

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM430 (3-[(2-tert-butyl-4-hydroxy-5-methylphenyl)sulfanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | -62.5 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM430 (3-[(2-tert-butyl-4-hydroxy-5-methylphenyl)sulfanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM472 (5-tert-butyl-4-{[(6S)-4-hydroxy-6-[2-(4-hydroxyphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50409174 (CHEMBL169119) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM472 (5-tert-butyl-4-{[(6S)-4-hydroxy-6-[2-(4-hydroxyphe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50457085 (CHEMBL4203542) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at rat A2A receptor assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 mins followed by CGS-21680 addition m... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457098 (CHEMBL4217582) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human A2A receptor expressed in HEK293 cell membranes assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 ... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50457098 (CHEMBL4217582) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at rat A2A receptor assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 mins followed by CGS-21680 addition m... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM465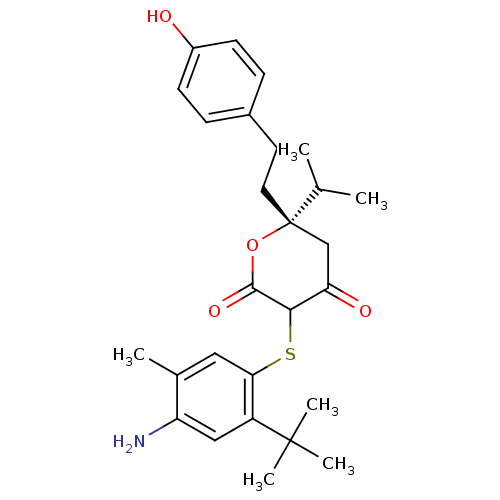 ((6S)-3-[(4-amino-2-tert-butyl-5-methylphenyl)sulfa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | 6.2 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards HIV protease was determined | Bioorg Med Chem Lett 9: 1481-6 (1999) BindingDB Entry DOI: 10.7270/Q2668CC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM465 ((6S)-3-[(4-amino-2-tert-butyl-5-methylphenyl)sulfa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards HIV protease was determined | Bioorg Med Chem Lett 9: 1481-6 (1999) BindingDB Entry DOI: 10.7270/Q2668CC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457084 (CHEMBL4217248) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human A2A receptor expressed in HEK293 cell membranes assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 ... