 Found 767 hits with Last Name = 'poulain' and Initial = 'r'
Found 767 hits with Last Name = 'poulain' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50427703

(CHEMBL2324220)Show SMILES NC(N)=NCCC[C@H](NC(=O)[C@@H](CC(=O)NO)Cc1ccc2ccccc2c1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCC(O)=O)C(N)=O |r,wU:7.7,45.48,11.11,wD:31.32,(15.26,-7.72,;16.62,-8.43,;17.92,-7.6,;16.69,-9.97,;15.39,-10.79,;15.46,-12.33,;14.16,-13.16,;14.24,-14.7,;12.94,-15.53,;11.61,-14.76,;11.61,-13.22,;10.27,-15.53,;10.27,-17.07,;8.94,-17.84,;7.6,-17.07,;8.94,-19.38,;7.6,-20.16,;8.97,-14.76,;8.94,-13.22,;7.58,-12.47,;7.55,-10.93,;8.87,-10.13,;8.84,-8.59,;10.15,-7.8,;11.51,-8.53,;11.55,-10.08,;10.22,-10.88,;10.26,-12.42,;15.6,-15.4,;15.66,-16.95,;16.9,-14.58,;18.26,-15.29,;18.34,-16.83,;19.7,-17.53,;21.15,-17.03,;22.08,-18.26,;21.2,-19.52,;21.54,-21.02,;20.42,-22.07,;18.95,-21.62,;18.6,-20.12,;19.73,-19.07,;19.56,-14.46,;19.49,-12.92,;20.92,-15.17,;22.22,-14.34,;22.16,-12.8,;23.45,-11.98,;23.38,-10.44,;22.02,-9.73,;24.68,-9.61,;23.58,-15.05,;24.88,-14.22,;23.66,-16.59,)| Show InChI InChI=1S/C37H45N9O8/c38-33(50)28(13-14-32(48)49)43-36(53)30(18-25-20-42-27-9-4-3-8-26(25)27)45-35(52)29(10-5-15-41-37(39)40)44-34(51)24(19-31(47)46-54)17-21-11-12-22-6-1-2-7-23(22)16-21/h1-4,6-9,11-12,16,20,24,28-30,42,54H,5,10,13-15,17-19H2,(H2,38,50)(H,43,53)(H,44,51)(H,45,52)(H,46,47)(H,48,49)(H4,39,40,41)/t24-,28+,29+,30+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDE expressed in CHO cells in presence of [125I]-insulin by HTRF assay |
Eur J Med Chem 179: 557-566 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.057
BindingDB Entry DOI: 10.7270/Q2XS5ZT5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50462575

(CHEMBL4238121)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](COCc2ccccc2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C27H31N5O5S/c28-26(29)23-13-11-20(12-14-23)15-30-25(33)16-31-27(34)24(18-37-17-21-7-3-1-4-8-21)32-38(35,36)19-22-9-5-2-6-10-22/h1-14,24,32H,15-19H2,(H3,28,29)(H,30,33)(H,31,34)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Inhibition human FA10a using Boc-LeuGly-Arg-AMC fluorogenic substrate |
J Med Chem 63: 3817-3833 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01183
BindingDB Entry DOI: 10.7270/Q2NZ8C0R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50514426

(CHEMBL4574713)Show SMILES NC(=N)c1ccc(NC(C(=O)NCc2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C22H22N4O/c23-21(24)18-11-13-19(14-12-18)26-20(17-9-5-2-6-10-17)22(27)25-15-16-7-3-1-4-8-16/h1-14,20,26H,15H2,(H3,23,24)(H,25,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Inhibition of thrombin (unknown origin) |
J Med Chem 63: 3817-3833 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01183
BindingDB Entry DOI: 10.7270/Q2NZ8C0R |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 5B
(Homo sapiens (Human)) | BDBM50514409
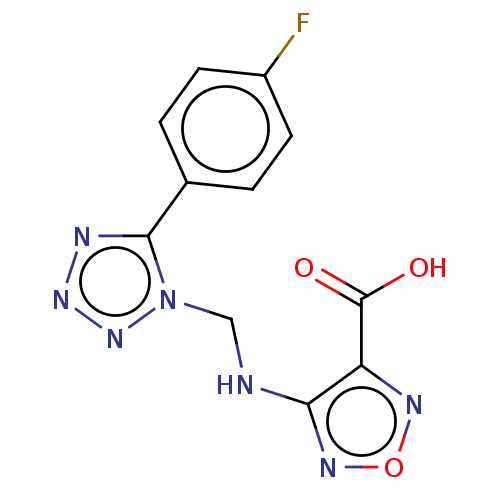
(CHEMBL4549938)Show InChI InChI=1S/C11H8FN7O3/c12-7-3-1-6(2-4-7)10-14-17-18-19(10)5-13-9-8(11(20)21)15-22-16-9/h1-4H,5H2,(H,13,16)(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Binding affinity to MBP-tagged recombinant STAT5b-SH2 domain (unknown origin) using carboxyfluoresceine-labeled phosphotyrosine octapeptide incubated... |
J Med Chem 63: 3817-3833 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01183
BindingDB Entry DOI: 10.7270/Q2NZ8C0R |
More data for this
Ligand-Target Pair | |
Bifunctional ligase/repressor BirA
(Staphylococcus aureus) | BDBM50514425

