 Found 2062 hits with Last Name = 'prasit' and Initial = 'p'
Found 2062 hits with Last Name = 'prasit' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19854
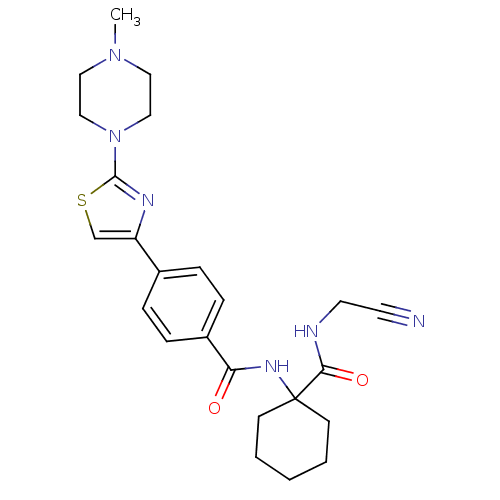
(CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...)Show SMILES CN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C24H30N6O2S/c1-29-13-15-30(16-14-29)23-27-20(17-33-23)18-5-7-19(8-6-18)21(31)28-24(9-3-2-4-10-24)22(32)26-12-11-25/h5-8,17H,2-4,9-10,12-16H2,1H3,(H,26,32)(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410611
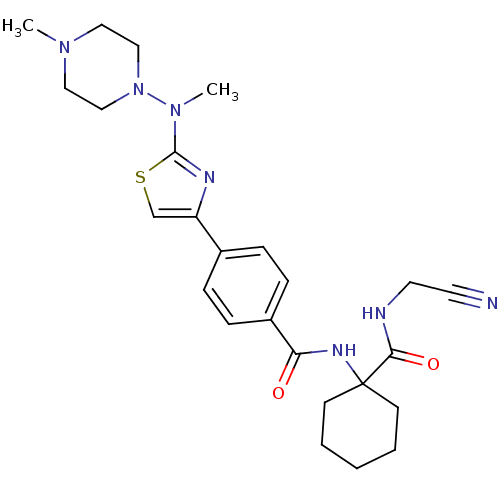
(CHEMBL414669)Show SMILES CN(N1CCN(C)CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C25H33N7O2S/c1-30-14-16-32(17-15-30)31(2)24-28-21(18-35-24)19-6-8-20(9-7-19)22(33)29-25(10-4-3-5-11-25)23(34)27-13-12-26/h6-9,18H,3-5,10-11,13-17H2,1-2H3,(H,27,34)(H,29,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410588
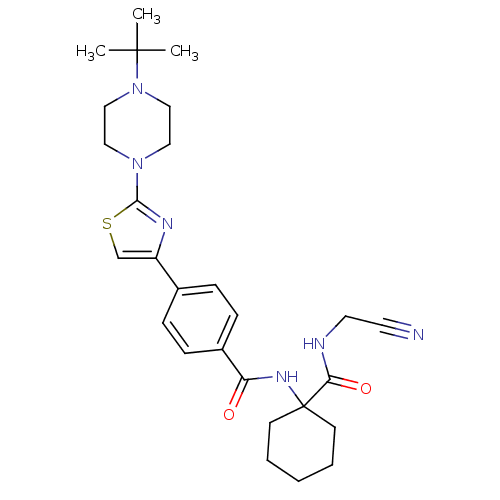
(CHEMBL200708)Show SMILES CC(C)(C)N1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C27H36N6O2S/c1-26(2,3)33-17-15-32(16-18-33)25-30-22(19-36-25)20-7-9-21(10-8-20)23(34)31-27(11-5-4-6-12-27)24(35)29-14-13-28/h7-10,19H,4-6,11-12,14-18H2,1-3H3,(H,29,35)(H,31,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410609
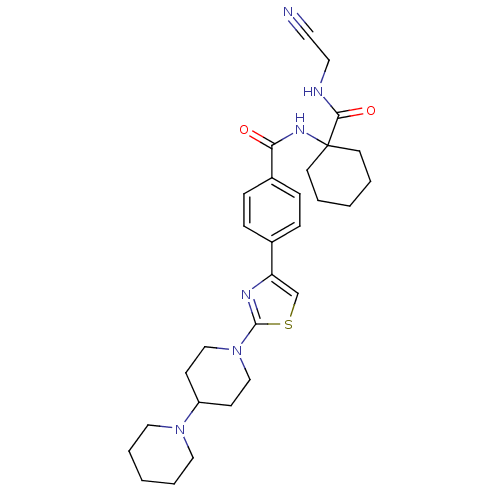
(CHEMBL198798)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1csc(n1)N1CCC(CC1)N1CCCCC1 Show InChI InChI=1S/C29H38N6O2S/c30-15-16-31-27(37)29(13-3-1-4-14-29)33-26(36)23-9-7-22(8-10-23)25-21-38-28(32-25)35-19-11-24(12-20-35)34-17-5-2-6-18-34/h7-10,21,24H,1-6,11-14,16-20H2,(H,31,37)(H,33,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410590
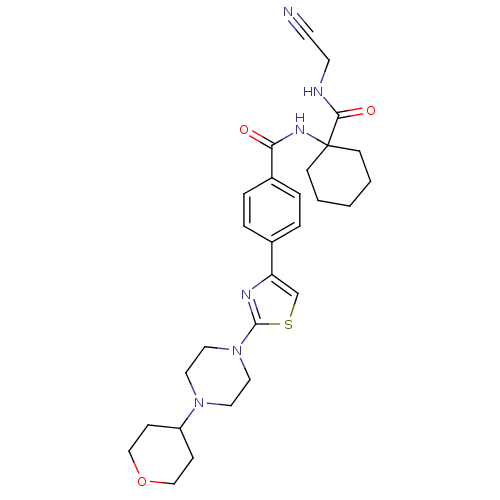
(CHEMBL200543)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1csc(n1)N1CCN(CC1)C1CCOCC1 Show InChI InChI=1S/C28H36N6O3S/c29-12-13-30-26(36)28(10-2-1-3-11-28)32-25(35)22-6-4-21(5-7-22)24-20-38-27(31-24)34-16-14-33(15-17-34)23-8-18-37-19-9-23/h4-7,20,23H,1-3,8-11,13-19H2,(H,30,36)(H,32,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410587