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM465 ((6S)-3-[(4-amino-2-tert-butyl-5-methylphenyl)sulfa...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2208 ((6S)-3-{[2-tert-butyl-4-(hydroxymethyl)-5-methylph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2204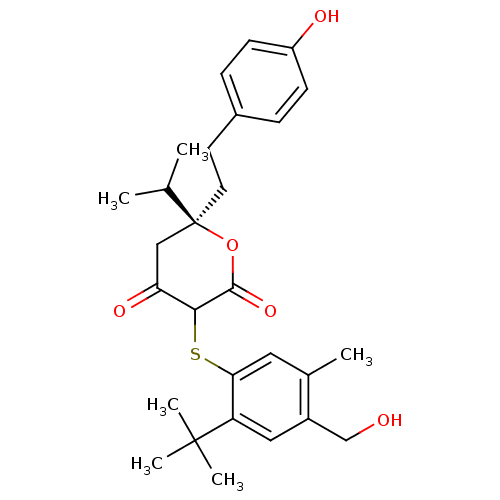 ((3-(2-tert-Butyl-4-hydroxymethyl-5-methyl-phenylsu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50078088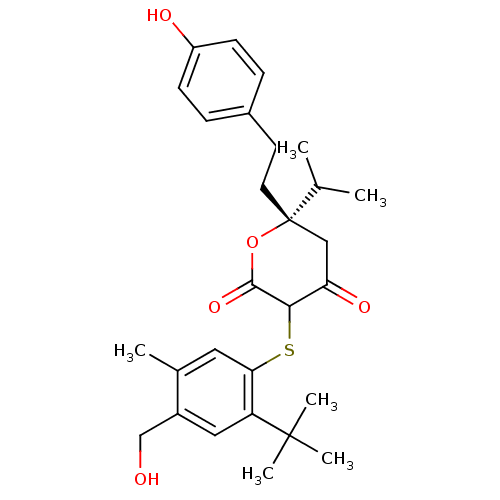 ((S)-3-(2-tert-Butyl-4-hydroxymethyl-5-methyl-pheny...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | 6.2 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against HIV protease at pH 6.2 was determined | Bioorg Med Chem Lett 9: 1481-6 (1999) BindingDB Entry DOI: 10.7270/Q2668CC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50078088 ((S)-3-(2-tert-Butyl-4-hydroxymethyl-5-methyl-pheny...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | 4.7 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against human immunodeficiency virus type 1(HIV-1) protease at pH 4.7 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50216785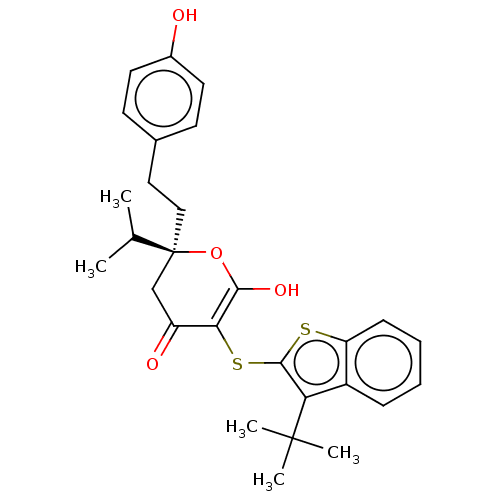 (CHEMBL61756) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | 4.7 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against human immunodeficiency virus type 1(HIV-1) protease at pH 6.2 | Bioorg Med Chem Lett 9: 2019-24 (1999) BindingDB Entry DOI: 10.7270/Q2PZ5814 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50368427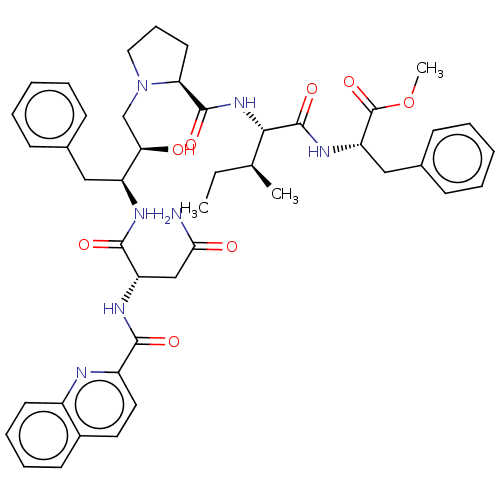 (CHEMBL1790880 | CHEMBL3349491) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Binding affinity to HIV protease | J Med Chem 35: 3803-12 (1992) BindingDB Entry DOI: 