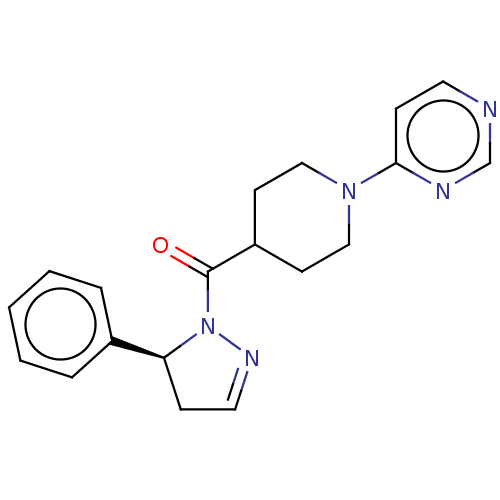 (CHEMBL4443534) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lanosterol synthase (Homo sapiens (Human)) | BDBM50271622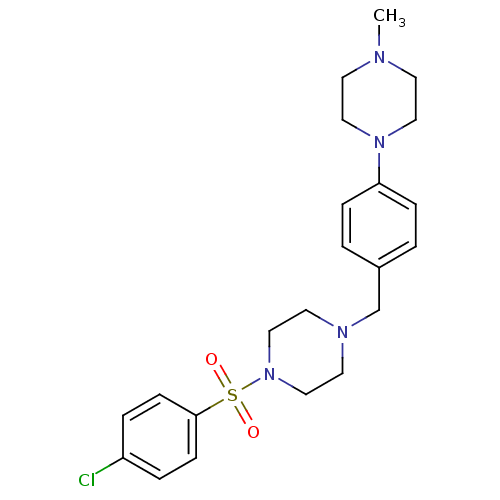 (1-[4-Chlorophenylsulfonyl]-4-[4-(4-methylpiperazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of 2,3-oxidosqualene-lanosterol cyclase (unknown origin) | Bioorg Med Chem 16: 6218-32 (2008) Article DOI: 10.1016/j.bmc.2008.04.034 BindingDB Entry DOI: 10.7270/Q2M61K14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513007 (CHEMBL4436215) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513041 (CHEMBL4576386) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513053 (CHEMBL4584779) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513040 (CHEMBL4593226) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RIP1 in human U937 cells assessed as reduction in TNFalpha/QVD-Oph-induced necroptosis measured after 24 hrs by cell titer-glo luminesc... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513013 (CHEMBL4450890) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RIP1 in human U937 cells assessed as reduction in TNFalpha/QVD-Oph-induced necroptosis measured after 24 hrs by cell titer-glo luminesc... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513012 (CHEMBL4561445) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513053 (CHEMBL4584779) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RIP1 in human U937 cells assessed as reduction in TNFalpha/QVD-Oph-induced necroptosis measured after 24 hrs by cell titer-glo luminesc... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513000 (CHEMBL4446915) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RIP1 in human U937 cells assessed as reduction in TNFalpha/QVD-Oph-induced necroptosis measured after 24 hrs by cell titer-glo luminesc... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50512997 (CHEMBL4545939) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of RIP1 in human whole blood assessed as reduction in TNFalpha/zVAD-fmk/SMAC-induced MIP1alpha production measured after 6 hrs by ELISA | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513017 (CHEMBL4556131) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor acetylhydrolase (Homo sapiens (Human)) | BDBM50182419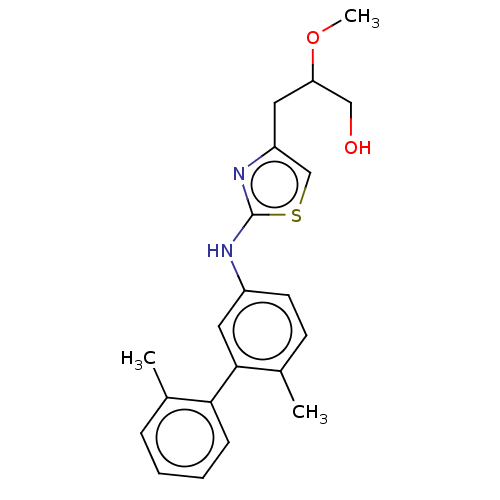 (CHEMBL3819444) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of human recombinant Lp-PLA2 using 2-thio-PAF as substrate after 20 mins by CPM-based fluorescence assay | J Med Chem 59: 5356-67 (2016) Article DOI: 10.1021/acs.jmedchem.6b00212 BindingDB Entry DOI: 10.7270/Q2Z60R0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513058 (CHEMBL4526661) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of (14-(2-{[3-({2-{[4-(cyanomethyl)phenyl]amino}-6-[(5-cyclopropyl-1H-pyrazol-3-yl)amino]-4-pyrimidinyl}amino)propyl]amino}-2-oxoethyl)-16... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513056 (CHEMBL4436896) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of (14-(2-{[3-({2-{[4-(cyanomethyl)phenyl]amino}-6-[(5-cyclopropyl-1H-pyrazol-3-yl)amino]-4-pyrimidinyl}amino)propyl]amino}-2-oxoethyl)-16... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513017 (CHEMBL4556131) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of (14-(2-{[3-({2-{[4-(cyanomethyl)phenyl]amino}-6-[(5-cyclopropyl-1H-pyrazol-3-yl)amino]-4-pyrimidinyl}amino)propyl]amino}-2-oxoethyl)-16... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50512990 (CHEMBL4446928) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of (14-(2-{[3-({2-{[4-(cyanomethyl)phenyl]amino}-6-[(5-cyclopropyl-1H-pyrazol-3-yl)amino]-4-pyrimidinyl}amino)propyl]amino}-2-oxoethyl)-16... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513026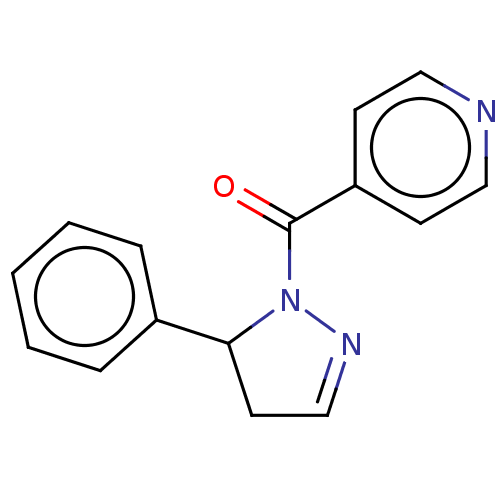 (CHEMBL4452678) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of (14-(2-{[3-({2-{[4-(cyanomethyl)phenyl]amino}-6-[(5-cyclopropyl-1H-pyrazol-3-yl)amino]-4-pyrimidinyl}amino)propyl]amino}-2-oxoethyl)-16... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513047 (CHEMBL4583157) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of (14-(2-{[3-({2-{[4-(cyanomethyl)phenyl]amino}-6-[(5-cyclopropyl-1H-pyrazol-3-yl)amino]-4-pyrimidinyl}amino)propyl]amino}-2-oxoethyl)-16... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513024 (CHEMBL4471642) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of (14-(2-{[3-({2-{[4-(cyanomethyl)phenyl]amino}-6-[(5-cyclopropyl-1H-pyrazol-3-yl)amino]-4-pyrimidinyl}amino)propyl]amino}-2-oxoethyl)-16... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513046 (CHEMBL4463322) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of (14-(2-{[3-({2-{[4-(cyanomethyl)phenyl]amino}-6-[(5-cyclopropyl-1H-pyrazol-3-yl)amino]-4-pyrimidinyl}amino)propyl]amino}-2-oxoethyl)-16... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50513033 (CHEMBL4454881) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of (14-(2-{[3-({2-{[4-(cyanomethyl)phenyl]amino}-6-[(5-cyclopropyl-1H-pyrazol-3-yl)amino]-4-pyrimidinyl}amino)propyl]amino}-2-oxoethyl)-16... | J Med Chem 62: 5096-5110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00318 BindingDB Entry DOI: 10.7270/Q24J0JFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 576 total ) | Next | Last >> |