

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus) | BDBM50093010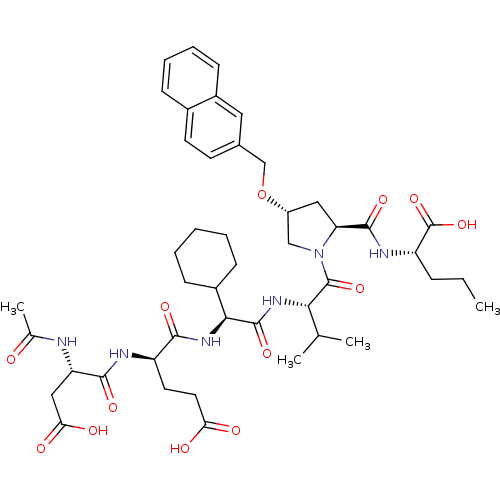 ((S)-2-{[(2S,4R)-1-((S)-2-{(S)-2-[(R)-2-((S)-2-Acet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against NS3 protease complexed with NS4A cofactor peptide (NS3-4A pep) | Bioorg Med Chem Lett 10: 2267-70 (2001) BindingDB Entry DOI: 10.7270/Q2S1831T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50071982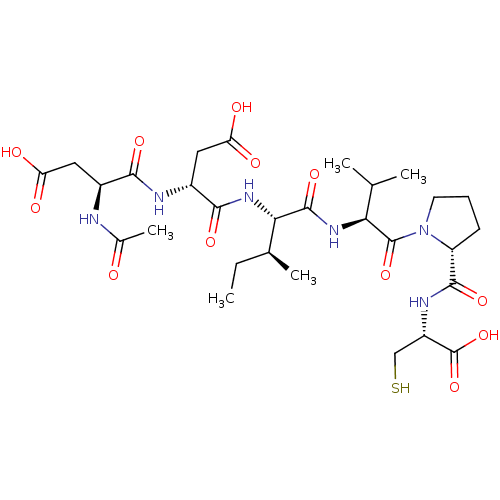 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3 protease. | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50070797 (CHEMBL2370476 | Hexapeptide analogue) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description The apparent Ki value against NS3-4Apep protease | Bioorg Med Chem Lett 8: 1713-8 (1999) BindingDB Entry DOI: 10.7270/Q2Z89CX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50366517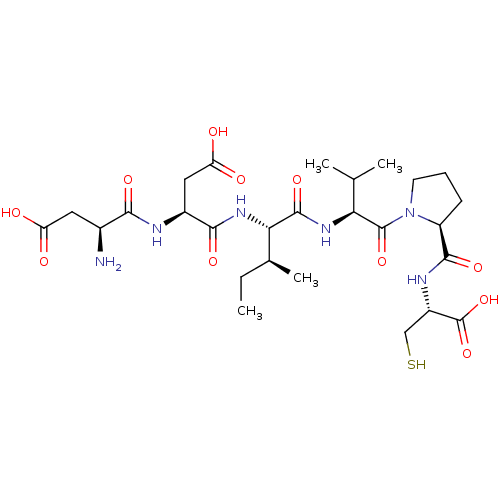 (CHEMBL1790303) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description The apparent Ki value against NS3-4Apep protease | Bioorg Med Chem Lett 8: 1713-8 (1999) BindingDB Entry DOI: 10.7270/Q2Z89CX4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142042 ((S)-2-[(1-Cyclohexyl-2-furan-3-yl-1H-benzoimidazol...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50093024 (1-{[1-(2-{2-[2-(2-Acetylamino-3-carboxy-propionyla...