(CHEMBL4462026)Show SMILES [H][C@]12CS[C@@H](CCCCc3cn(CCCNc4ccc([N+]([O-])=O)c5nonc45)nn3)[C@@]1([H])NC(=O)N2 |r| Show InChI InChI=1S/C20H25N9O4S/c30-20-22-14-11-34-16(17(14)23-20)5-2-1-4-12-10-28(27-24-12)9-3-8-21-13-6-7-15(29(31)32)19-18(13)25-33-26-19/h6-7,10,14,16-17,21H,1-5,8-9,11H2,(H2,22,23,30)/t14-,16-,17-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Binding affinity to Staphylococcus aureus biotin protein ligase |
J Med Chem 63: 3817-3833 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01183
BindingDB Entry DOI: 10.7270/Q2NZ8C0R |
More data for this
Ligand-Target Pair | |
NAD kinase 1
(Listeria monocytogenes serovar 1/2a (strain ATCC B...) | BDBM50514423

(CHEMBL4552749)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](CNC(=O)CSc2nc3c(N)ncnc3n2[C@@H]2O[C@H](CN=[N+]=[N-])[C@@H](O)[C@H]2O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H26N14O7S/c23-16-10-18(29-4-27-16)35(6-31-10)20-14(40)12(38)7(42-20)1-26-9(37)3-44-22-33-11-17(24)28-5-30-19(11)36(22)21-15(41)13(39)8(43-21)2-32-34-25/h4-8,12-15,20-21,38-41H,1-3H2,(H,26,37)(H2,23,27,29)(H2,24,28,30)/t7-,8-,12-,13-,14-,15-,20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Inhibition of Listeria monocytogenes NAD kinase assessed as suppression of reduced NADP formation by yeast glucose-6-phosphate dehydrogenase coupled ... |
J Med Chem 63: 3817-3833 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01183
BindingDB Entry DOI: 10.7270/Q2NZ8C0R |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 5B
(Homo sapiens (Human)) | BDBM23234

(1,2,5-oxadiazole, OXD2 | 4-amino-1,2,5-oxadiazole-...)Show InChI InChI=1S/C3H3N3O3/c4-2-1(3(7)8)5-9-6-2/h(H2,4,6)(H,7,8) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Binding affinity to MBP-tagged recombinant STAT5b-SH2 domain (unknown origin) using carboxyfluoresceine-labeled phosphotyrosine octapeptide incubated... |
J Med Chem 63: 3817-3833 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01183
BindingDB Entry DOI: 10.7270/Q2NZ8C0R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50232690

(4-Aminomethyl-Benzamidine | CHEMBL187301)Show InChI InChI=1S/C8H11N3/c9-5-6-1-3-7(4-2-6)8(10)11/h1-4H,5,9H2,(H3,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 6.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Inhibition human FA10a using Boc-LeuGly-Arg-AMC fluorogenic substrate |
J Med Chem 63: 3817-3833 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01183
BindingDB Entry DOI: 10.7270/Q2NZ8C0R |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 5B
(Homo sapiens (Human)) | BDBM50514419
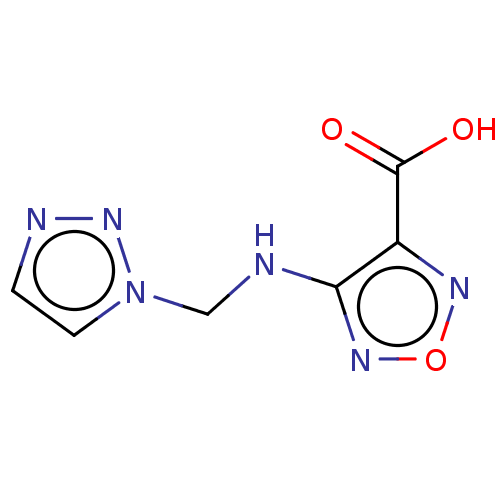
(CHEMBL4454339)Show InChI InChI=1S/C6H6N6O3/c13-6(14)4-5(10-15-9-4)7-3-12-2-1-8-11-12/h1-2H,3H2,(H,7,10)(H,13,14) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Binding affinity to MBP-tagged recombinant STAT5b-SH2 domain (unknown origin) using carboxyfluoresceine-labeled phosphotyrosine octapeptide incubated... |
J Med Chem 63: 3817-3833 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01183
BindingDB Entry DOI: 10.7270/Q2NZ8C0R |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 5B
(Homo sapiens (Human)) | BDBM50514420
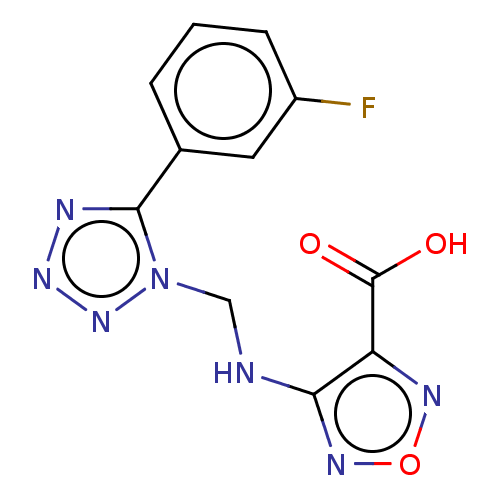
(CHEMBL4459159)Show InChI InChI=1S/C11H8FN7O3/c12-7-3-1-2-6(4-7)10-14-17-18-19(10)5-13-9-8(11(20)21)15-22-16-9/h1-4H,5H2,(H,13,16)(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Binding affinity to MBP-tagged recombinant STAT5b-SH2 domain (unknown origin) using carboxyfluoresceine-labeled phosphotyrosine octapeptide incubated... |
J Med Chem 63: 3817-3833 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01183
BindingDB Entry DOI: 10.7270/Q2NZ8C0R |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 5B
(Homo sapiens (Human)) | BDBM50514421