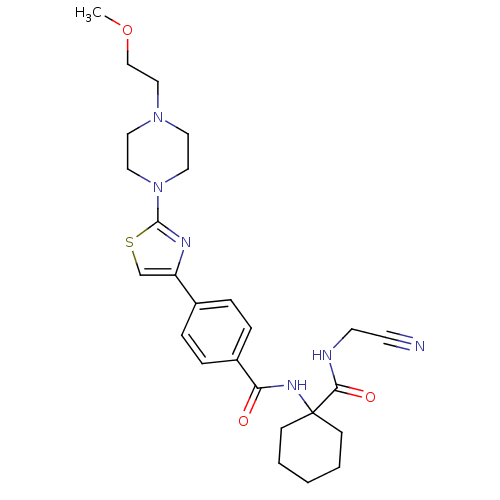
(CHEMBL200602)Show SMILES COCCN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C26H34N6O3S/c1-35-18-17-31-13-15-32(16-14-31)25-29-22(19-36-25)20-5-7-21(8-6-20)23(33)30-26(9-3-2-4-10-26)24(34)28-12-11-27/h5-8,19H,2-4,9-10,12-18H2,1H3,(H,28,34)(H,30,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19854

(CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...)Show SMILES CN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C24H30N6O2S/c1-29-13-15-30(16-14-29)23-27-20(17-33-23)18-5-7-19(8-6-18)21(31)28-24(9-3-2-4-10-24)22(32)26-12-11-25/h5-8,17H,2-4,9-10,12-16H2,1H3,(H,26,32)(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410607
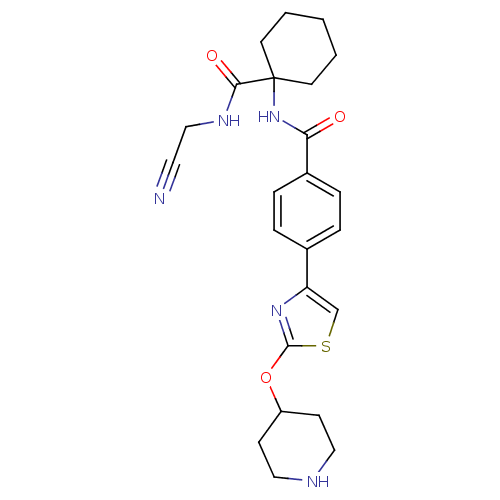
(CHEMBL200744)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1csc(OC2CCNCC2)n1 Show InChI InChI=1S/C24H29N5O3S/c25-12-15-27-22(31)24(10-2-1-3-11-24)29-21(30)18-6-4-17(5-7-18)20-16-33-23(28-20)32-19-8-13-26-14-9-19/h4-7,16,19,26H,1-3,8-11,13-15H2,(H,27,31)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410571
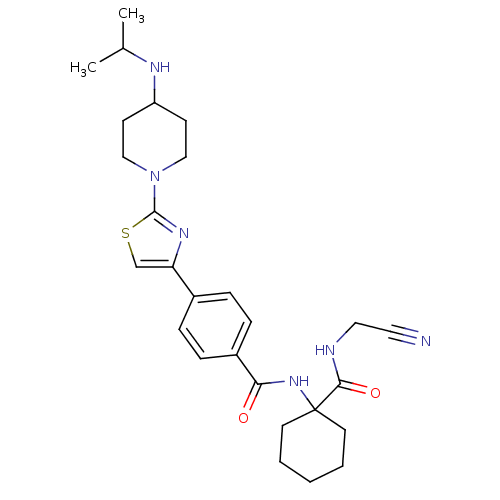
(CHEMBL200287)Show SMILES CC(C)NC1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C27H36N6O2S/c1-19(2)30-22-10-16-33(17-11-22)26-31-23(18-36-26)20-6-8-21(9-7-20)24(34)32-27(12-4-3-5-13-27)25(35)29-15-14-28/h6-9,18-19,22,30H,3-5,10-13,15-17H2,1-2H3,(H,29,35)(H,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410580
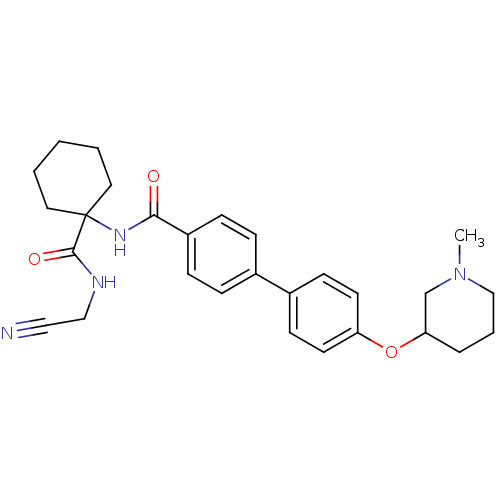
(CHEMBL435913)Show SMILES CN1CCCC(C1)Oc1ccc(cc1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C28H34N4O3/c1-32-19-5-6-25(20-32)35-24-13-11-22(12-14-24)21-7-9-23(10-8-21)26(33)31-28(15-3-2-4-16-28)27(34)30-18-17-29/h7-14,25H,2-6,15-16,18-20H2,1H3,(H,30,34)(H,31,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410575
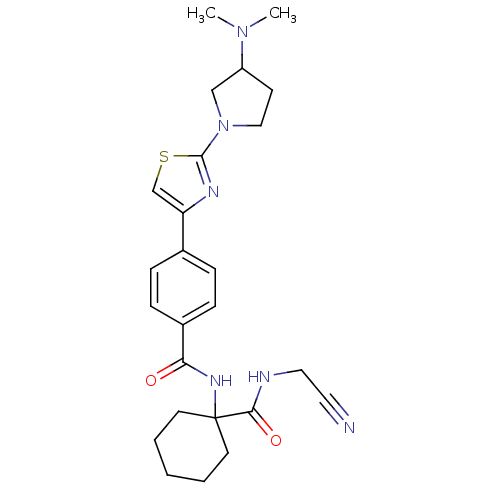