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibitory activity against NS3 protease complexed with NS4A cofactor peptide (NS3-4A pep) | Bioorg Med Chem Lett 10: 2267-70 (2001) BindingDB Entry DOI: 10.7270/Q2S1831T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142047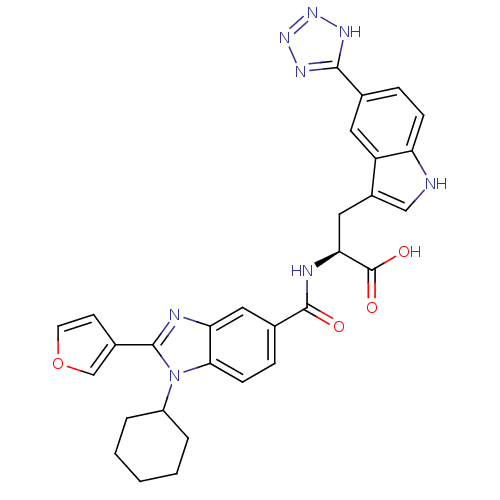 ((S)-2-[(1-Cyclohexyl-2-furan-3-yl-1H-benzoimidazol...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142043 ((2S)-3-(5-(carboxymethoxy)-1H-indol-3-yl)-2-(1-cyc...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142041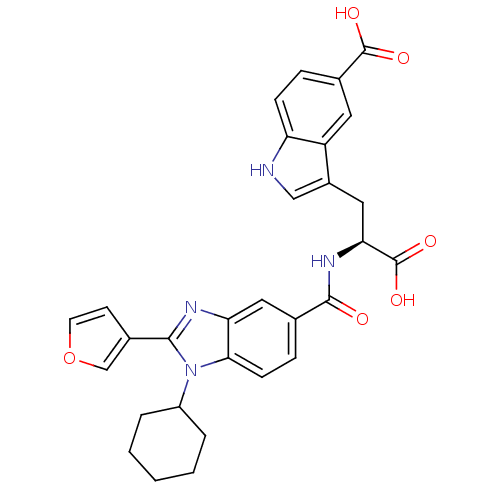 (3-{(S)-2-Carboxy-2-[(1-cyclohexyl-2-furan-3-yl-1H-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50093010 ((S)-2-{[(2S,4R)-1-((S)-2-{(S)-2-[(R)-2-((S)-2-Acet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibitory activity against NS3 protease complexed with NS4A cofactor peptide (NS3-4A pep) | Bioorg Med Chem Lett 10: 2267-70 (2001) BindingDB Entry DOI: 10.7270/Q2S1831T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50011004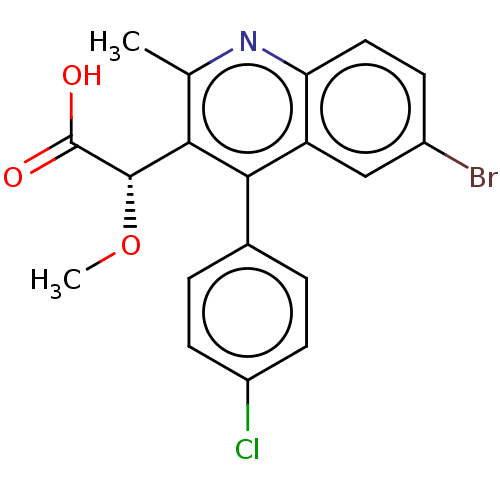 (CHEMBL3259893) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase by LTR cleavage assay | ACS Med Chem Lett 5: 422-7 (2014) Article DOI: 10.1021/ml500002n BindingDB Entry DOI: 10.7270/Q2K64KMK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50071966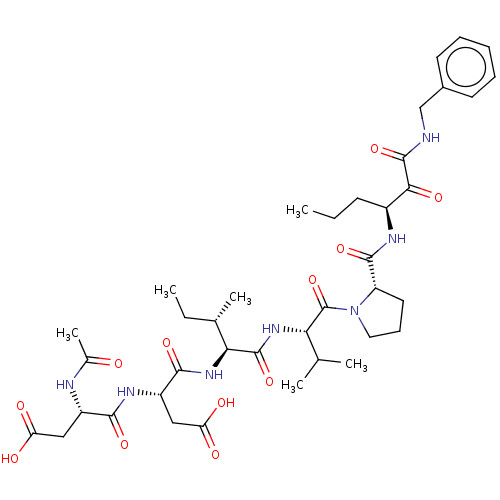 