(CHEMBL4438181)Show SMILES COC(=O)c1nonc1NCn1nnnc1-c1cccc(OC(F)(F)F)c1 Show InChI InChI=1S/C13H10F3N7O4/c1-25-12(24)9-10(20-27-19-9)17-6-23-11(18-21-22-23)7-3-2-4-8(5-7)26-13(14,15)16/h2-5H,6H2,1H3,(H,17,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Binding affinity to MBP-tagged recombinant STAT5b-SH2 domain (unknown origin) using carboxyfluoresceine-labeled phosphotyrosine octapeptide incubated... |
J Med Chem 63: 3817-3833 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01183
BindingDB Entry DOI: 10.7270/Q2NZ8C0R |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 5B
(Homo sapiens (Human)) | BDBM50514422

(CHEMBL4466928)Show InChI InChI=1S/C10H8N6O3/c17-10(18)8-9(14-19-13-8)11-5-16-7-4-2-1-3-6(7)12-15-16/h1-4H,5H2,(H,11,14)(H,17,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Binding affinity to MBP-tagged recombinant STAT5b-SH2 domain (unknown origin) using carboxyfluoresceine-labeled phosphotyrosine octapeptide incubated... |
J Med Chem 63: 3817-3833 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01183
BindingDB Entry DOI: 10.7270/Q2NZ8C0R |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 5B
(Homo sapiens (Human)) | BDBM50514412

(CHEMBL4462216)Show InChI InChI=1S/C11H9N7O3/c19-11(20)8-9(15-21-14-8)12-6-18-10(13-16-17-18)7-4-2-1-3-5-7/h1-5H,6H2,(H,12,15)(H,19,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Binding affinity to MBP-tagged recombinant STAT5b-SH2 domain (unknown origin) using carboxyfluoresceine-labeled phosphotyrosine octapeptide incubated... |
J Med Chem 63: 3817-3833 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01183
BindingDB Entry DOI: 10.7270/Q2NZ8C0R |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 5B
(Homo sapiens (Human)) | BDBM50514424
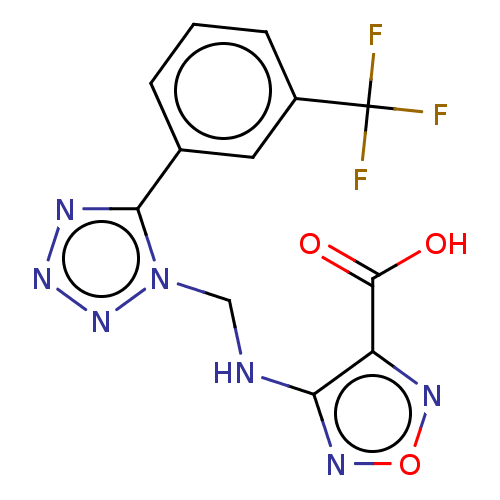
(CHEMBL4452459)Show InChI InChI=1S/C12H8F3N7O3/c13-12(14,15)7-3-1-2-6(4-7)10-17-20-21-22(10)5-16-9-8(11(23)24)18-25-19-9/h1-4H,5H2,(H,16,19)(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Binding affinity to MBP-tagged recombinant STAT5b-SH2 domain (unknown origin) using carboxyfluoresceine-labeled phosphotyrosine octapeptide incubated... |
J Med Chem 63: 3817-3833 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01183
BindingDB Entry DOI: 10.7270/Q2NZ8C0R |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 5B
(Homo sapiens (Human)) | BDBM50514411
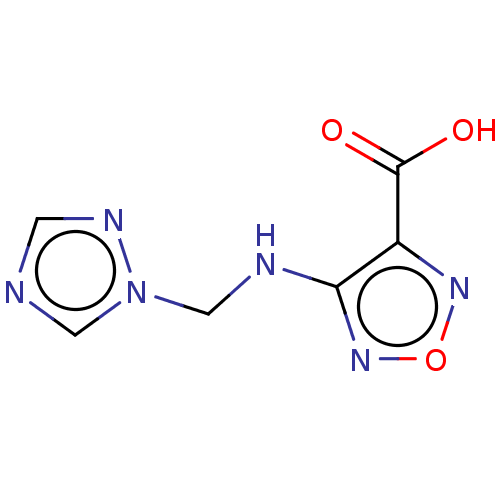
(CHEMBL4574617)Show InChI InChI=1S/C6H6N6O3/c13-6(14)4-5(11-15-10-4)8-3-12-2-7-1-9-12/h1-2H,3H2,(H,8,11)(H,13,14) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Binding affinity to MBP-tagged recombinant STAT5b-SH2 domain (unknown origin) using carboxyfluoresceine-labeled phosphotyrosine octapeptide incubated... |
J Med Chem 63: 3817-3833 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01183
BindingDB Entry DOI: 10.7270/Q2NZ8C0R |
More data for this
Ligand-Target Pair | |
Signal transducer and activator of transcription 5B
(Homo sapiens (Human)) | BDBM50514410

