

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor (unknown origin) | Bioorg Med Chem Lett 23: 2019-21 (2013) Article DOI: 10.1016/j.bmcl.2013.02.025 BindingDB Entry DOI: 10.7270/Q2WS8VM2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239073 (US9416103, CP-55,940) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 0.370 | -53.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description Competition receptor binding was performed as previously described [Shoemaker et al., J. Pharmacol. Exp. Ther., 314:868-75]. Briefly, 50 μg of mou... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50088439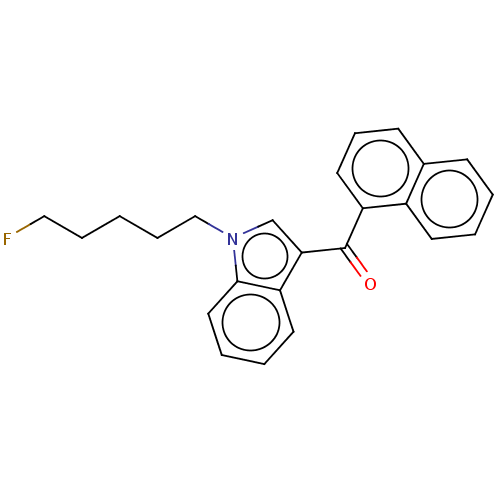 (CHEMBL3526578) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.395 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in B6SJL mouse brain membrane after 90 mins by liquid scintillation spectrophotometric analysis | Drug Metab Dispos 40: 2174-84 (2012) Article DOI: 10.1124/dmd.112.047530 BindingDB Entry DOI: 10.7270/Q23R0VK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239078 (US9416103, TV-5-157) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 min... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239078 (US9416103, TV-5-157) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description A functional assay screen for the inhibition of adenylate cyclase (AC) activity was chosen as the subsequent assay. This screen would allow us to gai... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50272598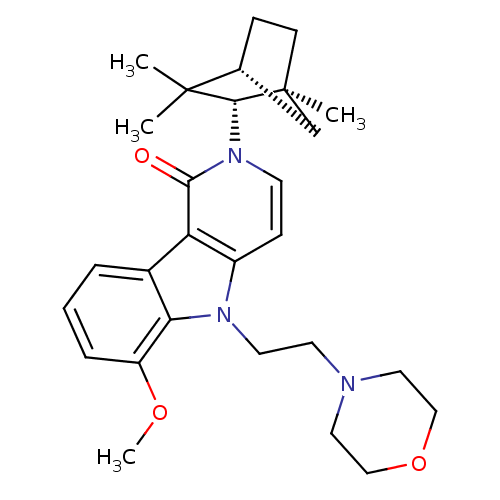 (6-Methoxy-5-(2-morpholin-4-yl-ethyl)-2-(1,3,3-trim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor (unknown origin) | Bioorg Med Chem Lett 23: 2019-21 (2013) Article DOI: 10.1016/j.bmcl.2013.02.025 BindingDB Entry DOI: 10.7270/Q2WS8VM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50353747 (CHEMBL561013 | JWH-018) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in B6SJL mouse brain membrane after 90 mins by liquid scintillation spectrophotometric analysis | Drug Metab Dispos 40: 2174-84 (2012) Article DOI: 10.1124/dmd.112.047530 BindingDB