(CHEMBL199470)Show SMILES CN(C)C1CCN(C1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C25H32N6O2S/c1-30(2)20-10-15-31(16-20)24-28-21(17-34-24)18-6-8-19(9-7-18)22(32)29-25(11-4-3-5-12-25)23(33)27-14-13-26/h6-9,17,20H,3-5,10-12,14-16H2,1-2H3,(H,27,33)(H,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410591

(CHEMBL200506)Show SMILES CN1CCN(Cc2nc(cs2)-c2ccc(cc2)C(=O)NC2(CCCCC2)C(=O)NCC#N)CC1 Show InChI InChI=1S/C25H32N6O2S/c1-30-13-15-31(16-14-30)17-22-28-21(18-34-22)19-5-7-20(8-6-19)23(32)29-25(9-3-2-4-10-25)24(33)27-12-11-26/h5-8,18H,2-4,9-10,12-17H2,1H3,(H,27,33)(H,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410612
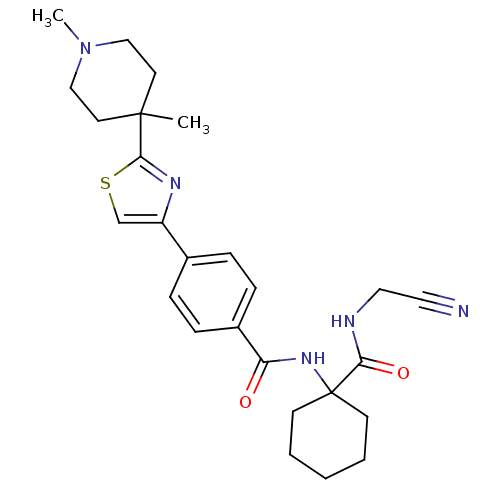
(CHEMBL200166)Show SMILES CN1CCC(C)(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C26H33N5O2S/c1-25(12-16-31(2)17-13-25)24-29-21(18-34-24)19-6-8-20(9-7-19)22(32)30-26(10-4-3-5-11-26)23(33)28-15-14-27/h6-9,18H,3-5,10-13,15-17H2,1-2H3,(H,28,33)(H,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410595
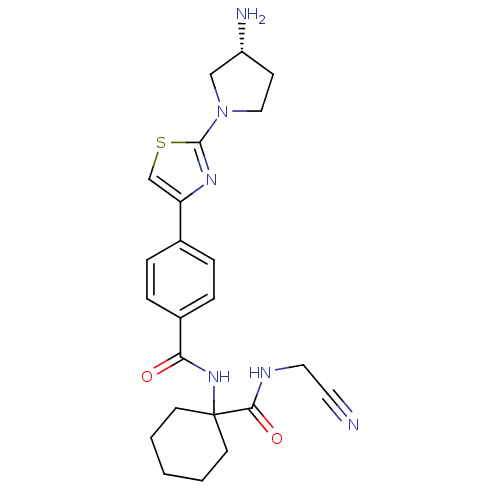
(CHEMBL200507)Show SMILES N[C@@H]1CCN(C1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C23H28N6O2S/c24-11-12-26-21(31)23(9-2-1-3-10-23)28-20(30)17-6-4-16(5-7-17)19-15-32-22(27-19)29-13-8-18(25)14-29/h4-7,15,18H,1-3,8-10,12-14,25H2,(H,26,31)(H,28,30)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19855
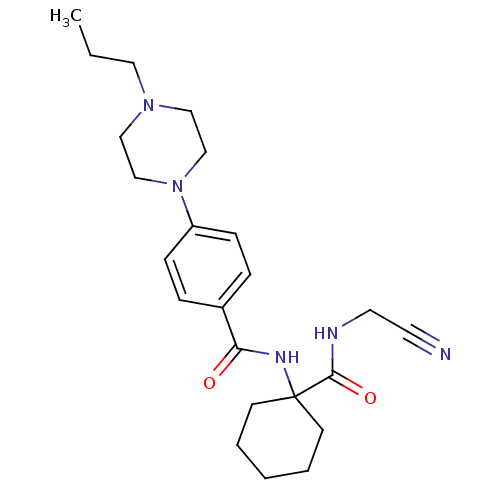
(Balicatib | CHEMBL371064 | N-[1-(cyanomethylcarbam...)Show SMILES CCCN1CCN(CC1)c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C23H33N5O2/c1-2-14-27-15-17-28(18-16-27)20-8-6-19(7-9-20)21(29)26-23(10-4-3-5-11-23)22(30)25-13-12-24/h6-9H,2-5,10-11,13-18H2,1H3,(H,25,30)(H,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410572
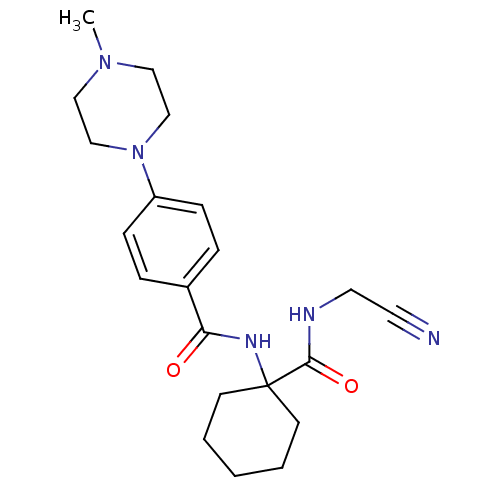
(CHEMBL440035)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C21H29N5O2/c1-25-13-15-26(16-14-25)18-7-5-17(6-8-18)19(27)24-21(9-3-2-4-10-21)20(28)23-12-11-22/h5-8H,2-4,9-10,12-16H2,1H3,(H,23,28)(H,24,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410594
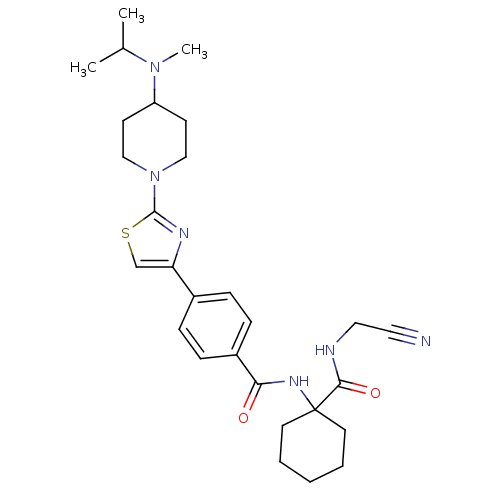