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human liver Cathepsin B | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50071966 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human leucocyte elastase (HLE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142056 ((S)-3-(5-Carbamoyl-1H-indol-3-yl)-2-[(1-cyclohexyl...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50011132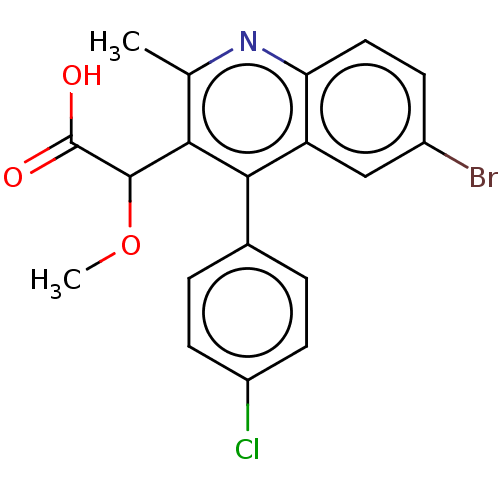 (ALLINI-1 | CHEMBL3259891) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase by LTR cleavage assay | ACS Med Chem Lett 5: 422-7 (2014) Article DOI: 10.1021/ml500002n BindingDB Entry DOI: 10.7270/Q2K64KMK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142052 ((2S)-2-(1-cyclohexyl-2-(furan-3-yl)-1H-benzo[d]imi...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50071973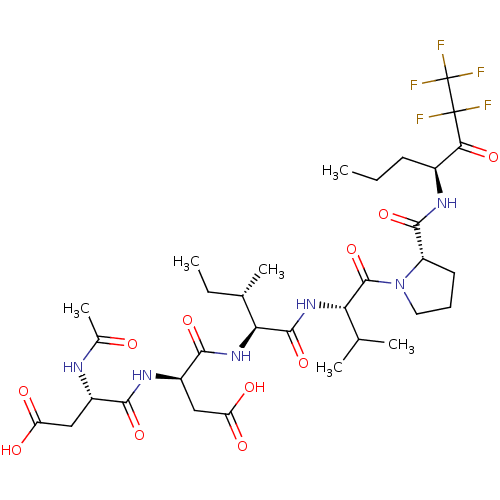 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human leucocyte elastase (HLE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 1/2A (Sus scrofa (Pig)) | BDBM50071968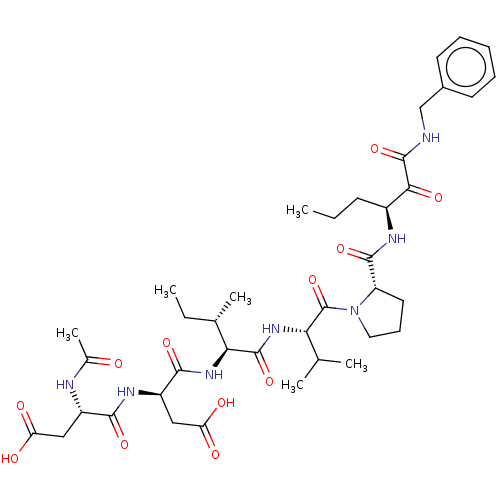 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of porcine pancreatic elastase (PPE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 1/2A (Sus scrofa (Pig)) | BDBM50071973 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of porcine pancreatic elastase (PPE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50071983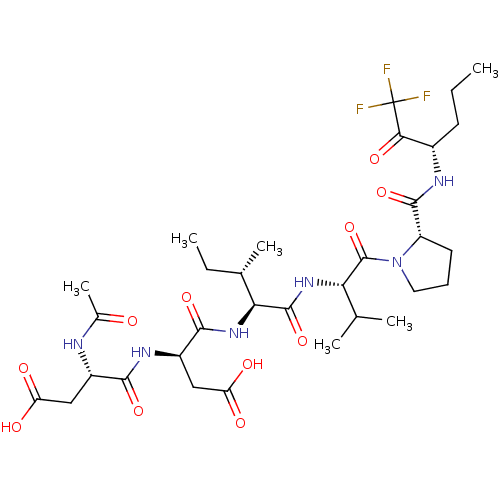 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of human leucocyte elastase (HLE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142045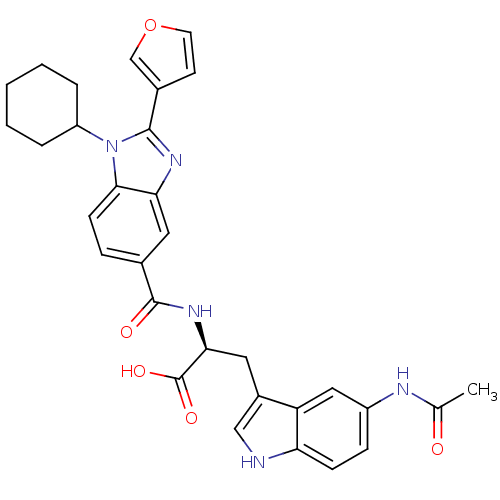 ((S)-3-(5-Acetylamino-1H-indol-3-yl)-2-[(1-cyclohex...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 1/2A (Sus scrofa (Pig)) | BDBM50071966 ((R)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of porcine pancreatic elastase (PPE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 2A (Sus scrofa) | BDBM50061511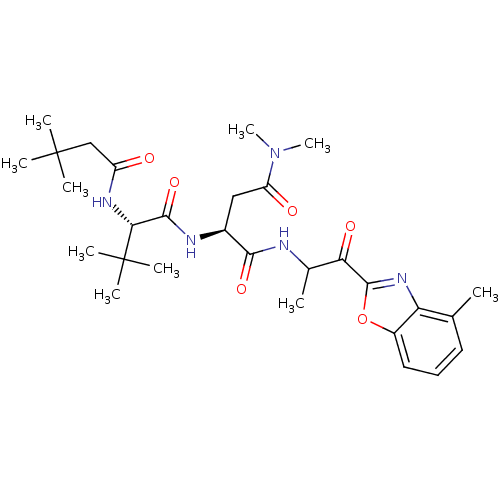 ((S)-2-[(S)-2-(3,3-Dimethyl-butyrylamino)-3,3-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Activity against serine protease porcine pancreatic elastase (PPE) | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50011131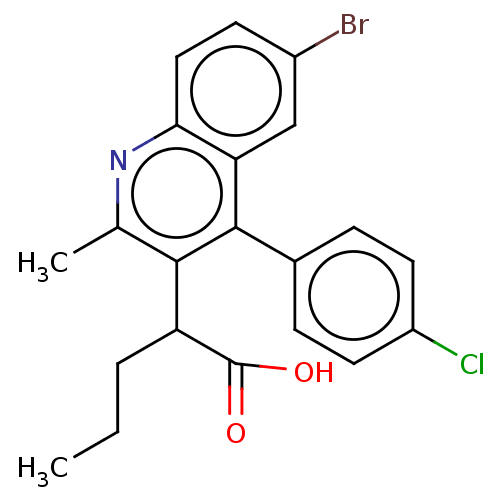 (CHEMBL3259890) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase by LTR cleavage assay | ACS Med Chem Lett 5: 422-7 (2014) Article DOI: 10.1021/ml500002n BindingDB Entry DOI: 10.7270/Q2K64KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 2A (Sus scrofa) | BDBM50061512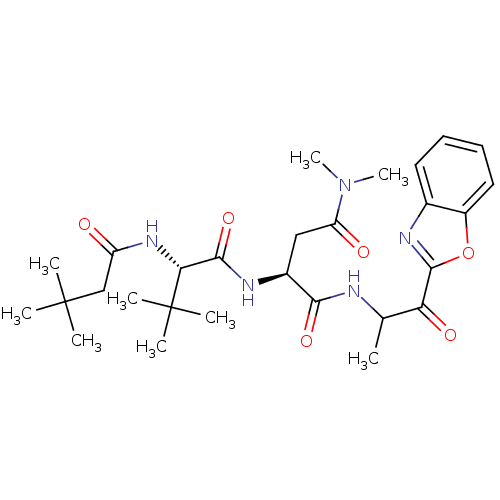 ((S)-N*1*-(2-Benzooxazol-2-yl-1-methyl-2-oxo-ethyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Activity against serine protease porcine pancreatic elastase (PPE) | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142053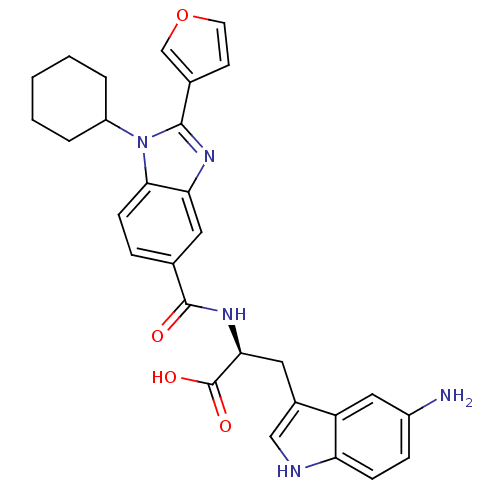 ((S)-3-(5-Amino-1H-indol-3-yl)-2-[(1-cyclohexyl-2-f...