10.7270/Q2348M0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50078087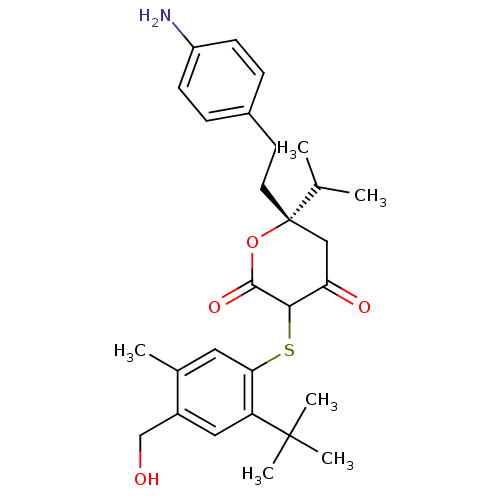 ((S)-6-[2-(4-Amino-phenyl)-ethyl]-3-(2-tert-butyl-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | 6.2 | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against HIV protease at pH 6.2 was determined | Bioorg Med Chem Lett 9: 1481-6 (1999) BindingDB Entry DOI: 10.7270/Q2668CC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2206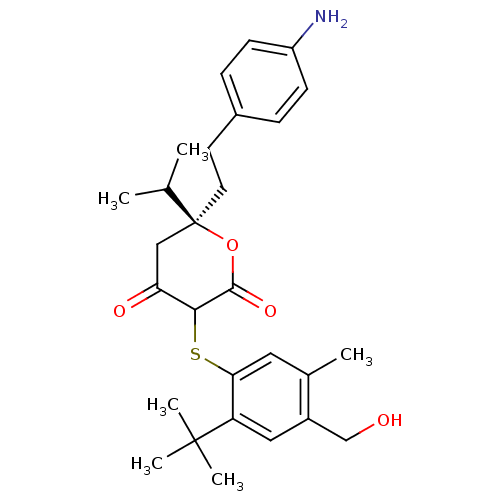 ((6S)-6-[2-(4-aminophenyl)ethyl]-3-{[2-tert-butyl-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457085 (CHEMBL4203542) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human A2A receptor expressed in HEK293 cell membranes assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 ... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM2533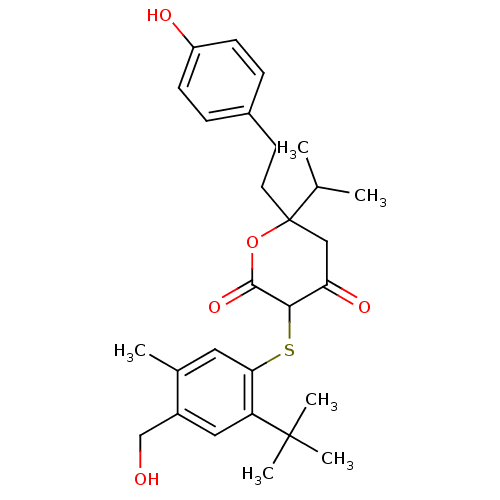 (3-{[2-tert-butyl-4-(hydroxymethyl)-5-methylphenyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.170 | -58.0 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM469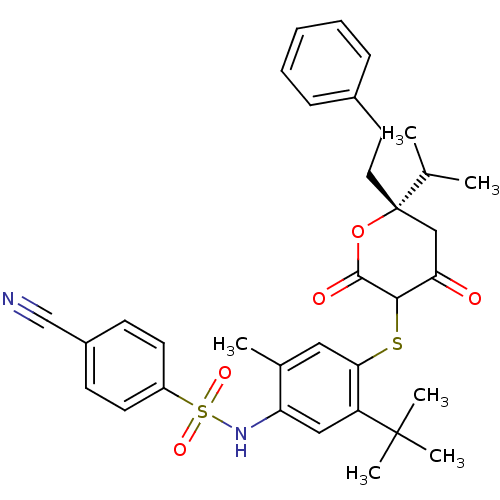 (CHEMBL2110206 | Dihydropyran-2-one deriv. 74 | N-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50235055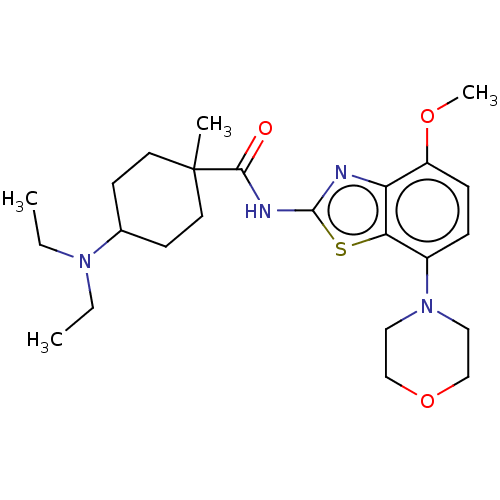 (CHEMBL4095355) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor expressed in HEK293 cell membranes assessed as decrease in CGS-21680/forskolin-induced cAMP level... | J Med Chem 60: 681-694 (2017) Article DOI: 10.1021/acs.jmedchem.6b01584 BindingDB Entry DOI: 10.7270/Q23J3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457093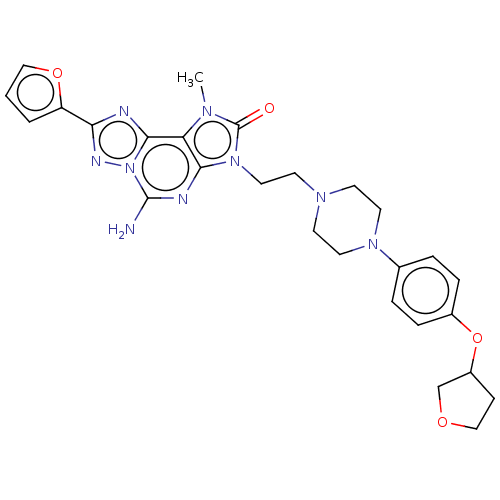 (CHEMBL4212821) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes after 90 mins radioligand binding assay | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM469 (CHEMBL2110206 | Dihydropyran-2-one deriv. 74 | N-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM402 (CHEMBL354027 | Dihydropyran-2-one deriv. 