(CHEMBL200596)Show SMILES CC(C)N(C)C1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C28H38N6O2S/c1-20(2)33(3)23-11-17-34(18-12-23)27-31-24(19-37-27)21-7-9-22(10-8-21)25(35)32-28(13-5-4-6-14-28)26(36)30-16-15-29/h7-10,19-20,23H,4-6,11-14,16-18H2,1-3H3,(H,30,36)(H,32,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410592
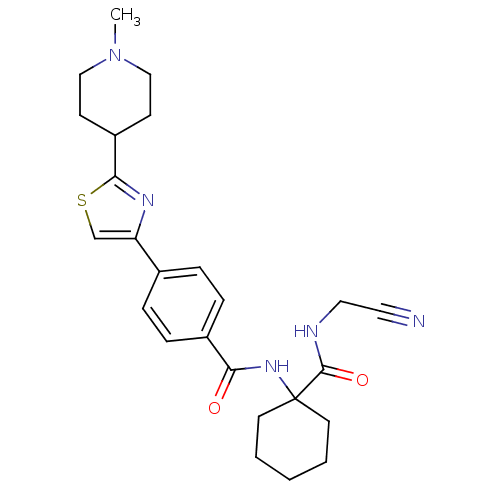
(CHEMBL200455)Show SMILES CN1CCC(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C25H31N5O2S/c1-30-15-9-20(10-16-30)23-28-21(17-33-23)18-5-7-19(8-6-18)22(31)29-25(11-3-2-4-12-25)24(32)27-14-13-26/h5-8,17,20H,2-4,9-12,14-16H2,1H3,(H,27,32)(H,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410606
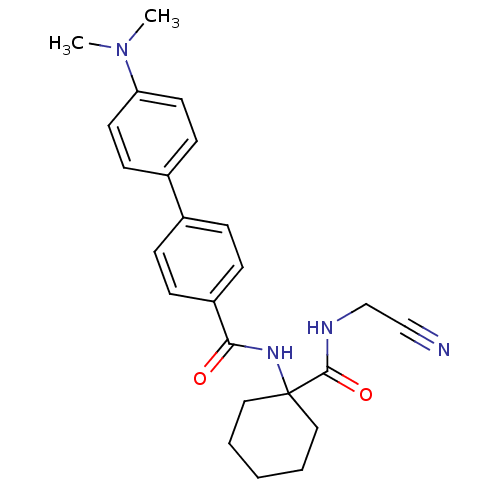
(CHEMBL383186)Show SMILES CN(C)c1ccc(cc1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C24H28N4O2/c1-28(2)21-12-10-19(11-13-21)18-6-8-20(9-7-18)22(29)27-24(14-4-3-5-15-24)23(30)26-17-16-25/h6-13H,3-5,14-15,17H2,1-2H3,(H,26,30)(H,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19857
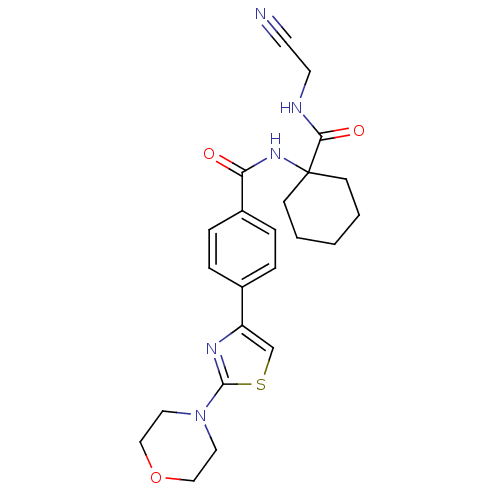
(N-{1-[(cyanomethyl)carbamoyl]cyclohexyl}-4-[2-(mor...)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1csc(n1)N1CCOCC1 Show InChI InChI=1S/C23H27N5O3S/c24-10-11-25-21(30)23(8-2-1-3-9-23)27-20(29)18-6-4-17(5-7-18)19-16-32-22(26-19)28-12-14-31-15-13-28/h4-7,16H,1-3,8-9,11-15H2,(H,25,30)(H,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410603
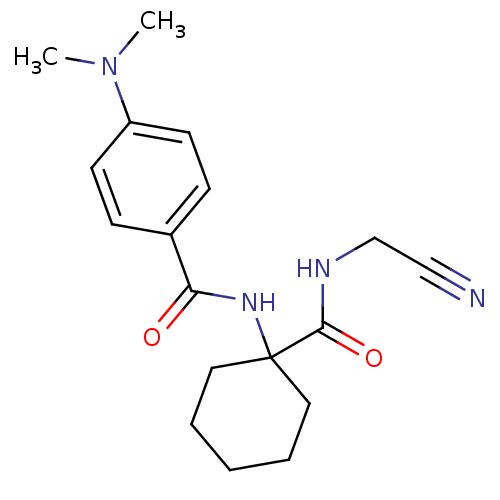
(CHEMBL198859)Show InChI InChI=1S/C18H24N4O2/c1-22(2)15-8-6-14(7-9-15)16(23)21-18(10-4-3-5-11-18)17(24)20-13-12-19/h6-9H,3-5,10-11,13H2,1-2H3,(H,20,24)(H,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410578
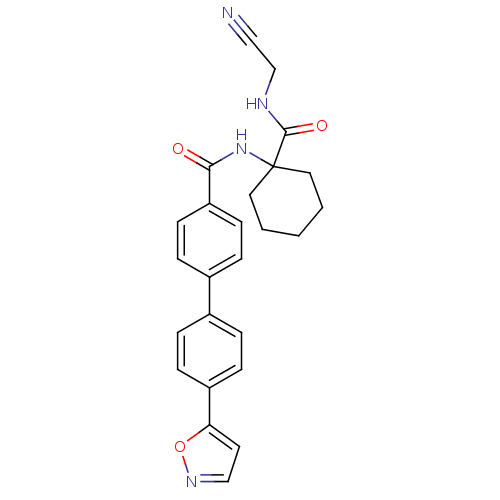
(CHEMBL199014)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1ccc(cc1)-c1ccno1 Show InChI InChI=1S/C25H24N4O3/c26-15-17-27-24(31)25(13-2-1-3-14-25)29-23(30)21-10-6-19(7-11-21)18-4-8-20(9-5-18)22-12-16-28-32-22/h4-12,16H,1-3,13-14,17H2,(H,27,31)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410593
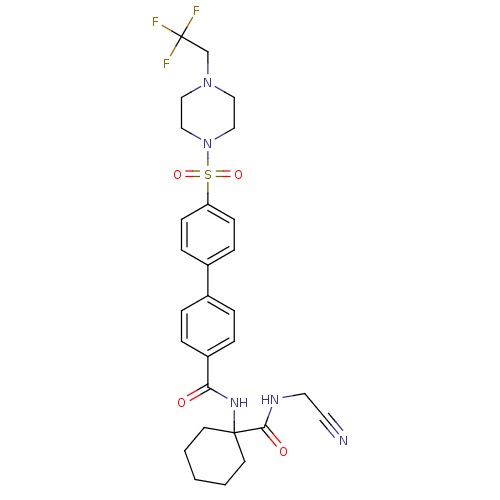