(CHEMBL4566571)Show InChI InChI=1S/C7H7N5O3/c13-7(14)5-6(11-15-10-5)8-4-12-3-1-2-9-12/h1-3H,4H2,(H,8,11)(H,13,14) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Binding affinity to MBP-tagged recombinant STAT5b-SH2 domain (unknown origin) using carboxyfluoresceine-labeled phosphotyrosine octapeptide incubated... |
J Med Chem 63: 3817-3833 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01183
BindingDB Entry DOI: 10.7270/Q2NZ8C0R |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50514418

(CHEMBL4572188)Show SMILES OC(=O)CNC(=O)[C@@H](COCc1ccccc1)NS(=O)(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C19H22N2O6S/c22-18(23)11-20-19(24)17(13-27-12-15-7-3-1-4-8-15)21-28(25,26)14-16-9-5-2-6-10-16/h1-10,17,21H,11-14H2,(H,20,24)(H,22,23)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Inhibition human FA10a using Boc-LeuGly-Arg-AMC fluorogenic substrate |
J Med Chem 63: 3817-3833 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01183
BindingDB Entry DOI: 10.7270/Q2NZ8C0R |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105091
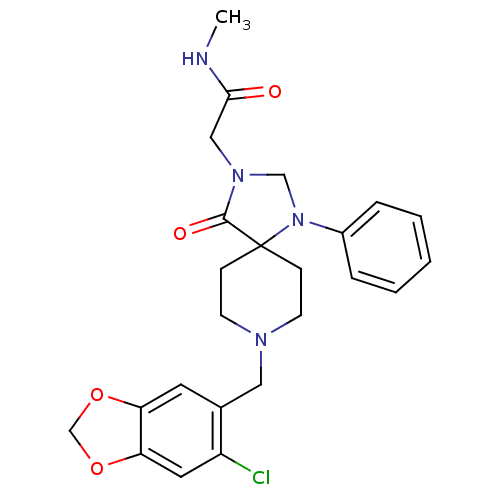
(2-[8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-4-oxo-...)Show SMILES CNC(=O)CN1CN(c2ccccc2)C2(CCN(Cc3cc4OCOc4cc3Cl)CC2)C1=O Show InChI InChI=1S/C24H27ClN4O4/c1-26-22(30)14-28-15-29(18-5-3-2-4-6-18)24(23(28)31)7-9-27(10-8-24)13-17-11-20-21(12-19(17)25)33-16-32-20/h2-6,11-12H,7-10,13-16H2,1H3,(H,26,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50026603

(Buprenorphine | CHEBI:3216)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@]([H])(C1)[C@](C)(O)C(C)(C)C)ccc3O |r,TLB:25:17:4.5.6:9.15.14,18:17:4.5.6:9.15.14,THB:10:9:17:4.5.6,3:4:17:9.15.14,26:23:16.1:18.19| Show InChI InChI=1S/C29H41NO4/c1-25(2,3)26(4,32)20-15-27-10-11-29(20,33-5)24-28(27)12-13-30(16-17-6-7-17)21(27)14-18-8-9-19(31)23(34-24)22(18)28/h8-9,17,20-21,24,31-32H,6-7,10-16H2,1-5H3/t20-,21-,24-,26+,27-,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against delta-opiate receptor (human) using [3H]-DPDPE radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105091

(2-[8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-4-oxo-...)Show SMILES CNC(=O)CN1CN(c2ccccc2)C2(CCN(Cc3cc4OCOc4cc3Cl)CC2)C1=O Show InChI InChI=1S/C24H27ClN4O4/c1-26-22(30)14-28-15-29(18-5-3-2-4-6-18)24(23(28)31)7-9-27(10-8-24)13-17-11-20-21(12-19(17)25)33-16-32-20/h2-6,11-12H,7-10,13-16H2,1H3,(H,26,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105085

