

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11694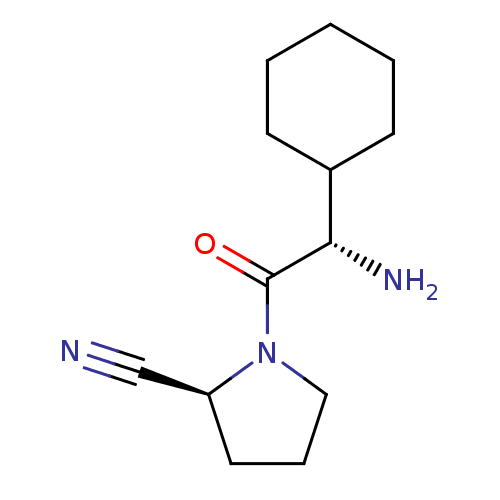 ((2S)-1-[(2S)-2-amino-2-cyclohexylacetyl]pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Binding affinity towards Dipeptidyl peptidase IV (DPP-IV) | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using midazolam as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using nifedipine as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using testosterone as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50088527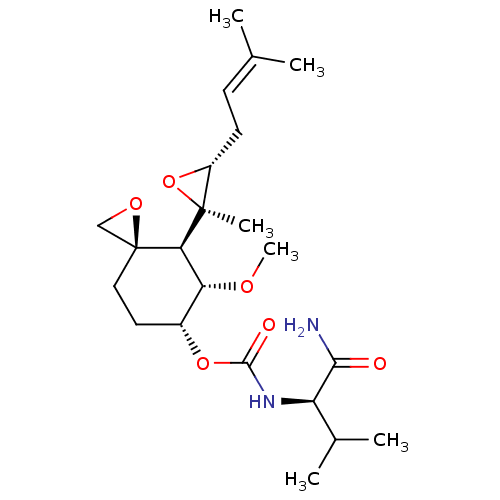 (CHEMBL3527358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using midazolam as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50088527 (CHEMBL3527358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 2.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using testosterone as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50088527 (CHEMBL3527358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 4.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using nifedipine as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11694 ((2S)-1-[(2S)-2-amino-2-cyclohexylacetyl]pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129897 (1-(2-Amino-3-methyl-butyryl)-pyrrolidine-2-carboni...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50129889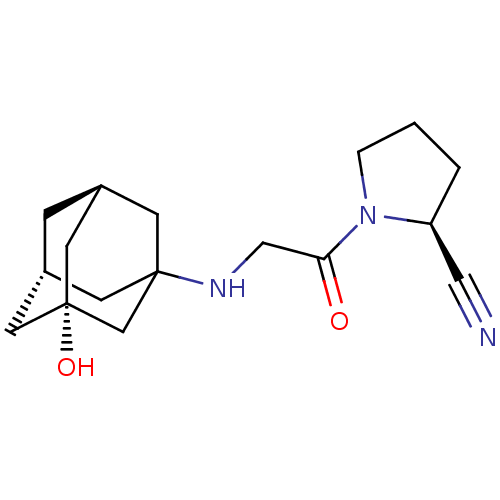 (1-[2-(3-Hydroxy-adamantan-1-ylamino)-acetyl]-pyrro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity against Dipeptidyl peptidase IV (DPP-IV) obtained from rat plasma | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129889 (1-[2-(3-Hydroxy-adamantan-1-ylamino)-acetyl]-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity against Dipeptidyl peptidase IV (DPP-IV) obtained from human plasma | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM11694 ((2S)-1-[(2S)-2-amino-2-cyclohexylacetyl]pyrrolidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity against Dipeptidyl peptidase IV (DPP-IV) obtained from rat plasma | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129945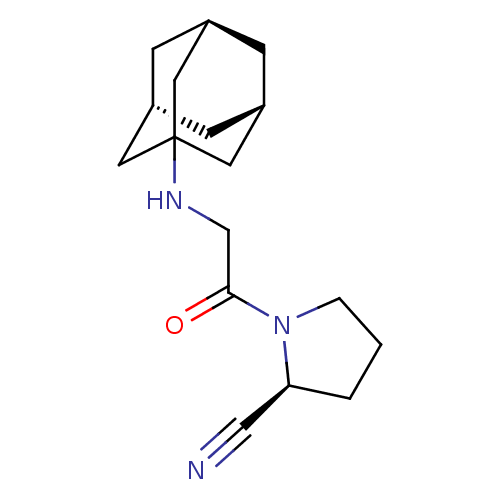 (1-[2-(Adamantan-1-ylamino)-acetyl]-pyrrolidine-2-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11694 ((2S)-1-[(2S)-2-amino-2-cyclohexylacetyl]pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity against Dipeptidyl peptidase IV (DPP-IV) obtained from human plasma | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129889 (1-[2-(3-Hydroxy-adamantan-1-ylamino)-acetyl]-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129884 (6-{4-[2-(2-Cyano-pyrrolidin-1-yl)-2-oxo-ethylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM11113 (6-{[2-({2-[(2S)-2-cyanopyrrolidin-1-yl]-2-oxoethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity against Dipeptidyl peptidase IV (DPP IV) obtained from rat plasma | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129865 (1-[2-(3-Ethyl-adamantan-1-ylamino)-acetyl]-pyrroli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11113 (6-{[2-({2-[(2S)-2-cyanopyrrolidin-1-yl]-2-oxoethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity against Dipeptidyl peptidase