(CHEMBL197440)Show SMILES FC(F)(F)CN1CCN(CC1)S(=O)(=O)c1ccc(cc1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C28H32F3N5O4S/c29-28(30,31)20-35-16-18-36(19-17-35)41(39,40)24-10-8-22(9-11-24)21-4-6-23(7-5-21)25(37)34-27(12-2-1-3-13-27)26(38)33-15-14-32/h4-11H,1-3,12-13,15-20H2,(H,33,38)(H,34,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410589
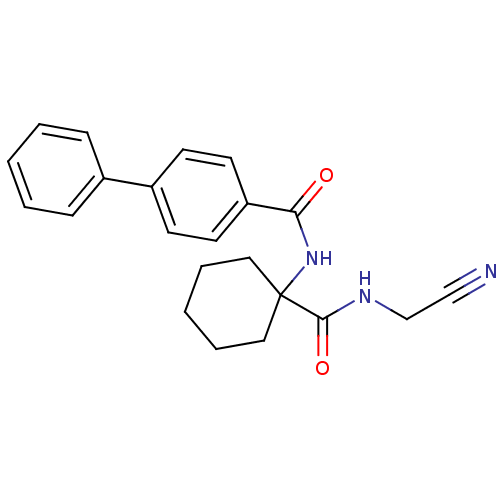
(CHEMBL200619)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C22H23N3O2/c23-15-16-24-21(27)22(13-5-2-6-14-22)25-20(26)19-11-9-18(10-12-19)17-7-3-1-4-8-17/h1,3-4,7-12H,2,5-6,13-14,16H2,(H,24,27)(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50174201
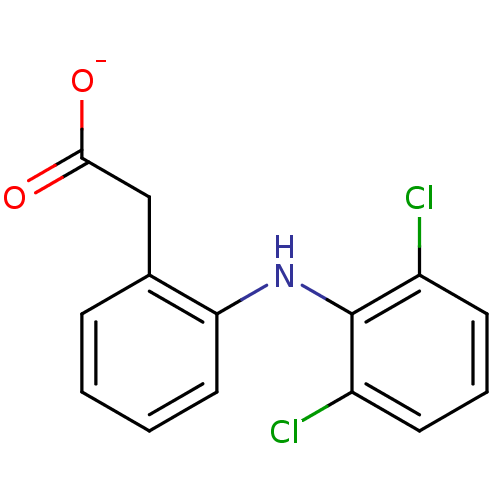
(ARTHROTEC | GP 45840 | SOLARAZE | Sodium; [2-(2,6-...)Show InChI InChI=1S/C14H11Cl2NO2/c15-10-5-3-6-11(16)14(10)17-12-7-2-1-4-9(12)8-13(18)19/h1-7,17H,8H2,(H,18,19)/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50287803

((2-Methyl-3-morpholin-4-ylmethyl-indol-1-yl)-napht...)Show SMILES Cc1c(CN2CCOCC2)c2ccccc2n1C(=O)c1cccc2ccccc12 Show InChI InChI=1S/C25H24N2O2/c1-18-23(17-26-13-15-29-16-14-26)21-10-4-5-12-24(21)27(18)25(28)22-11-6-8-19-7-2-3-9-20(19)22/h2-12H,13-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for binding affinity against recombinant human peripheral cannabinoid receptor 2 |
Bioorg Med Chem Lett 6: 2263-2268 (1996)
Article DOI: 10.1016/0960-894X(96)00426-X
BindingDB Entry DOI: 10.7270/Q2MS3SRN |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410574
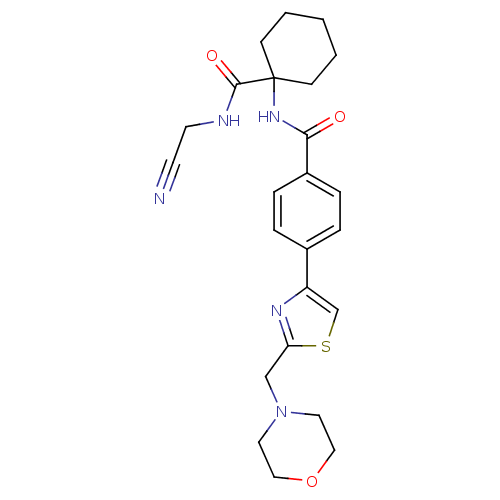
(CHEMBL381026)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1csc(CN2CCOCC2)n1 Show InChI InChI=1S/C24H29N5O3S/c25-10-11-26-23(31)24(8-2-1-3-9-24)28-22(30)19-6-4-18(5-7-19)20-17-33-21(27-20)16-29-12-14-32-15-13-29/h4-7,17H,1-3,8-9,11-16H2,(H,26,31)(H,28,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Rattus norvegicus (Rat)) | BDBM50151910

