

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50042235 (2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Endothelin A receptor | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50018850 (1-(3-Mercapto-2-methyl-propionyl)-4-phenylsulfanyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibitory constant against rabbit lung Angiotensin I converting enzyme | J Med Chem 31: 1148-60 (1988) BindingDB Entry DOI: 10.7270/Q2V125DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50042235 (2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50042235 (2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367254 (ENALAPRILAT) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung angiotensin-1 converting enzyme | J Med Chem 31: 1148-60 (1988) BindingDB Entry DOI: 10.7270/Q2V125DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50091105 (4''-Oxazol-2-yl-biphenyl-2-sulfonic acid (3,4-dime...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Endothelin A receptor | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50018849 (4-Cyclohexyl-1-{2-[hydroxy-(4-phenyl-butyl)-phosph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung angiotensin-1 converting enzyme | J Med Chem 31: 1148-60 (1988) BindingDB Entry DOI: 10.7270/Q2V125DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung angiotensin-1 converting enzyme | J Med Chem 31: 1148-60 (1988) BindingDB Entry DOI: 10.7270/Q2V125DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50117910 (4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Endothelin A receptor | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50049201 (2-Cyclopropyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Receptor binding affinity determined from competitive binding assay using 1251 labelled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 201-206 (1994) Article DOI: 10.1016/S0960-894X(01)81147-1 BindingDB Entry DOI: 10.7270/Q2TH8MMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50117911 (4'-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM19264 ((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Codon Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 2 (IMPDH type II isoform); Range is 6-10 nM | J Med Chem 41: 618-22 (1998) Article DOI: 10.1021/jm970705k BindingDB Entry DOI: 10.7270/Q20C4WGZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50117910 (4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards rat Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50117910 (4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Angiotensin II receptor, type 1 | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50018848 (1-{2-[Hydroxy-(4-phenyl-butyl)-phosphinoyl]-acetyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibitory activity against rabbit lung angiotensin-1 converting enzyme | J Med Chem 31: 1148-60 (1988) BindingDB Entry DOI: 10.7270/Q2V125DX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 1 (Homo sapiens (Human)) | BDBM19264 ((4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Codon Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 1 (IMPDH type I isoform); Range is 33-37 nM | J Med Chem 41: 618-22 (1998) Article DOI: 10.1021/jm970705k BindingDB Entry DOI: 10.7270/Q20C4WGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50117911 (4'-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity towards human Endothelin A receptor | J Med Chem 45: 3829-35 (2002) BindingDB Entry DOI: 10.7270/Q2Z60NDM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inosine-5'-monophosphate dehydrogenase 2 (Homo sapiens (Human)) | BDBM50369419 (CHEMBL610421) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Codon Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human Inosine-5'-monophosphate dehydrogenase 2 (IMPDH type II isoform) | J Med Chem 41: 618-22 (1998) Article DOI: 10.1021/jm970705k BindingDB Entry DOI: 10.7270/Q20C4WGZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434318 (CHEMBL2386566) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434329 (CHEMBL2386554) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434329 (CHEMBL2386554) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434316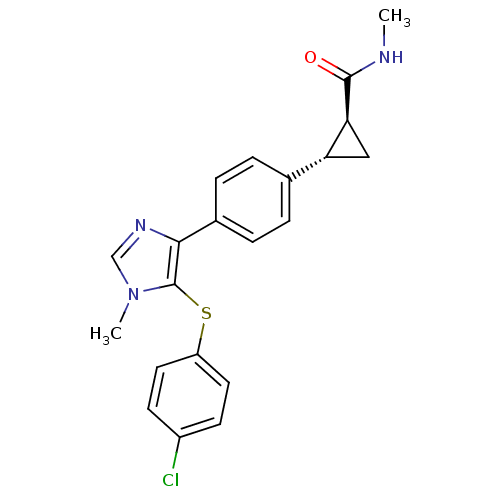 (CHEMBL2386568) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434318 (CHEMBL2386566) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434317 (CHEMBL2386567) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434313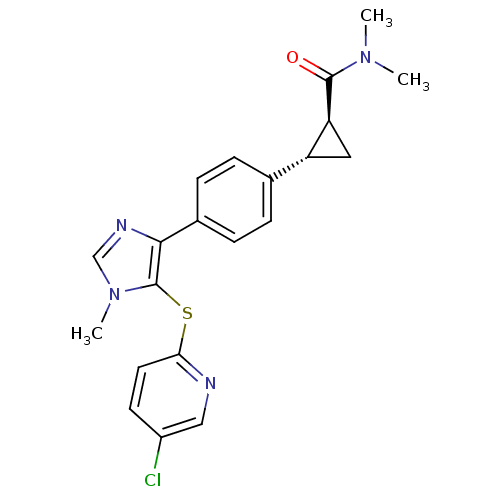 (CHEMBL2386571) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434315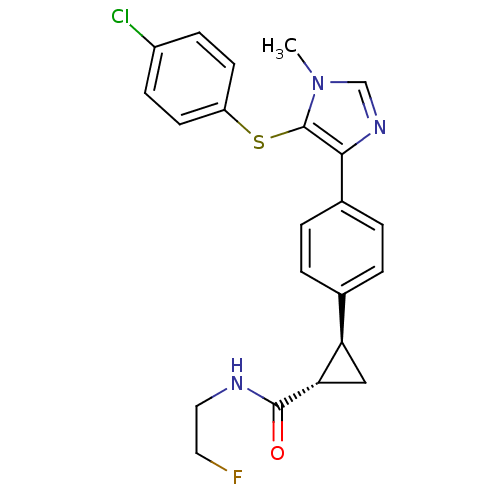 (CHEMBL2386569) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434322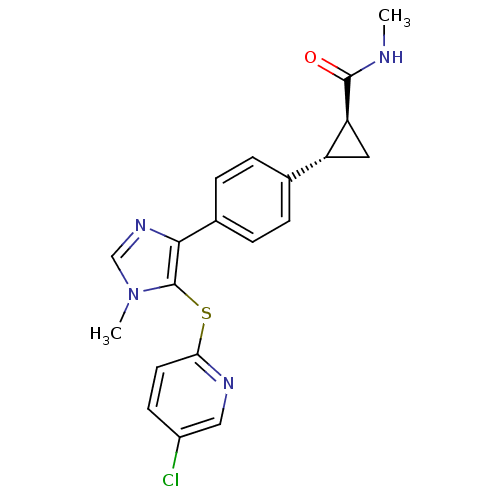 (CHEMBL2386562) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434314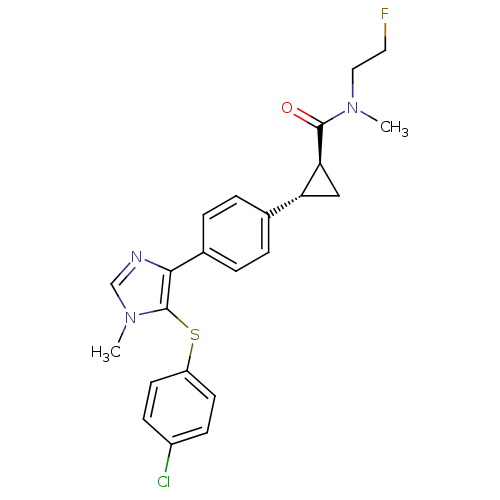 (CHEMBL2386570) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434314 (CHEMBL2386570) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434316 (CHEMBL2386568) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434315 (CHEMBL2386569) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434320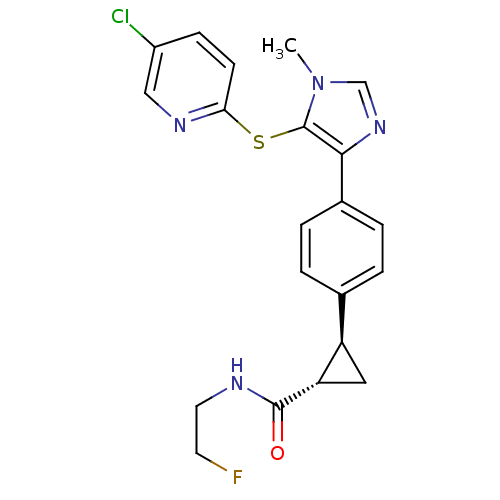 (CHEMBL2386564) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434317 (CHEMBL2386567) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434313 (CHEMBL2386571) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434319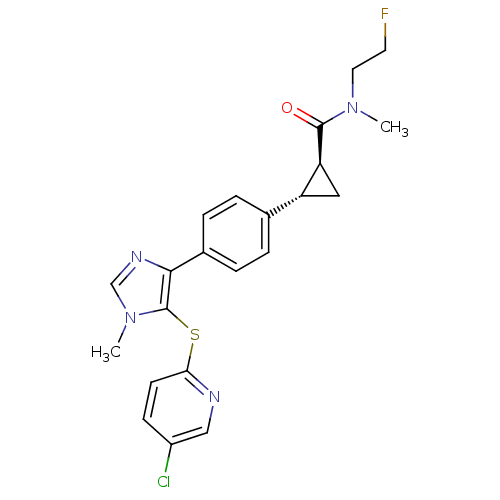 (CHEMBL2386565) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434319 (CHEMBL2386565) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434321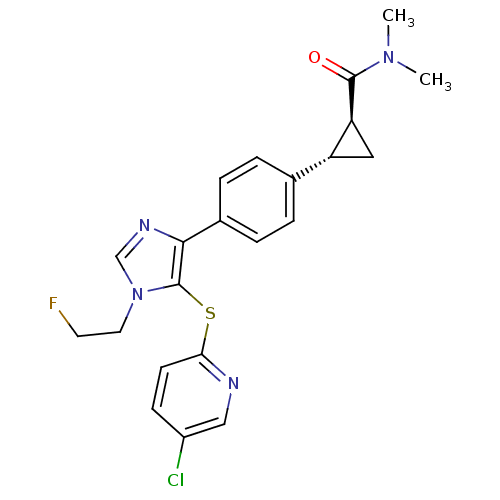 (CHEMBL2386563) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434323 (CHEMBL2386561) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM35948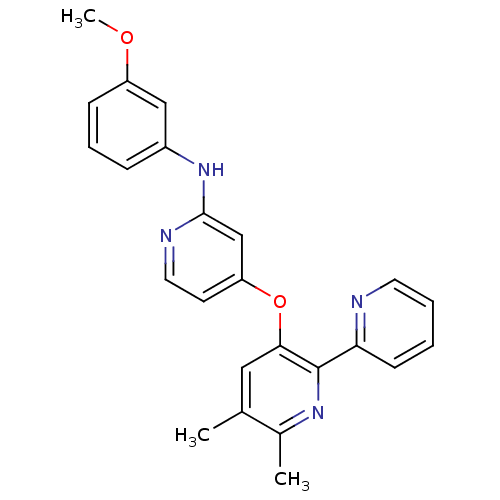 (4-pyridinoxy-2-anilinopyridine-based compound, 12) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | 32 | n/a | n/a | 7.4 | 23 |
AstraZeneca | Assay Description A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... | J Med Chem 52: 7901-5 (2009) Article DOI: 10.1021/jm900807w BindingDB Entry DOI: 10.7270/Q2KS6PXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434328 (CHEMBL2386555) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434320 (CHEMBL2386564) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434321 (CHEMBL2386563) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50434328 (CHEMBL2386555) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434322 (CHEMBL2386562) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM35949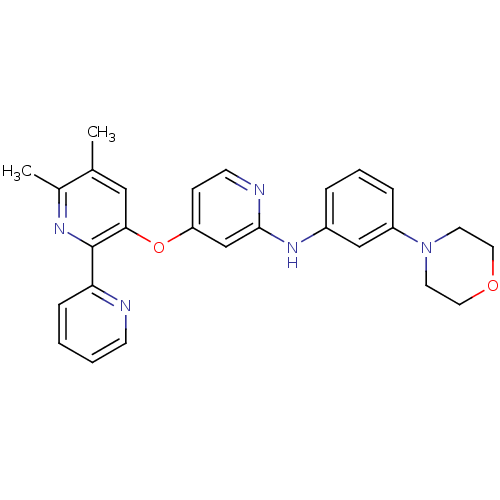 (4-pyridinoxy-2-anilinopyridine-based compound, 13) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | 62 | n/a | n/a | 7.4 | 23 |
AstraZeneca | Assay Description A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... | J Med Chem 52: 7901-5 (2009) Article DOI: 10.1021/jm900807w BindingDB Entry DOI: 10.7270/Q2KS6PXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM35946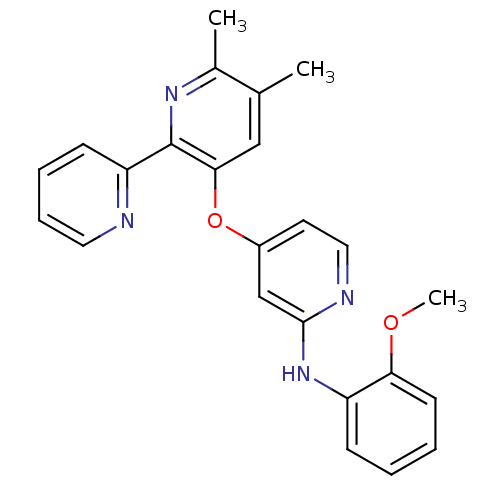 (4-pyridinoxy-2-anilinopyridine-based compound, 10) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | 114 | n/a | n/a | 7.4 | 23 |
AstraZeneca | Assay Description A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... | J Med Chem 52: 7901-5 (2009) Article DOI: 10.1021/jm900807w BindingDB Entry DOI: 10.7270/Q2KS6PXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50434323 (CHEMBL2386561) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of rat FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin p... | ACS Med Chem Lett 4: 509-13 (2013) Article DOI: 10.1021/ml4000996 BindingDB Entry DOI: 10.7270/Q21R6RWJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM35953 (4-pyridinoxy-2-anilinopyridine-based compound, 17) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | 31 | n/a | n/a | 7.4 | 23 |
AstraZeneca | Assay Description A fluorescence polarization assay was used to assess the ALK5 binding capacity and biochemical activity of compounds. ALK5 protein was added to each ... | J Med Chem 52: 7901-5 (2009) Article DOI: 10.1021/jm900807w BindingDB Entry DOI: 10.7270/Q2KS6PXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 252 total ) | Next | Last >> |