(17-cyclobutylmethyl-4,5alpha-epoxymorphinan-3,6alp...)Show SMILES O[C@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CCC1)c45 |r| Show InChI InChI=1S/C21H27NO4/c23-14-5-4-13-10-16-21(25)7-6-15(24)19-20(21,17(13)18(14)26-19)8-9-22(16)11-12-2-1-3-12/h4-5,12,15-16,19,23-25H,1-3,6-11H2/t15-,16+,19-,20-,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM21641

(2-(2-benzyl-3-sulfanylpropanamido)acetic acid | CH...)Show InChI InChI=1S/C12H15NO3S/c14-11(15)7-13-12(16)10(8-17)6-9-4-2-1-3-5-9/h1-5,10,17H,6-8H2,(H,13,16)(H,14,15) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human NEP-mediated amyloid beta hydrolysis |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50026603

(Buprenorphine | CHEBI:3216)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@]([H])(C1)[C@](C)(O)C(C)(C)C)ccc3O |r,TLB:25:17:4.5.6:9.15.14,18:17:4.5.6:9.15.14,THB:10:9:17:4.5.6,3:4:17:9.15.14,26:23:16.1:18.19| Show InChI InChI=1S/C29H41NO4/c1-25(2,3)26(4,32)20-15-27-10-11-29(20,33-5)24-28(27)12-13-30(16-17-6-7-17)21(27)14-18-8-9-19(31)23(34-24)22(18)28/h8-9,17,20-21,24,31-32H,6-7,10-16H2,1-5H3/t20-,21-,24-,26+,27-,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 using [3H]- U-69,593 radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105094
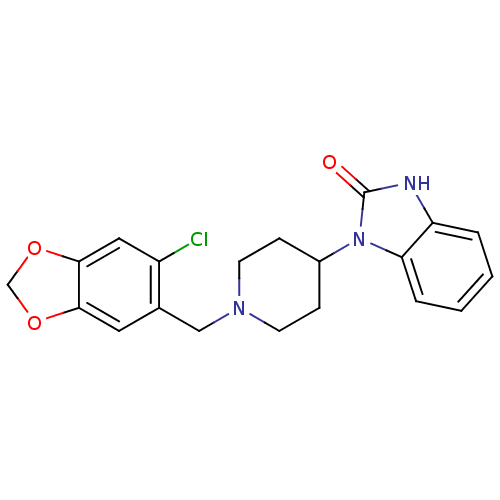
(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Clc1cc2OCOc2cc1CN1CCC(CC1)n1c2ccccc2[nH]c1=O Show InChI InChI=1S/C20H20ClN3O3/c21-15-10-19-18(26-12-27-19)9-13(15)11-23-7-5-14(6-8-23)24-17-4-2-1-3-16(17)22-20(24)25/h1-4,9-10,14H,5-8,11-12H2,(H,22,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105094

(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Clc1cc2OCOc2cc1CN1CCC(CC1)n1c2ccccc2[nH]c1=O Show InChI InChI=1S/C20H20ClN3O3/c21-15-10-19-18(26-12-27-19)9-13(15)11-23-7-5-14(6-8-23)24-17-4-2-1-3-16(17)22-20(24)25/h1-4,9-10,14H,5-8,11-12H2,(H,22,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50008984

(4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...)Show InChI InChI=1S/C22H28N2O/c1-2-22(25)24(20-11-7-4-8-12-20)21-14-17-23(18-15-21)16-13-19-9-5-3-6-10-19/h3-12,21H,2,13-18H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000788