(3-Fluoro-5-(5-pyridin-2-yl-tetrazol-2-yl)-benzonit...)Show InChI InChI=1S/C13H7FN6/c14-10-5-9(8-15)6-11(7-10)20-18-13(17-19-20)12-3-1-2-4-16-12/h1-7H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from glutamate 5 receptor of rat cortical membranes |
J Med Chem 47: 4645-8 (2004)
Article DOI: 10.1021/jm049828c
BindingDB Entry DOI: 10.7270/Q2DJ5F3G |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50234418
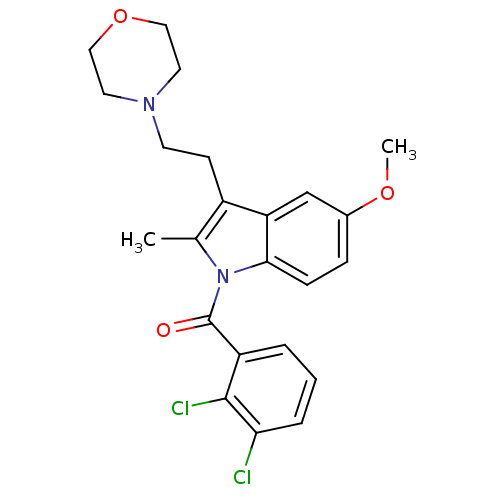
((2,3-Dichloro-phenyl)-[5-methoxy-2-methyl-3-(2-mor...)Show SMILES COc1ccc2n(C(=O)c3cccc(Cl)c3Cl)c(C)c(CCN3CCOCC3)c2c1 Show InChI InChI=1S/C23H24Cl2N2O3/c1-15-17(8-9-26-10-12-30-13-11-26)19-14-16(29-2)6-7-21(19)27(15)23(28)18-4-3-5-20(24)22(18)25/h3-7,14H,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for binding affinity against recombinant human peripheral cannabinoid receptor 2 |
Bioorg Med Chem Lett 6: 2263-2268 (1996)
Article DOI: 10.1016/0960-894X(96)00426-X
BindingDB Entry DOI: 10.7270/Q2MS3SRN |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410597
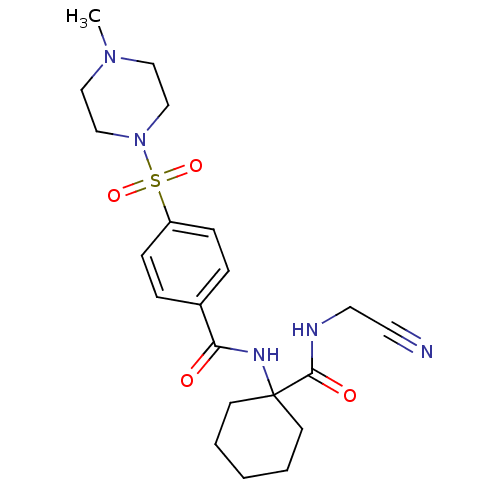
(CHEMBL199488)Show SMILES CN1CCN(CC1)S(=O)(=O)c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C21H29N5O4S/c1-25-13-15-26(16-14-25)31(29,30)18-7-5-17(6-8-18)19(27)24-21(9-3-2-4-10-21)20(28)23-12-11-22/h5-8H,2-4,9-10,12-16H2,1H3,(H,23,28)(H,24,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410583
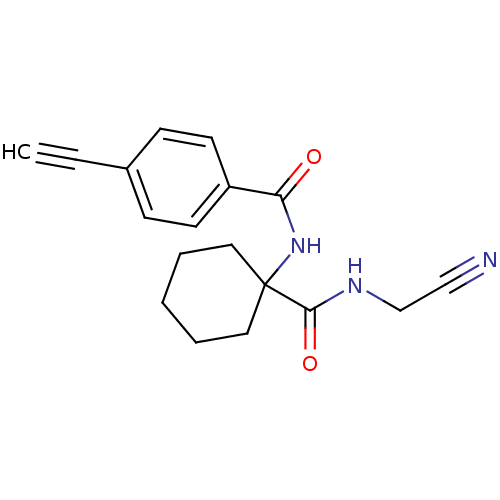
(CHEMBL200599)Show InChI InChI=1S/C18H19N3O2/c1-2-14-6-8-15(9-7-14)16(22)21-18(10-4-3-5-11-18)17(23)20-13-12-19/h1,6-9H,3-5,10-11,13H2,(H,20,23)(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410601
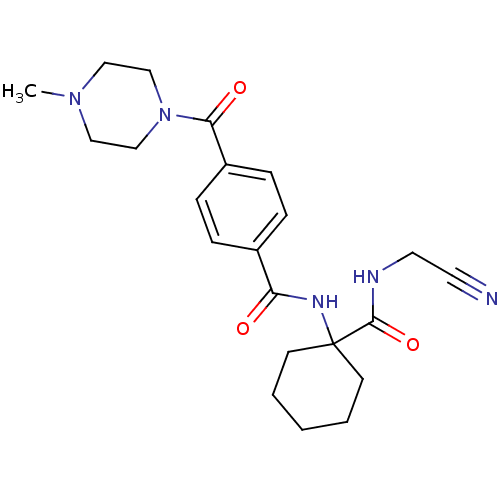
(CHEMBL199487)Show SMILES CN1CCN(CC1)C(=O)c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C22H29N5O3/c1-26-13-15-27(16-14-26)20(29)18-7-5-17(6-8-18)19(28)25-22(9-3-2-4-10-22)21(30)24-12-11-23/h5-8H,2-4,9-10,12-16H2,1H3,(H,24,30)(H,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50009864