Entry DOI: 10.7270/Q23R0VK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50432198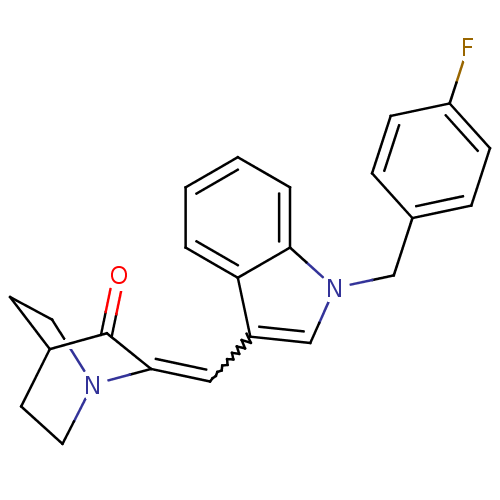 (CHEMBL240913) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]CP 55940 from human CB2 receptor expressed in CHO cells after 90 mins by liquid scintillation counting | Bioorg Med Chem Lett 23: 2019-21 (2013) Article DOI: 10.1016/j.bmcl.2013.02.025 BindingDB Entry DOI: 10.7270/Q2WS8VM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50572486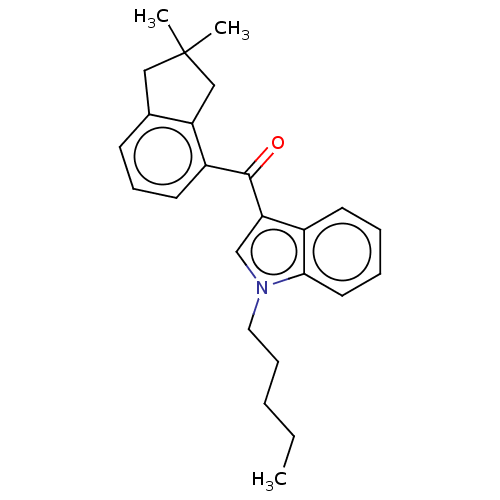 (CHEMBL4860950) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50572486 (CHEMBL4860950) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239078 (US9416103, TV-5-157) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in mouse Neuro2a cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 mins by liq... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239078 (US9416103, TV-5-157) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description A functional assay screen for the inhibition of adenylate cyclase (AC) activity was chosen as the subsequent assay. This screen would allow us to gai... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Binding affinity to CB1 receptor (unknown origin) | Bioorg Med Chem Lett 23: 2019-21 (2013) Article DOI: 10.1016/j.bmcl.2013.02.025 BindingDB Entry DOI: 10.7270/Q2WS8VM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50432193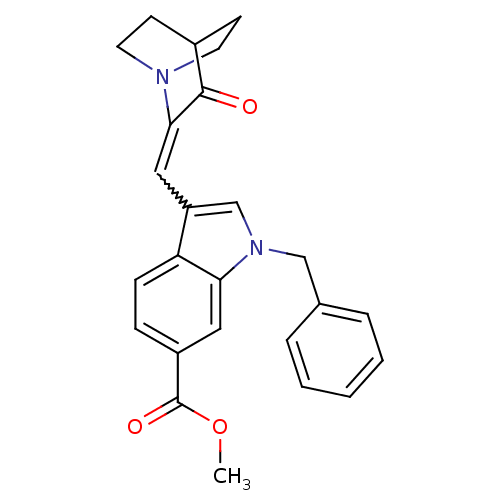 (CHEMBL2346979) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]CP 55940 from human CB2 receptor expressed in CHO cells after 90 mins by liquid scintillation counting | Bioorg Med Chem Lett 23: 2019-21 (2013) Article DOI: 10.1016/j.bmcl.2013.02.025 BindingDB Entry DOI: 10.7270/Q2WS8VM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50353747 (CHEMBL561013 | JWH-018) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50041958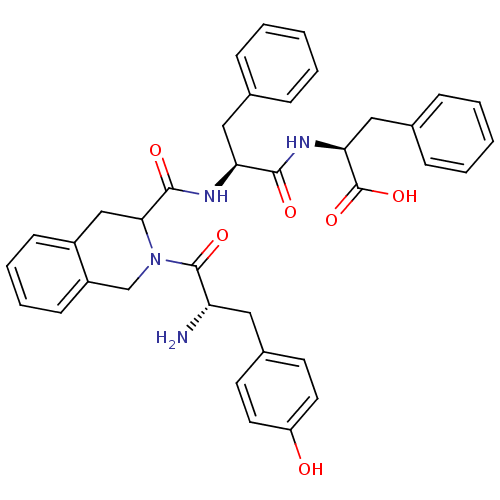 (2-(1-{2-[2-amino-3-(4-hydroxyphenyl)propanoyl]-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by PDSP Ki Database | J Pharmacol Exp Ther 301: 661-71 (2002) Article