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061532 ((S)-N*1*-{2-[(Benzo[1,3]dioxol-5-ylmethyl)-carbamo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142044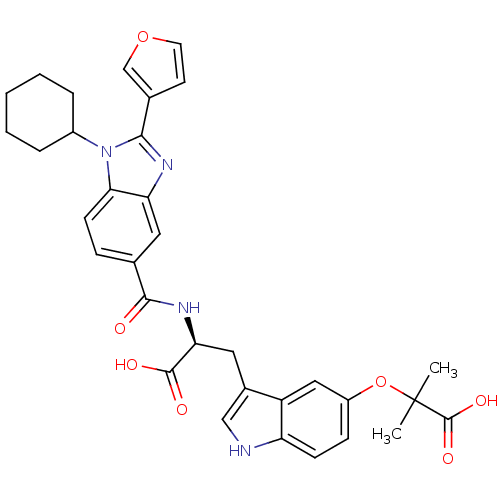 ((S)-3-[5-(1-Carboxy-1-methyl-ethoxy)-1H-indol-3-yl...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061518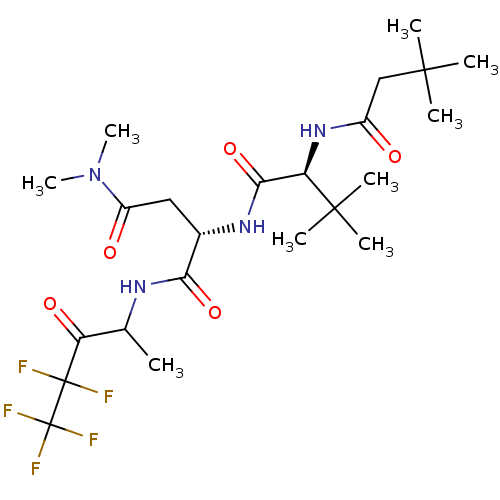 ((S)-2-[(S)-2-(3,3-Dimethyl-butyrylamino)-3,3-dimet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061537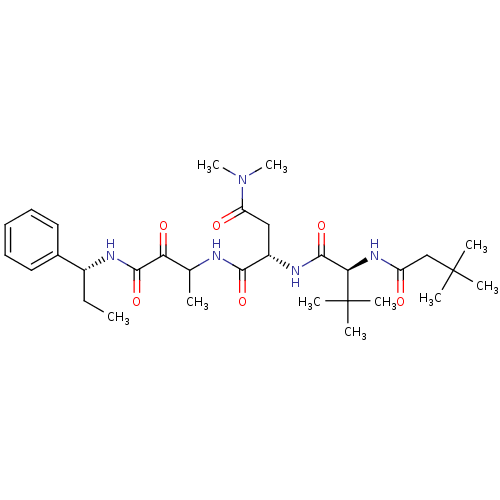 ((S)-2-[(S)-2-(3,3-Dimethyl-butyrylamino)-3,3-dimet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142049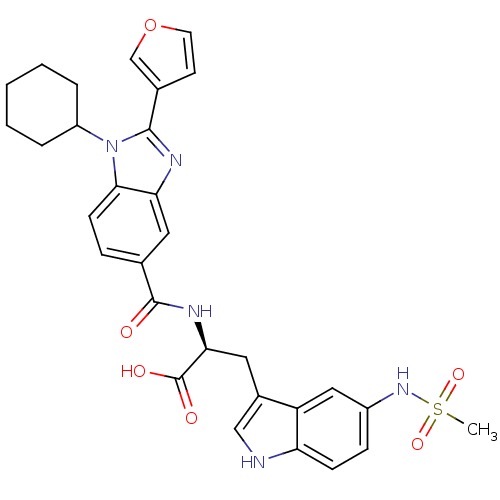 ((S)-2-[(1-Cyclohexyl-2-furan-3-yl-1H-benzoimidazol...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142050 ((S)-2-(1-cyclohexyl-2-(pyridin-2-yl)-1H-benzo[d]im...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061515 ((S)-N*1*-[2-(2-Benzyloxy-ethylcarbamoyl)-1-methyl-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142048 ((S)-2-[(1-Cyclohexyl-2-furan-3-yl-1H-benzoimidazol...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142069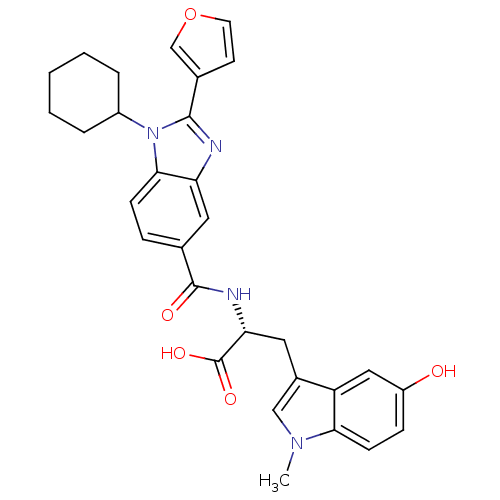 ((R)-2-[(1-Cyclohexyl-2-furan-3-yl-1H-benzoimidazol...