(CHEMBL274833 | [2-Methyl-1-(2-morpholin-4-yl-ethyl...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2ccccc2n1CCN1CCOCC1 Show InChI InChI=1S/C26H26N2O2/c1-19-25(26(29)22-11-6-8-20-7-2-3-9-21(20)22)23-10-4-5-12-24(23)28(19)14-13-27-15-17-30-18-16-27/h2-12H,13-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for binding affinity against recombinant human peripheral cannabinoid receptor 2 |
Bioorg Med Chem Lett 6: 2263-2268 (1996)
Article DOI: 10.1016/0960-894X(96)00426-X
BindingDB Entry DOI: 10.7270/Q2MS3SRN |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50240608
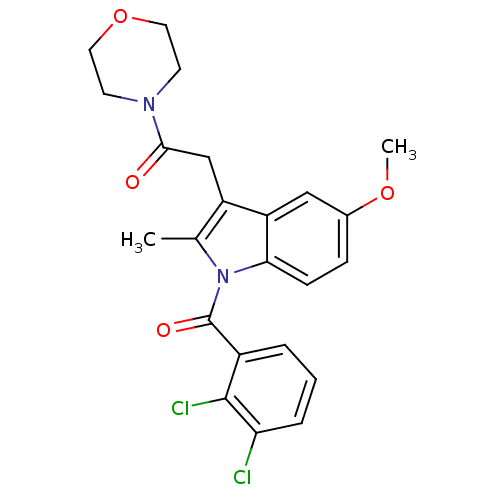
(2-(1-(2,3-dichlorobenzoyl)-5-methoxy-2-methyl-1H-i...)Show SMILES COc1ccc2n(C(=O)c3cccc(Cl)c3Cl)c(C)c(CC(=O)N3CCOCC3)c2c1 Show InChI InChI=1S/C23H22Cl2N2O4/c1-14-17(13-21(28)26-8-10-31-11-9-26)18-12-15(30-2)6-7-20(18)27(14)23(29)16-4-3-5-19(24)22(16)25/h3-7,12H,8-11,13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for binding affinity against recombinant human peripheral cannabinoid receptor 2 |
Bioorg Med Chem Lett 6: 2263-2268 (1996)
Article DOI: 10.1016/0960-894X(96)00426-X
BindingDB Entry DOI: 10.7270/Q2MS3SRN |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410600
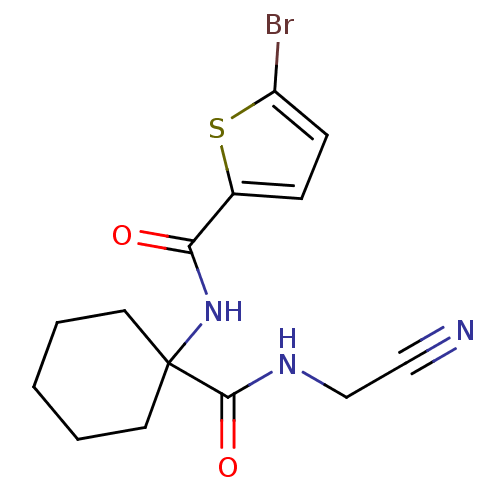
(CHEMBL200167)Show InChI InChI=1S/C14H16BrN3O2S/c15-11-5-4-10(21-11)12(19)18-14(6-2-1-3-7-14)13(20)17-9-8-16/h4-5H,1-3,6-7,9H2,(H,17,20)(H,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410602
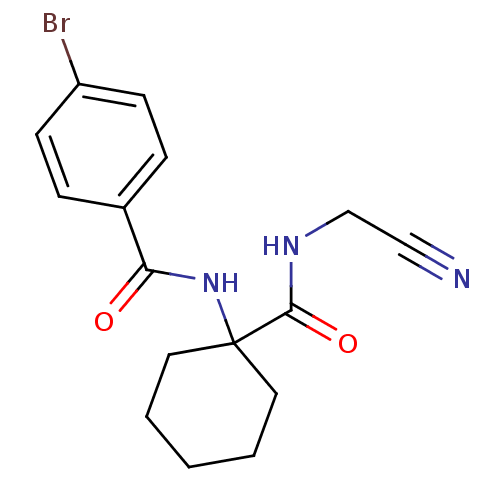
(CHEMBL370786)Show InChI InChI=1S/C16H18BrN3O2/c17-13-6-4-12(5-7-13)14(21)20-16(8-2-1-3-9-16)15(22)19-11-10-18/h4-7H,1-3,8-9,11H2,(H,19,22)(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(RAT) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19858
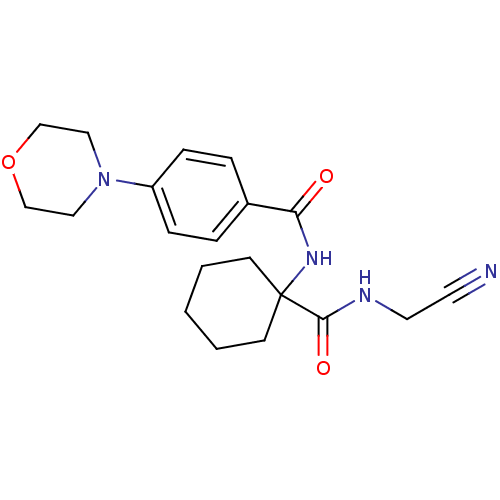
(N-{1-[(cyanomethyl)carbamoyl]cyclohexyl}-4-(morpho...)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)N1CCOCC1 Show InChI InChI=1S/C20H26N4O3/c21-10-11-22-19(26)20(8-2-1-3-9-20)23-18(25)16-4-6-17(7-5-16)24-12-14-27-15-13-24/h4-7H,1-3,8-9,11-15H2,(H,22,26)(H,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 19.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 296: 558-66 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4VPJ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 19.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50095493