((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 using [3H]- U-69,593 radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105080
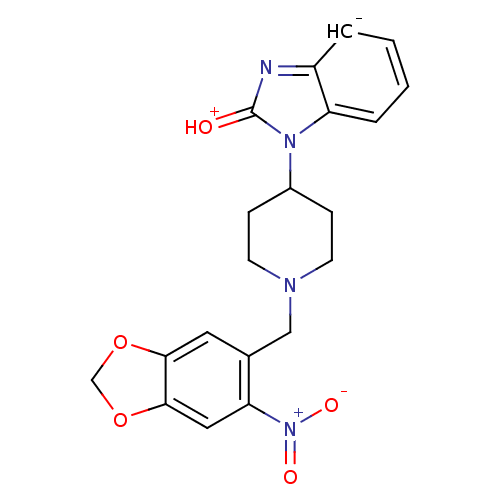
(1-[1-(6-Nitro-benzo[1,3]dioxol-5-ylmethyl)-piperid...)Show SMILES [O-][N+](=O)c1cc2OCOc2cc1CN1CCC(CC1)n1[c-]2ccccc2nc1=[OH+] Show InChI InChI=1S/C20H19N4O5/c25-20-21-15-3-1-2-4-16(15)23(20)14-5-7-22(8-6-14)11-13-9-18-19(29-12-28-18)10-17(13)24(26)27/h1-4,9-10,14H,5-8,11-12H2/q-1/p+1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50017698
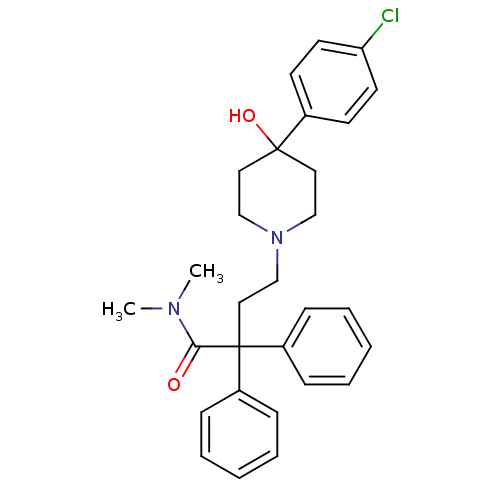
(4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-N,N...)Show SMILES CN(C)C(=O)C(CCN1CCC(O)(CC1)c1ccc(Cl)cc1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H33ClN2O2/c1-31(2)27(33)29(24-9-5-3-6-10-24,25-11-7-4-8-12-25)19-22-32-20-17-28(34,18-21-32)23-13-15-26(30)16-14-23/h3-16,34H,17-22H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105080

(1-[1-(6-Nitro-benzo[1,3]dioxol-5-ylmethyl)-piperid...)Show SMILES [O-][N+](=O)c1cc2OCOc2cc1CN1CCC(CC1)n1[c-]2ccccc2nc1=[OH+] Show InChI InChI=1S/C20H19N4O5/c25-20-21-15-3-1-2-4-16(15)23(20)14-5-7-22(8-6-14)11-13-9-18-19(29-12-28-18)10-17(13)24(26)27/h1-4,9-10,14H,5-8,11-12H2/q-1/p+1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50026603

(Buprenorphine | CHEBI:3216)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@]([H])(C1)[C@](C)(O)C(C)(C)C)ccc3O |r,TLB:25:17:4.5.6:9.15.14,18:17:4.5.6:9.15.14,THB:10:9:17:4.5.6,3:4:17:9.15.14,26:23:16.1:18.19| Show InChI InChI=1S/C29H41NO4/c1-25(2,3)26(4,32)20-15-27-10-11-29(20,33-5)24-28(27)12-13-30(16-17-6-7-17)21(27)14-18-8-9-19(31)23(34-24)22(18)28/h8-9,17,20-21,24,31-32H,6-7,10-16H2,1-5H3/t20-,21-,24-,26+,27-,28+,29-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105094

(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Clc1cc2OCOc2cc1CN1CCC(CC1)n1c2ccccc2[nH]c1=O Show InChI InChI=1S/C20H20ClN3O3/c21-15-10-19-18(26-12-27-19)9-13(15)11-23-7-5-14(6-8-23)24-17-4-2-1-3-16(17)22-20(24)25/h1-4,9-10,14H,5-8,11-12H2,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 using [3H]- U-69,593 radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105094

(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Clc1cc2OCOc2cc1CN1CCC(CC1)n1c2ccccc2[nH]c1=O Show InChI InChI=1S/C20H20ClN3O3/c21-15-10-19-18(26-12-27-19)9-13(15)11-23-7-5-14(6-8-23)24-17-4-2-1-3-16(17)22-20(24)25/h1-4,9-10,14H,5-8,11-12H2,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 by using [3H]U-69593 as radioligand |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000788

((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105072
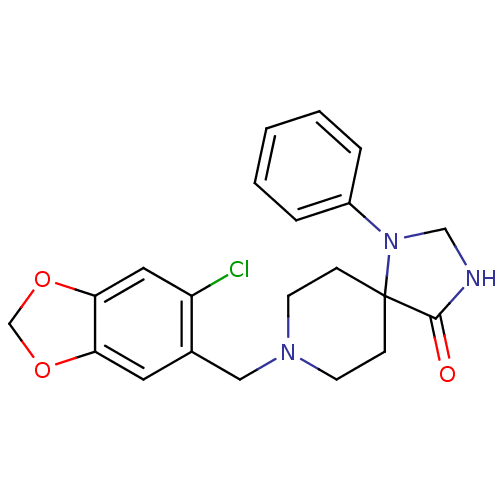
(8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-1-phenyl-...)Show SMILES Clc1cc2OCOc2cc1CN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C21H22ClN3O3/c22-17-11-19-18(27-14-28-19)10-15(17)12-24-8-6-21(7-9-24)20(26)23-13-25(21)16-4-2-1-3-5-16/h1-5,10-11H,6-9,12-14H2,(H,23,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105072