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 1/2A (Sus scrofa (Pig)) | BDBM50071983 ((S)-3-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Compound was tested for inhibition of porcine pancreatic elastase (PPE) | Bioorg Med Chem Lett 8: 2719-24 (1999) BindingDB Entry DOI: 10.7270/Q2V69HRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 2A (Sus scrofa) | BDBM50061528 ((S)-2-[(S)-2-(3,3-Dimethyl-butyrylamino)-3,3-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Activity against serine protease porcine pancreatic elastase (PPE) | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 2A (Sus scrofa) | BDBM50061509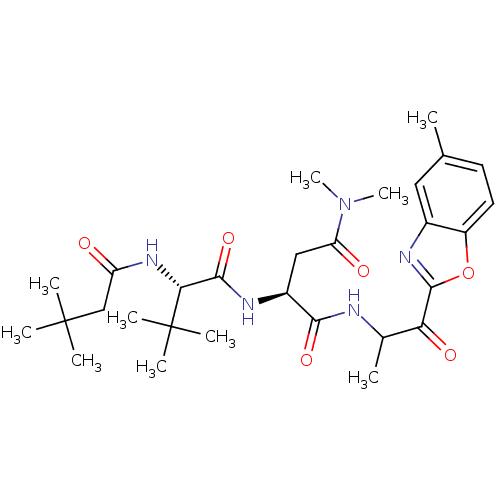 ((S)-2-[(S)-2-(3,3-Dimethyl-butyrylamino)-3,3-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Activity against serine protease porcine pancreatic elastase (PPE) | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061503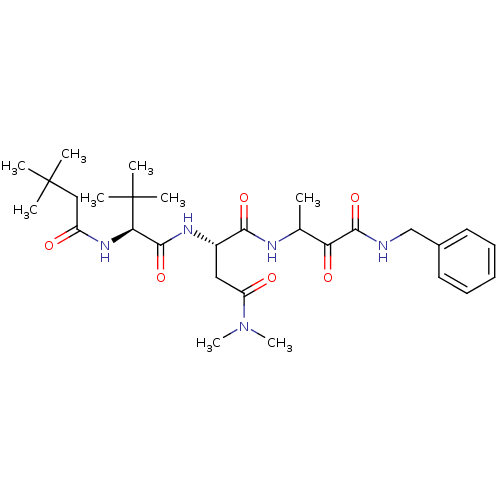 ((S)-N*1*-(2-Benzylcarbamoyl-1-methyl-2-oxo-ethyl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061506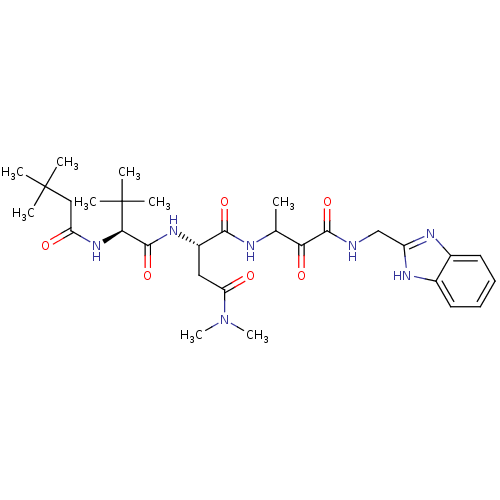 ((S)-N*1*-{2-[(1H-Benzoimidazol-2-ylmethyl)-carbamo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50011044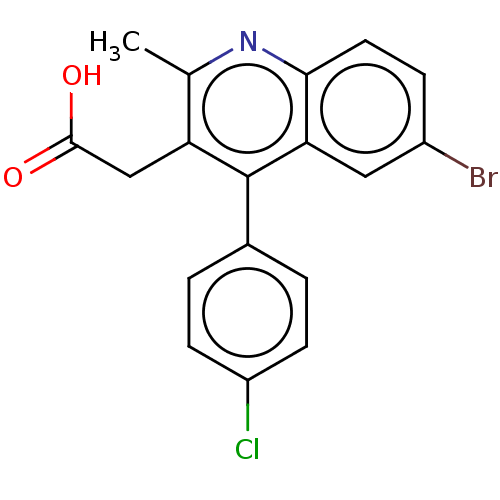 (CHEMBL3259887) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase by LTR cleavage assay | ACS Med Chem Lett 5: 422-7 (2014) Article DOI: 10.1021/ml500002n BindingDB Entry DOI: 10.7270/Q2K64KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50061544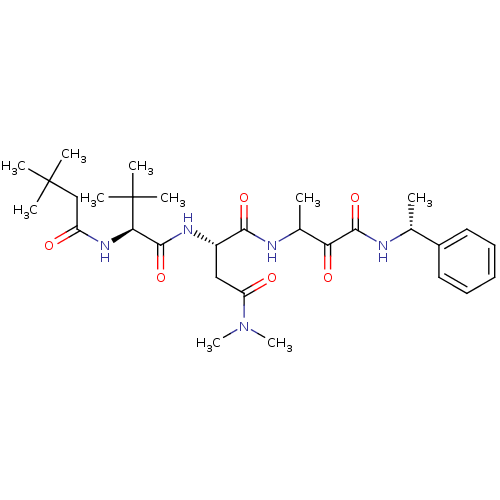 ((S)-2-[(S)-2-(3,3-Dimethyl-butyrylamino)-3,3-dimet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) protease | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50398047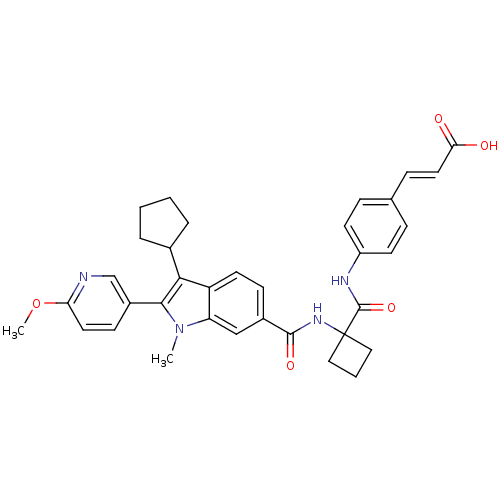 (CHEMBL2181641) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C19 | J Med Chem 55: 7650-66 (2012) Article DOI: 10.1021/jm3006788 BindingDB Entry DOI: 10.7270/Q2ZS2XNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 2A (Sus scrofa) | BDBM50061497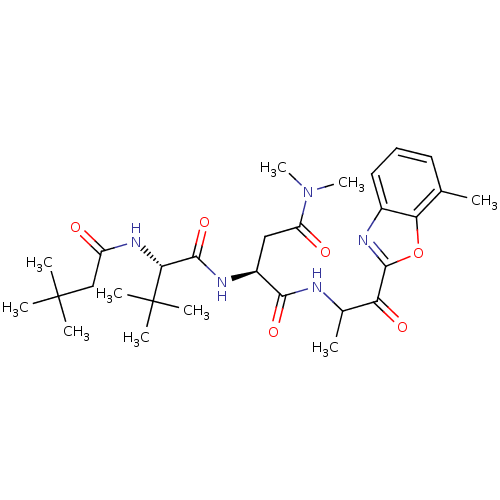 ((S)-2-[(S)-2-(3,3-Dimethyl-butyrylamino)-3,3-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Ltd. Curated by ChEMBL | Assay Description Activity against serine protease porcine pancreatic elastase (PPE) | J Med Chem 40: 4113-35 (1998) Article DOI: 10.1021/jm970104t BindingDB Entry DOI: 10.7270/Q2PC31HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50011130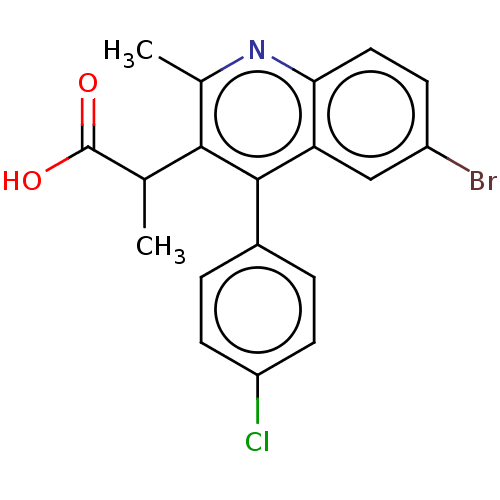 (CHEMBL3259889) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase by LTR cleavage assay | ACS Med Chem Lett 5: 422-7 (2014) Article DOI: 10.1021/ml500002n BindingDB Entry DOI: 