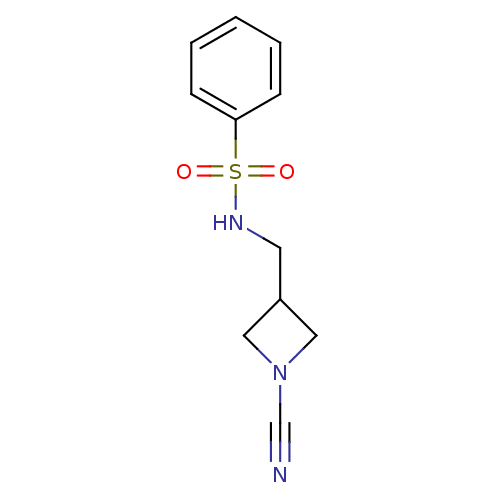
(CHEMBL276169 | N-(1-Cyano-azetidin-3-ylmethyl)-ben...)Show InChI InChI=1S/C11H13N3O2S/c12-9-14-7-10(8-14)6-13-17(15,16)11-4-2-1-3-5-11/h1-5,10,13H,6-8H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory constant of the compound against human cathepsin K |
J Med Chem 44: 94-104 (2001)
BindingDB Entry DOI: 10.7270/Q2DV1J4R |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50240605
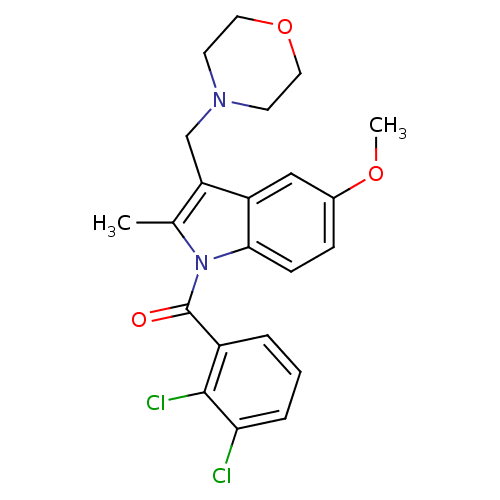
((2,3-Dichloro-phenyl)-(5-methoxy-2-methyl-3-morpho...)Show SMILES COc1ccc2n(C(=O)c3cccc(Cl)c3Cl)c(C)c(CN3CCOCC3)c2c1 Show InChI InChI=1S/C22H22Cl2N2O3/c1-14-18(13-25-8-10-29-11-9-25)17-12-15(28-2)6-7-20(17)26(14)22(27)16-4-3-5-19(23)21(16)24/h3-7,12H,8-11,13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for binding affinity against recombinant human peripheral cannabinoid receptor 2 |
Bioorg Med Chem Lett 6: 2263-2268 (1996)
Article DOI: 10.1016/0960-894X(96)00426-X
BindingDB Entry DOI: 10.7270/Q2MS3SRN |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 290: 551-60 (1999)
BindingDB Entry DOI: 10.7270/Q2S18110 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50287806
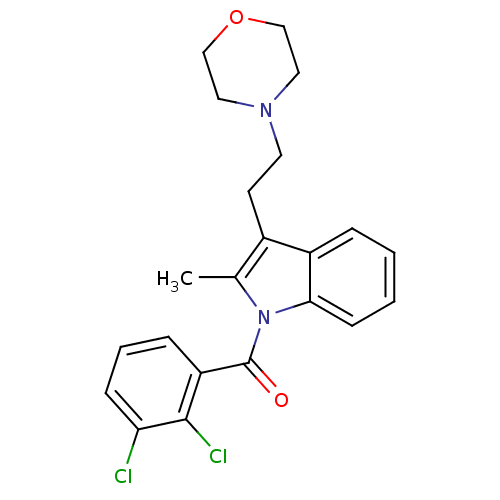
((2,3-Dichloro-phenyl)-[2-methyl-3-(2-morpholin-4-y...)Show SMILES Cc1c(CCN2CCOCC2)c2ccccc2n1C(=O)c1cccc(Cl)c1Cl Show InChI InChI=1S/C22H22Cl2N2O2/c1-15-16(9-10-25-11-13-28-14-12-25)17-5-2-3-8-20(17)26(15)22(27)18-6-4-7-19(23)21(18)24/h2-8H,9-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for binding affinity against recombinant human peripheral cannabinoid receptor 2 |
Bioorg Med Chem Lett 6: 2263-2268 (1996)
Article DOI: 10.1016/0960-894X(96)00426-X
BindingDB Entry DOI: 10.7270/Q2MS3SRN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data