(8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-1-phenyl-...)Show SMILES Clc1cc2OCOc2cc1CN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C21H22ClN3O3/c22-17-11-19-18(27-14-28-19)10-15(17)12-24-8-6-21(7-9-24)20(26)23-13-25(21)16-4-2-1-3-5-16/h1-5,10-11H,6-9,12-14H2,(H,23,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105091

(2-[8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-4-oxo-...)Show SMILES CNC(=O)CN1CN(c2ccccc2)C2(CCN(Cc3cc4OCOc4cc3Cl)CC2)C1=O Show InChI InChI=1S/C24H27ClN4O4/c1-26-22(30)14-28-15-29(18-5-3-2-4-6-18)24(23(28)31)7-9-27(10-8-24)13-17-11-20-21(12-19(17)25)33-16-32-20/h2-6,11-12H,7-10,13-16H2,1H3,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 using [3H]- U-69,593 radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105091

(2-[8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-4-oxo-...)Show SMILES CNC(=O)CN1CN(c2ccccc2)C2(CCN(Cc3cc4OCOc4cc3Cl)CC2)C1=O Show InChI InChI=1S/C24H27ClN4O4/c1-26-22(30)14-28-15-29(18-5-3-2-4-6-18)24(23(28)31)7-9-27(10-8-24)13-17-11-20-21(12-19(17)25)33-16-32-20/h2-6,11-12H,7-10,13-16H2,1H3,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 by using [3H]U-69593 as radioligand |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105066

(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Cc1ccc2[nH]c(=O)n(C3CCN(Cc4cc5OCOc5cc4Cl)CC3)c2c1 Show InChI InChI=1S/C21H22ClN3O3/c1-13-2-3-17-18(8-13)25(21(26)23-17)15-4-6-24(7-5-15)11-14-9-19-20(10-16(14)22)28-12-27-19/h2-3,8-10,15H,4-7,11-12H2,1H3,(H,23,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105066

(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Cc1ccc2[nH]c(=O)n(C3CCN(Cc4cc5OCOc5cc4Cl)CC3)c2c1 Show InChI InChI=1S/C21H22ClN3O3/c1-13-2-3-17-18(8-13)25(21(26)23-17)15-4-6-24(7-5-15)11-14-9-19-20(10-16(14)22)28-12-27-19/h2-3,8-10,15H,4-7,11-12H2,1H3,(H,23,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50351798

(CHEMBL1824184)Show SMILES CCCCn1ncc(C(O)=O)c1Nc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C21H21N7O2/c1-2-3-12-28-20(18(13-22-28)21(29)30)23-15-10-8-14(9-11-15)16-6-4-5-7-17(16)19-24-26-27-25-19/h4-11,13,23H,2-3,12H2,1H3,(H,29,30)(H,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [125I][Sar1,Ile8]-AT-II from human recombinant AT1 receptor expressed in HEK293 cells after 120 mins by liquid scintillation counting |
Eur J Med Chem 46: 3867-76 (2011)
Article DOI: 10.1016/j.ejmech.2011.05.056
BindingDB Entry DOI: 10.7270/Q2R78FKW |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105066

(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Cc1ccc2[nH]c(=O)n(C3CCN(Cc4cc5OCOc5cc4Cl)CC3)c2c1 Show InChI InChI=1S/C21H22ClN3O3/c1-13-2-3-17-18(8-13)25(21(26)23-17)15-4-6-24(7-5-15)11-14-9-19-20(10-16(14)22)28-12-27-19/h2-3,8-10,15H,4-7,11-12H2,1H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 using [3H]- U-69,593 radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105066