IV (DPP IV) obtained from human plasma | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129868 (1-{2-[4-(5-Trifluoromethyl-pyridin-2-ylamino)-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50113678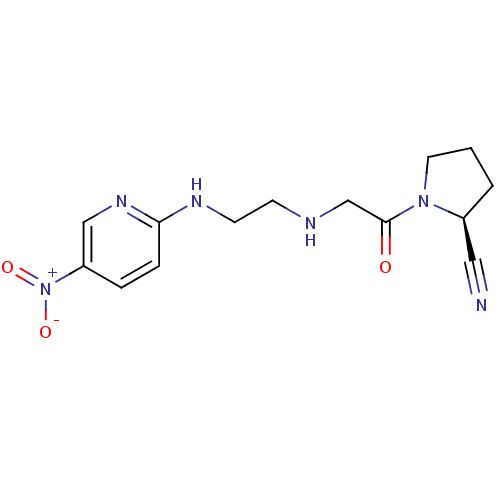 (1-{2-[2-(5-Nitro-pyridin-2-ylamino)-ethylamino]-ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50113678 (1-{2-[2-(5-Nitro-pyridin-2-ylamino)-ethylamino]-ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.73 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity against Dipeptidyl peptidase IV (DPP IV) obtained from human plasma | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes assessed as dibenzo fluuorescene oxidation up to 40 uM | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129898 (1-{2-[4-(Benzothiazol-2-ylamino)-cyclohexylamino]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129909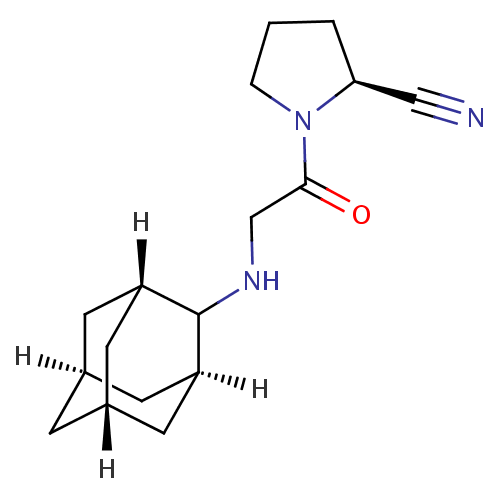 (1-[2-(Adamantan-2-ylamino)-acetyl]-pyrrolidine-2-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129925 (1-{2-[4-(4-Chloro-phenoxy)-cyclohexylamino]-acetyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129912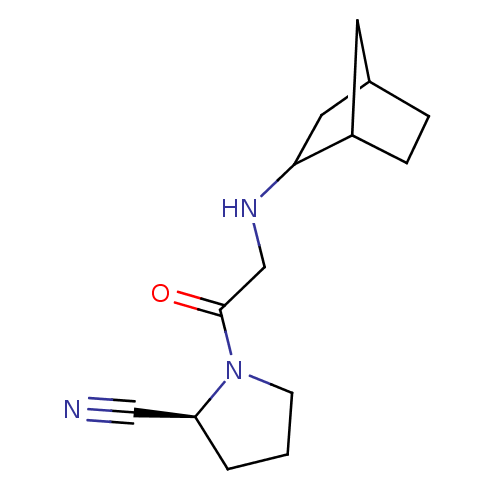 (1-[2-(Bicyclo[2.2.1]hept-2-ylamino)-acetyl]-pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129900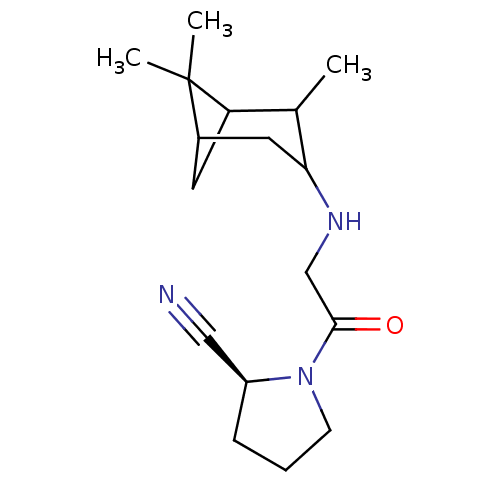 ((1R,2R,3R,5S)1-[2-(2,6,6-Trimethyl-bicyclo[3.1.1]h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129943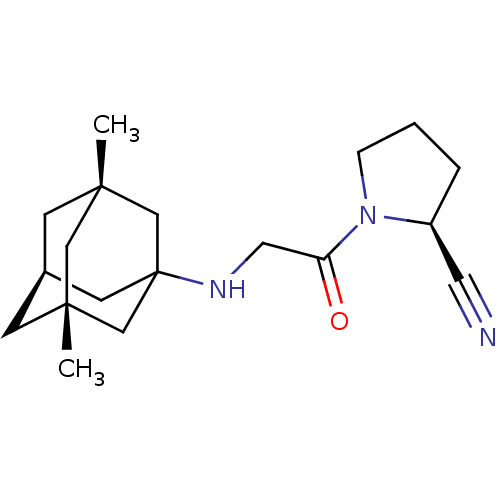 (1-[2-(3,5-Dimethyl-adamantan-1-ylamino)-acetyl]-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50113678 (1-{2-[2-(5-Nitro-pyridin-2-ylamino)-ethylamino]-ac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibitory activity against Dipeptidyl peptidase IV (DPP IV) obtained from rat plasma | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129926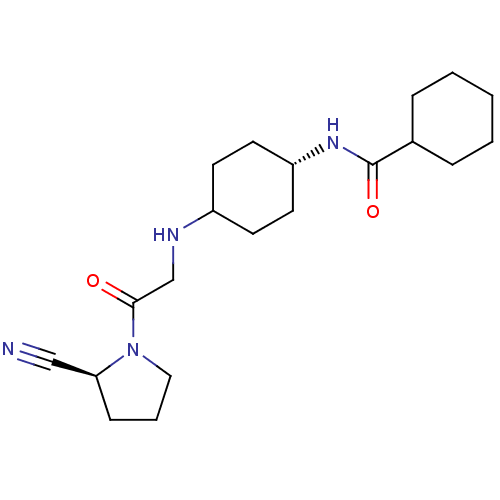 (CHEMBL92021 | Cyclohexanecarboxylic acid {4-[2-(2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11113 (6-{[2-({2-[(2S)-2-cyanopyrrolidin-1-yl]-2-oxoethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129872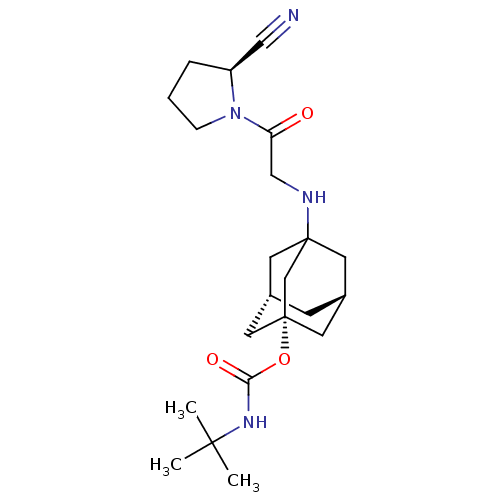 (CHEMBL319115 | tert-Butyl-carbamic acid 3-[2-(2-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129908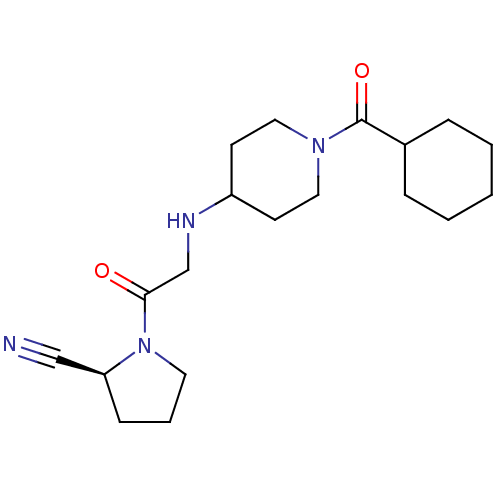 (1-[2-(1-Cyclohexanecarbonyl-piperidin-4-ylamino)-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129924 (1-[2-(1,1-Dimethyl-2-phenyl-ethylamino)-acetyl]-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129915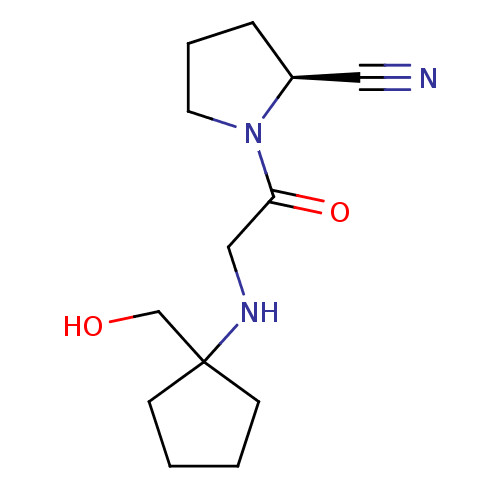 (1-[2-(1-Hydroxymethyl-cyclopentylamino)-acetyl]-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129902 (6-{2-[2-(2-Cyano-pyrrolidin-1-yl)-2-oxo-ethylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129937 (1-{2-[2-(Pyridin-2-ylamino)-ethylamino]-acetyl}-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12644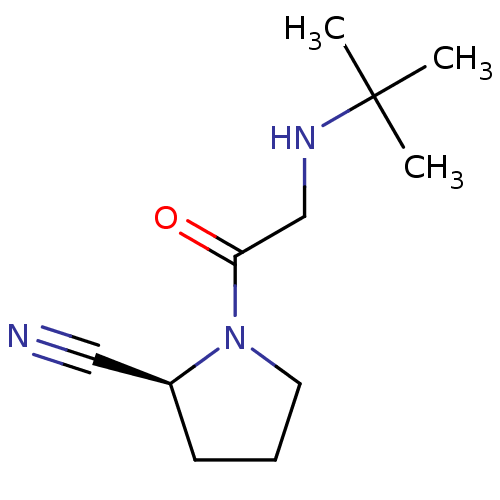 ((2S)-1-[2-(tert-butylamino)acetyl]pyrrolidine-2-ca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129940 (1-{2-[2-(5-Chloro-pyridin-2-ylamino)-ethylamino]-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129881 (1-(2-Cyclooctylamino-acetyl)-pyrrolidine-2-carboni...