DOI: 10.1124/jpet.301.2.661 BindingDB Entry DOI: 10.7270/Q27D2SQM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50572487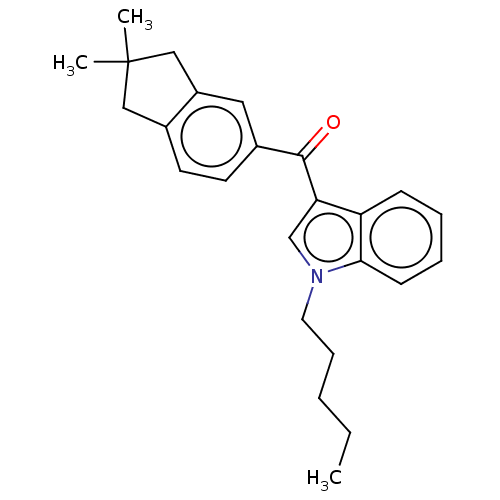 (CHEMBL4856192) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50041958 (2-(1-{2-[2-amino-3-(4-hydroxyphenyl)propanoyl]-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by PDSP Ki Database | J Pharmacol Exp Ther 301: 661-71 (2002) Article DOI: 10.1124/jpet.301.2.661 BindingDB Entry DOI: 10.7270/Q27D2SQM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50353747 (CHEMBL561013 | JWH-018) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50432198 (CHEMBL240913) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]CP 55940 from CB1 receptor in mouse whole brain membrane homogenates after 90 mins by liquid scintillation counting | Bioorg Med Chem Lett 23: 2019-21 (2013) Article DOI: 10.1016/j.bmcl.2013.02.025 BindingDB Entry DOI: 10.7270/Q2WS8VM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM85804 (1-Naphthyl(1-butyl-1H-indole-3-yl)methanone | JWH-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 min... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM85804 (1-Naphthyl(1-butyl-1H-indole-3-yl)methanone | JWH-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description A functional assay screen for the inhibition of adenylate cyclase (AC) activity was chosen as the subsequent assay. This screen would allow us to gai... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239077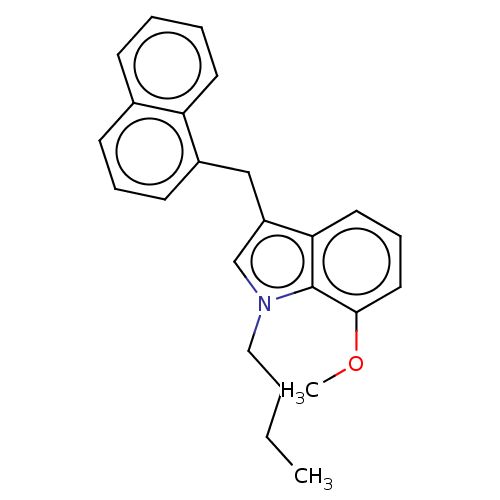 (US9416103, TV-5-249) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description A functional assay screen for the inhibition of adenylate cyclase (AC) activity was chosen as the subsequent assay. This screen would allow us to gai... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239077 (US9416103, TV-5-249) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 min... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50432199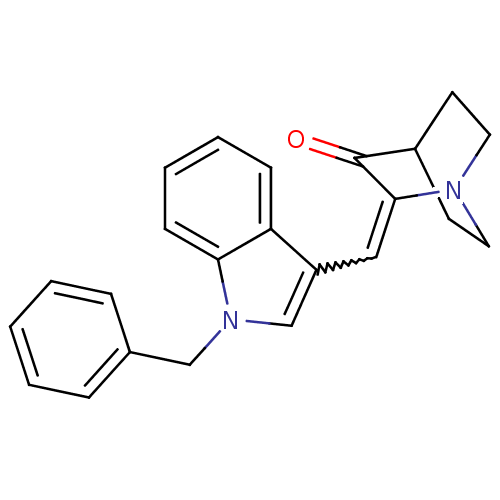 (CHEMBL238082) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]CP 55940 from human CB2 receptor expressed in CHO cells after 90 mins by liquid scintillation counting | Bioorg Med Chem Lett 23: 2019-21 (2013) Article DOI: 10.1016/j.bmcl.2013.02.025 BindingDB Entry DOI: 10.7270/Q2WS8VM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM85804 (1-Naphthyl(1-butyl-1H-indole-3-yl)methanone | JWH-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 12.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description A functional assay screen for the inhibition of adenylate cyclase (AC) activity was chosen as the subsequent assay. This screen would allow us to gai... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM85804 (1-Naphthyl(1-butyl-1H-indole-3-yl)methanone | JWH-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 12.9 | -45.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description Competition receptor binding was performed as previously described [Shoemaker et al., J. Pharmacol. Exp. Ther., 314:868-75]. Briefly, 50 μg of mou... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM85804 (1-Naphthyl(1-butyl-1H-indole-3-yl)methanone | JWH-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in mouse Neuro2a cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 mins by liq... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239074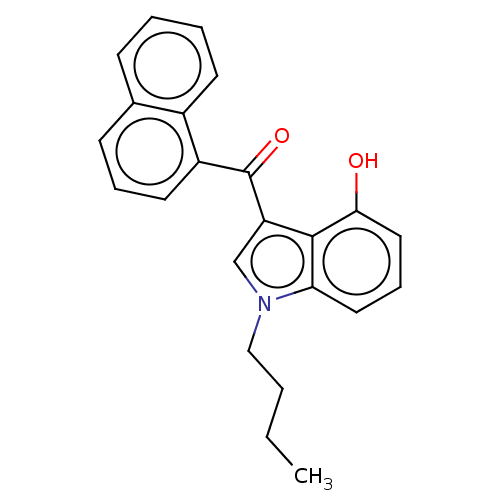 (US9416103, JWH-073-M1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 14.1 | -44.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description Competition receptor binding was performed as previously described [Shoemaker et al., J. Pharmacol. Exp. Ther., 314:868-75]. Briefly, 50 μg of mou... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21008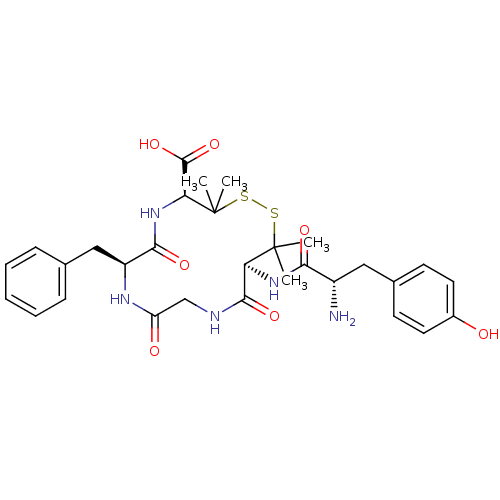 ((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 14.