(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Cc1ccc2[nH]c(=O)n(C3CCN(Cc4cc5OCOc5cc4Cl)CC3)c2c1 Show InChI InChI=1S/C21H22ClN3O3/c1-13-2-3-17-18(8-13)25(21(26)23-17)15-4-6-24(7-5-15)11-14-9-19-20(10-16(14)22)28-12-27-19/h2-3,8-10,15H,4-7,11-12H2,1H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 by using [3H]U-69593 as radioligand |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Insulin-degrading enzyme
(Homo sapiens (Human)) | BDBM50525490
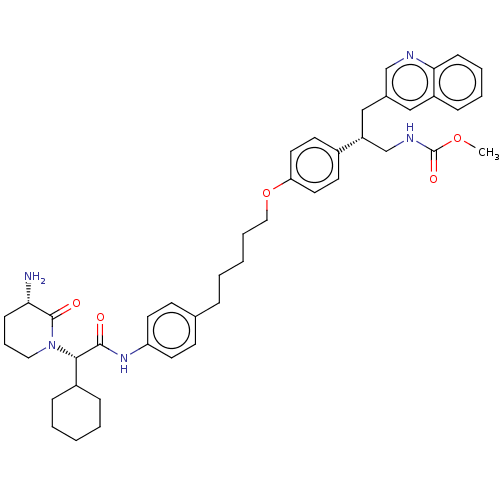
(CHEMBL4437643)Show SMILES COC(=O)NC[C@@H](Cc1cnc2ccccc2c1)c1ccc(OCCCCCc2ccc(NC(=O)[C@H](C3CCCCC3)N3CCC[C@H](N)C3=O)cc2)cc1 |r| Show InChI InChI=1S/C44H55N5O5/c1-53-44(52)47-30-36(28-32-27-35-14-7-8-16-40(35)46-29-32)33-19-23-38(24-20-33)54-26-9-3-4-11-31-17-21-37(22-18-31)48-42(50)41(34-12-5-2-6-13-34)49-25-10-15-39(45)43(49)51/h7-8,14,16-24,27,29,34,36,39,41H,2-6,9-13,15,25-26,28,30,45H2,1H3,(H,47,52)(H,48,50)/t36-,39+,41+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant IDE exosite (unknown origin) expressed in Escherichia coli using insulin as substrate incubated for 4 hrs by AlphaLisa assa... |
Eur J Med Chem 179: 557-566 (2019)
Article DOI: 10.1016/j.ejmech.2019.06.057
BindingDB Entry DOI: 10.7270/Q2XS5ZT5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM82507
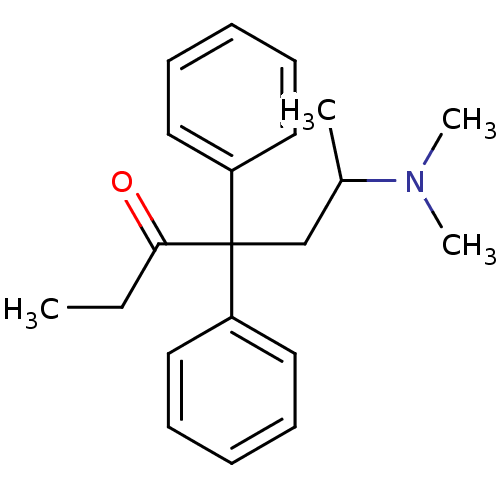
((+/-)-Methadone | CAS_5967-73-7 | METHADONE | Meth...)Show InChI InChI=1S/C21H27NO/c1-5-20(23)21(16-17(2)22(3)4,18-12-8-6-9-13-18)19-14-10-7-11-15-19/h6-15,17H,5,16H2,1-4H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105072

(8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-1-phenyl-...)Show SMILES Clc1cc2OCOc2cc1CN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C21H22ClN3O3/c22-17-11-19-18(27-14-28-19)10-15(17)12-24-8-6-21(7-9-24)20(26)23-13-25(21)16-4-2-1-3-5-16/h1-5,10-11H,6-9,12-14H2,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 by using [3H]U-69593 as radioligand |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105072

(8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-1-phenyl-...)Show SMILES Clc1cc2OCOc2cc1CN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C21H22ClN3O3/c22-17-11-19-18(27-14-28-19)10-15(17)12-24-8-6-21(7-9-24)20(26)23-13-25(21)16-4-2-1-3-5-16/h1-5,10-11H,6-9,12-14H2,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 using [3H]- U-69,593 radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
M1 family aminopeptidase
(Plasmodium falciparum (isolate FcB1 / Columbia)) | BDBM50206554

(CHEMBL219777 | N-(4-fluoro-benzyl)-N'-hydroxy-2-[1...)Show InChI InChI=1S/C17H15FN2O3/c18-14-8-6-13(7-9-14)11-19-16(21)15(17(22)20-23)10-12-4-2-1-3-5-12/h1-10,23H,11H2,(H,19,21)(H,20,22)/b15-10- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Inserm
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum FcB1 M1 aminopeptidase |
J Med Chem 50: 1322-34 (2007)
Article DOI: 10.1021/jm061169b
BindingDB Entry DOI: 10.7270/Q2M908B3 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
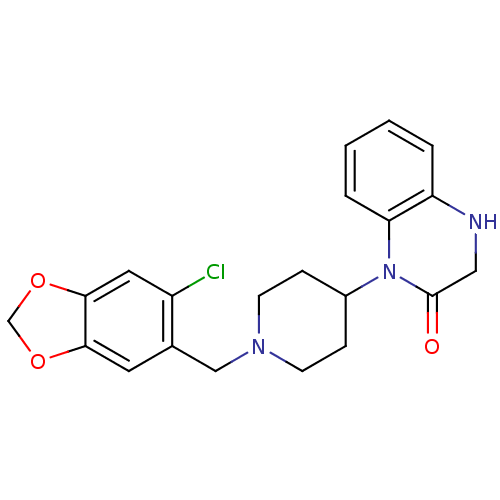
(Homo sapiens (Human)) | BDBM50105060
(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Clc1cc2OCOc2cc1CN1CCC(CC1)N1C(=O)CNc2ccccc12 Show InChI InChI=1S/C21H22ClN3O3/c22-16-10-20-19(27-13-28-20)9-14(16)12-24-7-5-15(6-8-24)25-18-4-2-1-3-17(18)23-11-21(25)26/h1-4,9-10,15,23H,5-8,11-13H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data