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM12645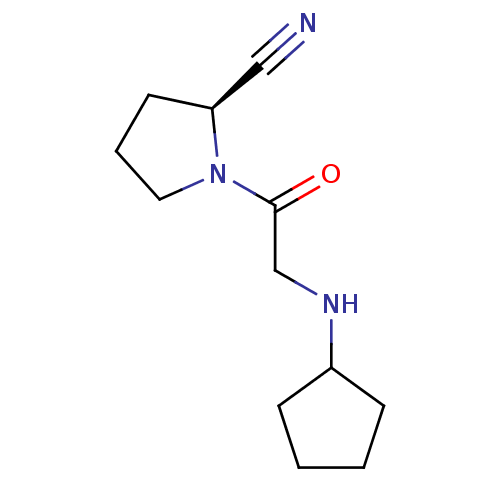 ((2S)-1-[2-(cyclopentylamino)acetyl]pyrrolidine-2-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129919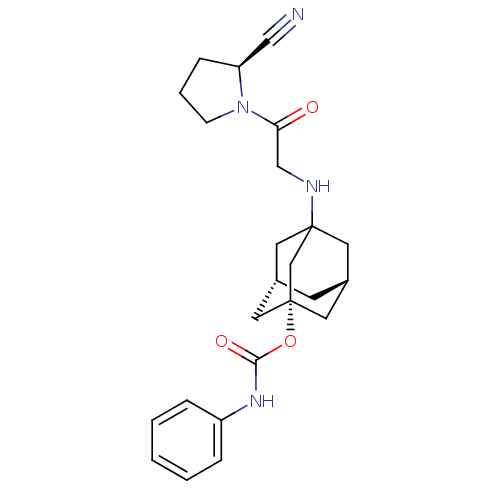 (CHEMBL330689 | Phenyl-carbamic acid 3-[2-(2-cyano-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129932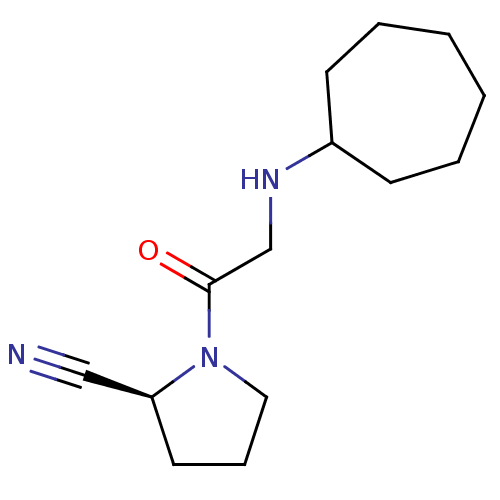 (1-(2-Cycloheptylamino-acetyl)-pyrrolidine-2-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129907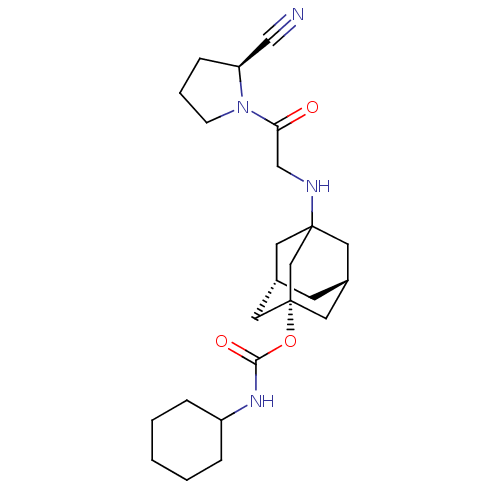 (CHEMBL95093 | Cyclohexyl-carbamic acid 3-[2-(2-cya...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129920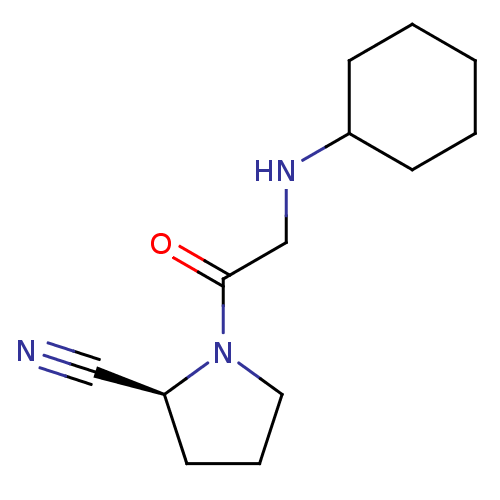 (1-(2-Cyclohexylamino-acetyl)-pyrrolidine-2-carboni...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129906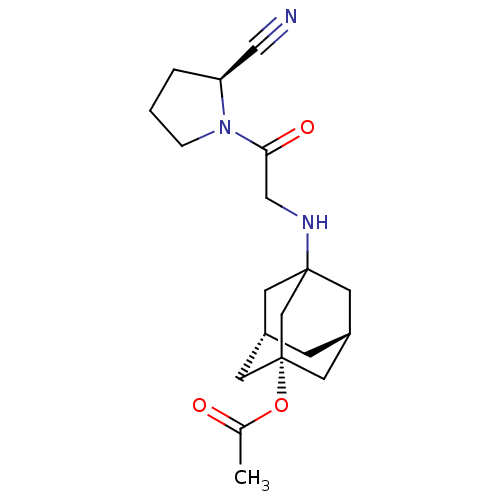 (Acetic acid 3-[2-(2-cyano-pyrrolidin-1-yl)-2-oxo-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129866 (1-(2-Cyclobutylamino-acetyl)-pyrrolidine-2-carboni...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129911 (1-{2-[2-(5-Trifluoromethyl-pyridin-2-ylamino)-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50129894 (6-{3-[2-(2-Cyano-pyrrolidin-1-yl)-2-oxo-ethylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human dipeptidyl peptidase IV (DPP IV) obtained from human colonic carcinoma cells | J Med Chem 46: 2774-89 (2003) Article DOI: 10.1021/jm030091l BindingDB Entry DOI: 10.7270/Q20001HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 147 total ) | Next | Last >> |