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas Curated by PDSP Ki Database | J Pharmacol Exp Ther 301: 661-71 (2002) Article DOI: 10.1124/jpet.301.2.661 BindingDB Entry DOI: 10.7270/Q27D2SQM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM60994 ((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in B6SJL mouse brain membrane after 90 mins by liquid scintillation spectrophotometric analysis | Drug Metab Dispos 40: 2174-84 (2012) Article DOI: 10.1124/dmd.112.047530 BindingDB Entry DOI: 10.7270/Q23R0VK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239077 (US9416103, TV-5-249) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in mouse Neuro2a cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 mins by liq... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50088440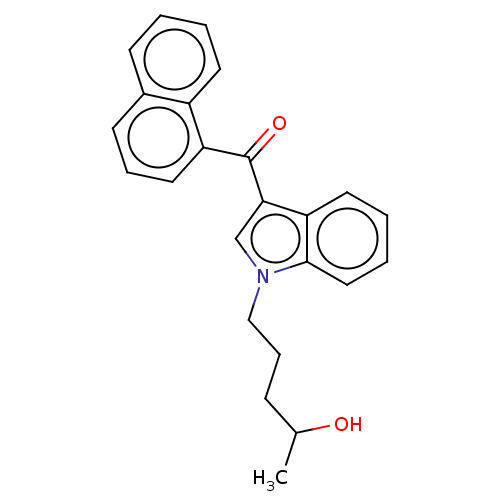 (CHEMBL3526291) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in B6SJL mouse brain membrane after 90 mins by liquid scintillation spectrophotometric analysis | Drug Metab Dispos 40: 2174-84 (2012) Article DOI: 10.1124/dmd.112.047530 BindingDB Entry DOI: 10.7270/Q23R0VK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239077 (US9416103, TV-5-249) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 15.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description A functional assay screen for the inhibition of adenylate cyclase (AC) activity was chosen as the subsequent assay. This screen would allow us to gai... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50572485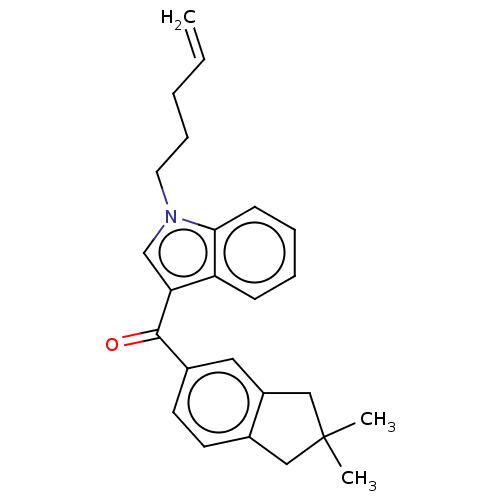 (CHEMBL4855206) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50272598 (6-Methoxy-5-(2-morpholin-4-yl-ethyl)-2-(1,3,3-trim...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Binding affinity to CB1 receptor (unknown origin) | Bioorg Med Chem Lett 23: 2019-21 (2013) Article DOI: 10.1016/j.bmcl.2013.02.025 BindingDB Entry DOI: 10.7270/Q2WS8VM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50432197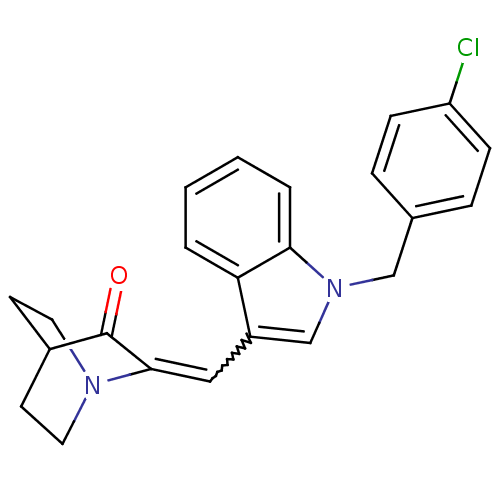 (CHEMBL240697) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]CP 55940 from human CB2 receptor expressed in CHO cells after 90 mins by liquid scintillation counting | Bioorg Med Chem Lett 23: 2019-21 (2013) Article DOI: 10.1016/j.bmcl.2013.02.025 BindingDB Entry DOI: 10.7270/Q2WS8VM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239075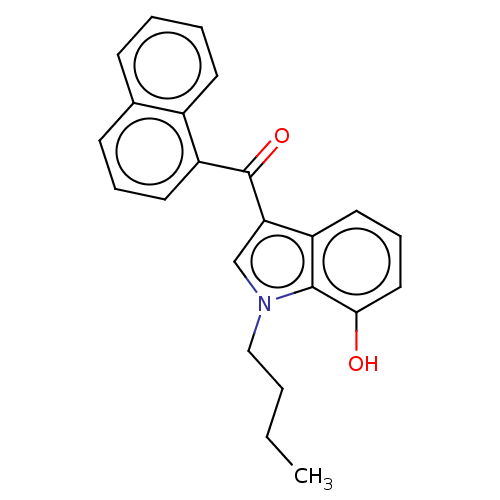 (US9416103, JWH-073-M4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in mouse Neuro2a cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 mins by liq... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50572489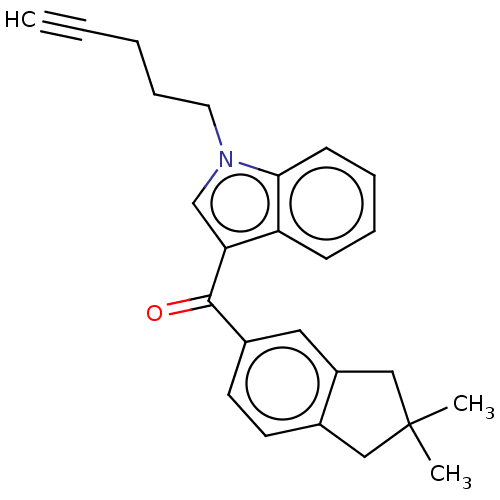 (CHEMBL4877815) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50553588 (CHEMBL4747163) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP-55940 from CB2 receptor (unknown origin) by competitive binding assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127501 BindingDB Entry DOI: 10.7270/Q2NV9NW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239075 (US9416103, JWH-073-M4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 24.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description A functional assay screen for the inhibition of adenylate cyclase (AC) activity was chosen as the subsequent assay. This screen would allow us to gai... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM239079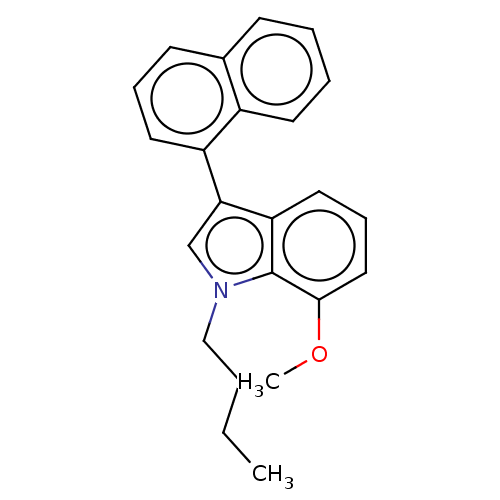 (US9416103, TV-6-41) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 26.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description A functional assay screen for the inhibition of adenylate cyclase (AC) activity was chosen as the subsequent assay. This screen would allow us to gai... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50491525 (CHEMBL2380408) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor transfected in CHO cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 min... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50432197 (CHEMBL240697) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]CP 55940 from CB1 receptor in mouse whole brain membrane homogenates after 90 mins by liquid scintillation counting | Bioorg Med Chem Lett 23: 2019-21 (2013) Article DOI: 10.1016/j.bmcl.2013.02.025 BindingDB Entry DOI: 10.7270/Q2WS8VM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50088444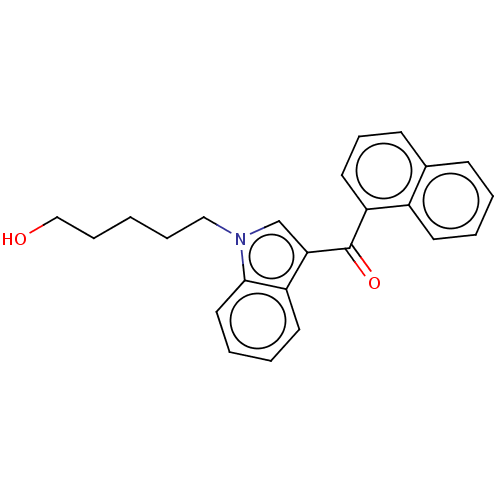 (CHEMBL3526177) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in