10.7270/Q2K64KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50011043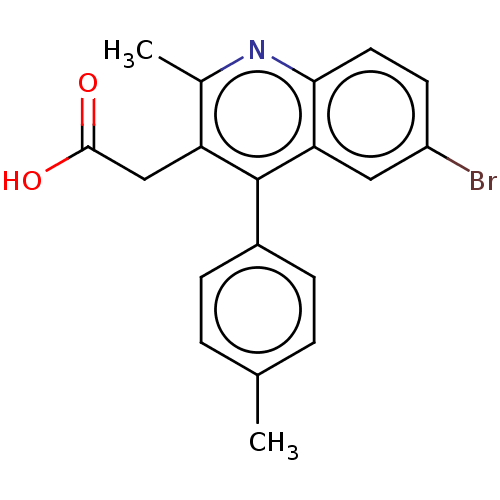 (CHEMBL3259886) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase by LTR cleavage assay | ACS Med Chem Lett 5: 422-7 (2014) Article DOI: 10.1021/ml500002n BindingDB Entry DOI: 10.7270/Q2K64KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50093025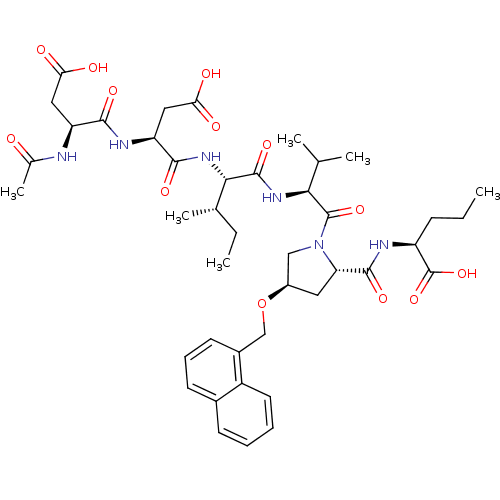 ((S)-2-{[(2S,4R)-1-((S)-2-{(2S,3S)-2-[(S)-2-((S)-2-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibitory activity against NS3 protease complexed with NS4A cofactor peptide (NS3-4A pep) | Bioorg Med Chem Lett 10: 2267-70 (2001) BindingDB Entry DOI: 10.7270/Q2S1831T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142064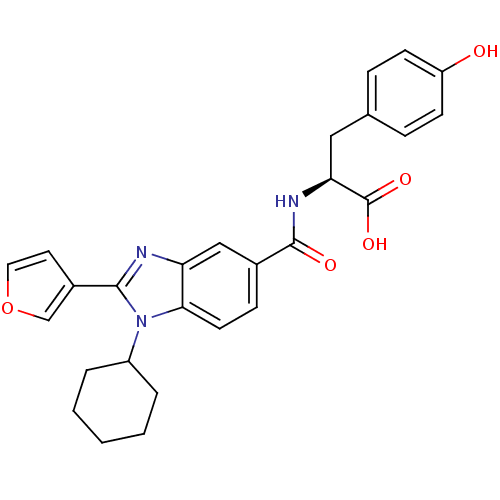 ((S)-2-[(1-Cyclohexyl-2-furan-3-yl-1H-benzoimidazol...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50158528 ((S)-1-cyclohexyl-2-(furan-3-yl)-N-(2-(5-hydroxy-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of human liver CYP3A4 | Bioorg Med Chem Lett 20: 196-200 (2010) Article DOI: 10.1016/j.bmcl.2009.10.136 BindingDB Entry DOI: 10.7270/Q2TD9XFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142061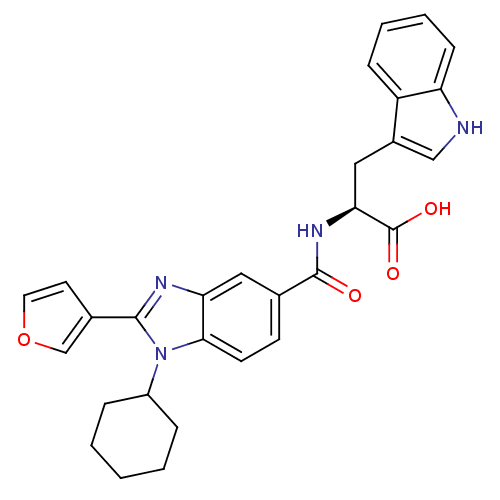 ((2S)-2-(1-cyclohexyl-2-(furan-3-yl)-1H-benzo[d]imi...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 436 total ) | Next | Last >> |