7 | N-[5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | -57.5 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM402 (CHEMBL354027 | Dihydropyran-2-one deriv. 7 | N-[5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457099 (CHEMBL4213177) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human A2A receptor expressed in HEK293 cell membranes assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 ... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457093 (CHEMBL4212821) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human A2A receptor expressed in HEK293 cell membranes assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 ... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50368430 (CHEMBL1790874 | CHEMBL3349485) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Binding affinity to HIV protease | J Med Chem 35: 3803-12 (1992) BindingDB Entry DOI: 10.7270/Q2348M0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50235053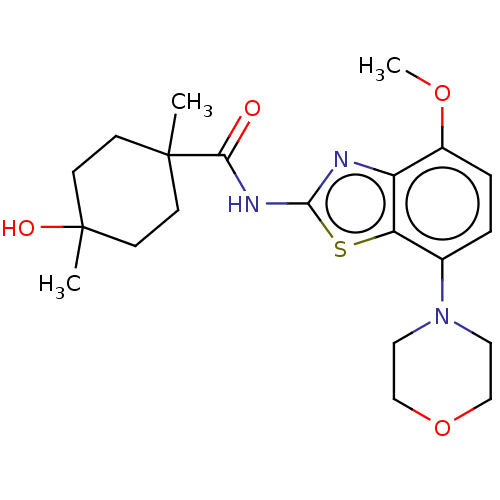 (CHEMBL4064207) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor expressed in HEK293 cell membranes assessed as decrease in CGS-21680/forskolin-induced cAMP level... | J Med Chem 60: 681-694 (2017) Article DOI: 10.1021/acs.jmedchem.6b01584 BindingDB Entry DOI: 10.7270/Q23J3G74 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457097 (CHEMBL4205884) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human A2A receptor expressed in HEK293 cell membranes assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 ... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457092 (CHEMBL4207914) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human A2A receptor expressed in HEK293 cell membranes assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 ... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457103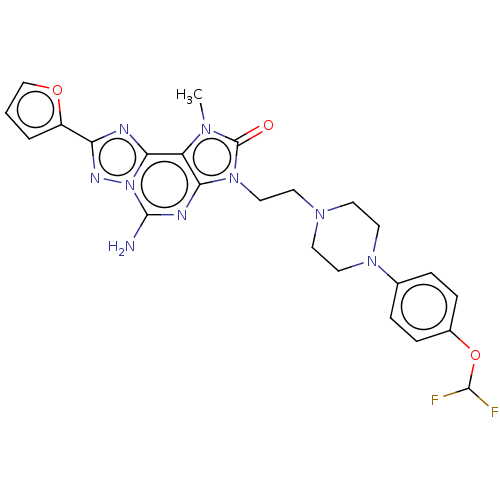 (CHEMBL4217668) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human A2A receptor expressed in HEK293 cell membranes assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 ... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50457087 (CHEMBL4205341) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at rat A2A receptor assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 mins followed by CGS-21680 addition m... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457092 (CHEMBL4207914) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes after 90 mins radioligand binding assay | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50011294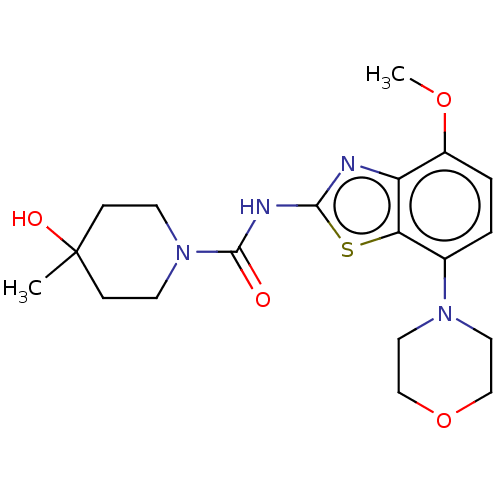 (A2A | Ro-4494351 | Ro-4494351-002 | Ro-4494351000 ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2A receptor expressed in HEK293 cell membranes assessed as decrease in CGS-21680/forskolin-induced cAMP level... | J Med Chem 60: 681-694 (2017) Article DOI: 10.1021/acs.jmedchem.6b01584 BindingDB Entry DOI: 10.7270/Q23J3G74 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457105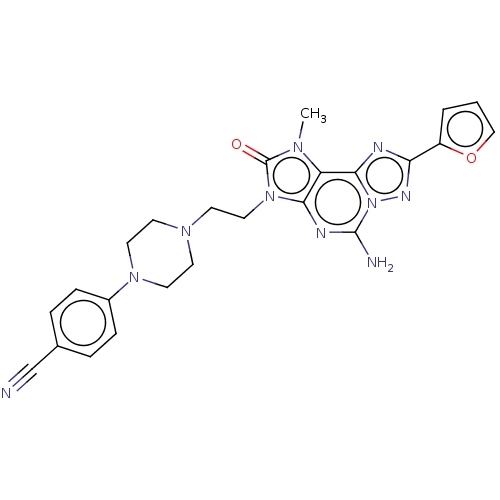 (CHEMBL4212278) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human A2A receptor expressed in HEK293 cell membranes assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 ... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457097 (CHEMBL4205884) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes after 90 mins radioligand binding assay | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM470 (CHEMBL2110205 | Dihydropyran-2-one deriv. 75 | N-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM467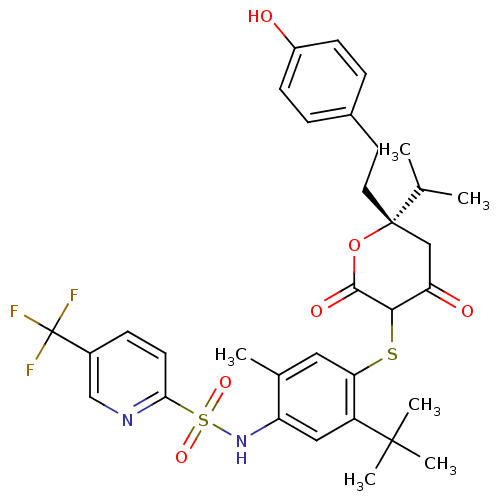 ((S)-N-(5-tert-Butyl-4-{4-hydroxy-6-[2-(4-hydroxyph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Tested for binding affinity against HIV protease | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM467 ((S)-N-(5-tert-Butyl-4-{4-hydroxy-6-[2-(4-hydroxyph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 43: 843-58 (2000) Article DOI: 10.1021/jm990281p BindingDB Entry DOI: 10.7270/Q21N7Z9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457085 (CHEMBL4203542) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes after 90 mins radioligand binding assay | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457098 (CHEMBL4217582) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes after 90 mins radioligand binding assay | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457091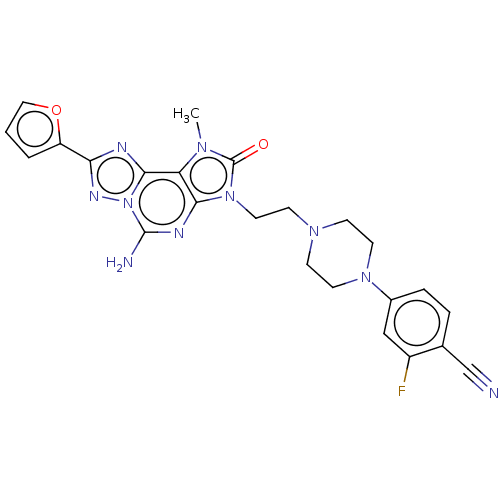 (CHEMBL4207104) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human A2A receptor expressed in HEK293 cell membranes assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 ... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50368420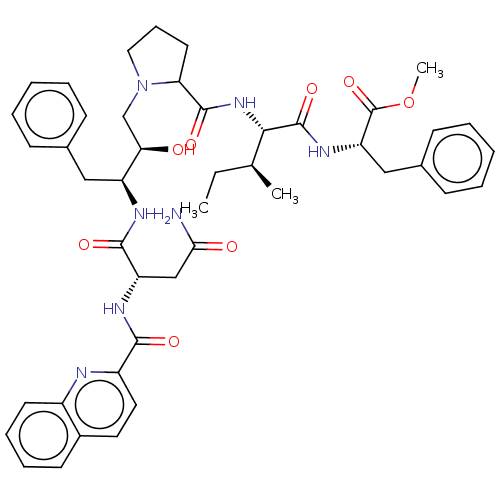 (CHEMBL1790890 | CHEMBL3349500) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wisconsin-Madison Curated by ChEMBL | Assay Description Binding affinity to HIV protease | J Med Chem 35: 3803-12 (1992) BindingDB Entry DOI: 10.7270/Q2348M0D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457107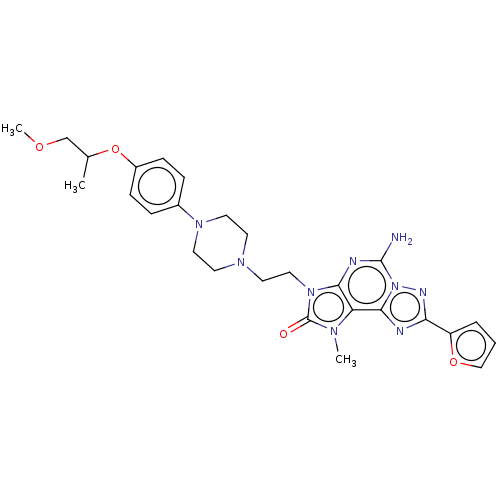 (CHEMBL4209408) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]ZM241385 from human A2A receptor expressed in HEK293 cell membranes after 90 mins radioligand binding assay | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457086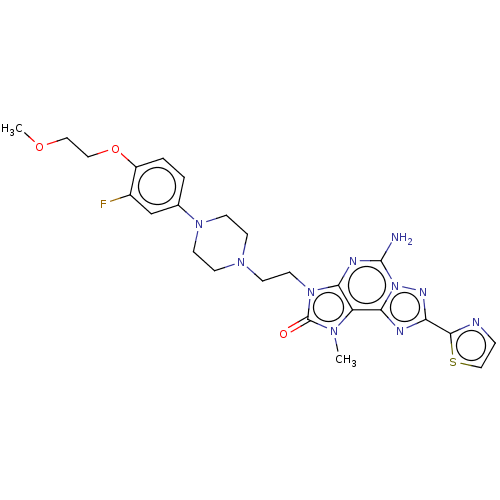 (CHEMBL4215754) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human A2A receptor expressed in HEK293 cell membranes assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 ... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50457104 (CHEMBL4217235) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human A2A receptor expressed in HEK293 cell membranes assessed as reduction in CGS-21680-induced cAMP level pretreated for 15 ... | ACS Med Chem Lett 8: 835-840 (2017) Article DOI: 10.1021/acsmedchemlett.7b00175 BindingDB Entry DOI: 10.7270/Q2571FMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1219 total ) | Next | Last >> |