B6SJL mouse brain membrane after 90 mins by liquid scintillation spectrophotometric analysis | Drug Metab Dispos 40: 2174-84 (2012) Article DOI: 10.1124/dmd.112.047530 BindingDB Entry DOI: 10.7270/Q23R0VK3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50491525 (CHEMBL2380408) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description Agonist activity at CB1 receptor in mouse Neuro2a cells assessed as inhibition of forskolin-stimulated adenylyl cyclase activity after 15 mins by liq... | J Med Chem 56: 4537-50 (2013) Article DOI: 10.1021/jm400268b BindingDB Entry DOI: 10.7270/Q25M68M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM239079 (US9416103, TV-6-41) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 37.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Board of Trustees of the University of Arkansas; The University of Kansas US Patent | Assay Description A functional assay screen for the inhibition of adenylate cyclase (AC) activity was chosen as the subsequent assay. This screen would allow us to gai... | US Patent US9416103 (2016) BindingDB Entry DOI: 10.7270/Q2HX1BKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50572487 (CHEMBL4856192) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Mus musculus (Mouse)) | BDBM50432195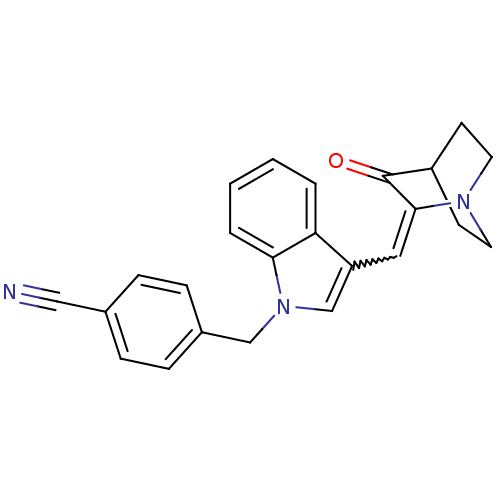 (CHEMBL2346977) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]CP 55940 from CB1 receptor in mouse whole brain membrane homogenates after 90 mins by liquid scintillation counting | Bioorg Med Chem Lett 23: 2019-21 (2013) Article DOI: 10.1016/j.bmcl.2013.02.025 BindingDB Entry DOI: 10.7270/Q2WS8VM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50432192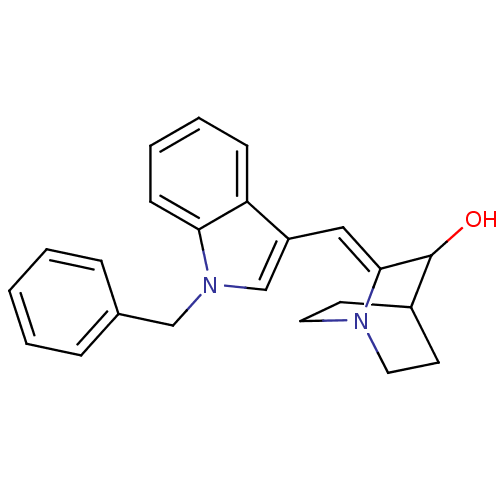 (CHEMBL238083) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]CP 55940 from human CB2 receptor expressed in CHO cells after 90 mins by liquid scintillation counting | Bioorg Med Chem Lett 23: 2019-21 (2013) Article DOI: 10.1016/j.bmcl.2013.02.025 BindingDB Entry DOI: 10.7270/Q2WS8VM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 98 total ) | Next | Last >> |