

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50100221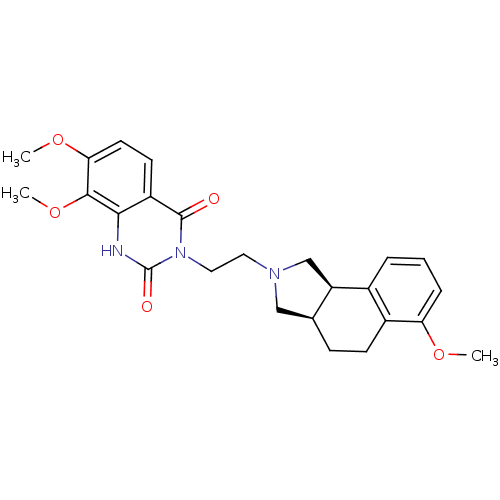 (7,8-Dimethoxy-3-[2-(6-methoxy-1,3,3a,4,5,9b-hexahy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to alpha-1A adrenergic receptor in rat submaxillary gland | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50100210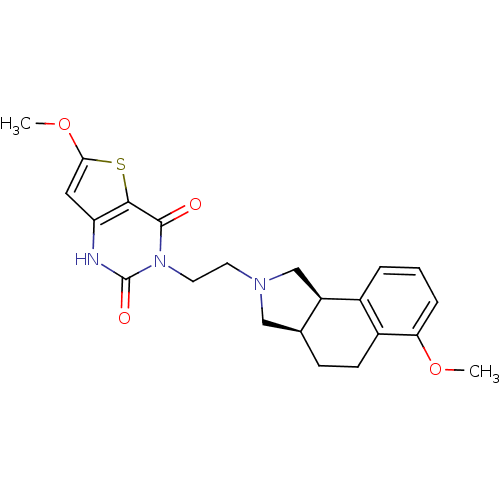 (6-Methoxy-3-[2-(6-methoxy-1,3,3a,4,5,9b-hexahydro-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to alpha-1A adrenergic receptor in rat submaxillary gland | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50100214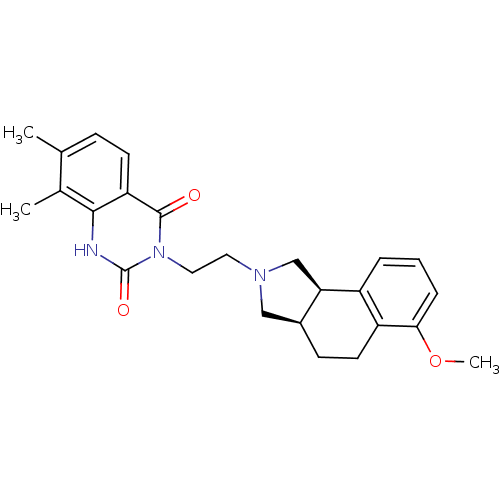 (3-[2-(6-Methoxy-1,3,3a,4,5,9b-hexahydro-benzo[e]is...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to alpha-1A adrenergic receptor in rat submaxillary gland | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50100238 (7-Methoxy-3-[2-(6-methoxy-1,3,3a,4,5,9b-hexahydro-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to alpha-1A adrenergic receptor in rat submaxillary gland | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50100229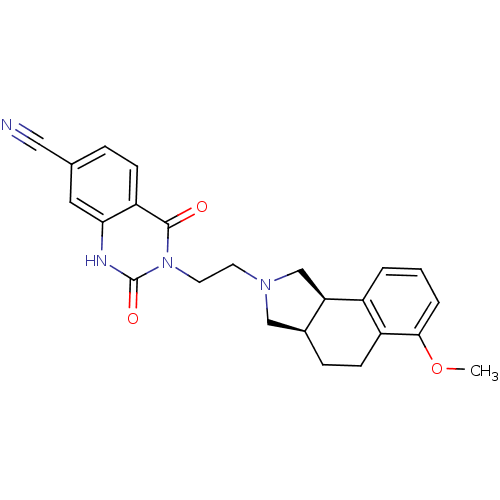 (3-[2-(6-Methoxy-1,3,3a,4,5,9b-hexahydro-benzo[e]is...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to alpha-1A adrenergic receptor in rat submaxillary gland | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50000041 ((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Evaluated for the binding affinity towards NK1 receptor in the striatal membranes of guinea pig | J Med Chem 36: 3197-201 (1993) BindingDB Entry DOI: 10.7270/Q29887NM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50000041 ((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Evaluated for the binding affinity towards NK1 receptor in the striatal membranes of guinea pig | J Med Chem 36: 3197-201 (1993) BindingDB Entry DOI: 10.7270/Q29887NM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Alpha-1D adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50100238 (7-Methoxy-3-[2-(6-methoxy-1,3,3a,4,5,9b-hexahydro-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound to rat alpha-1D adrenergic receptor expressed in LTK cells | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50100210 (6-Methoxy-3-[2-(6-methoxy-1,3,3a,4,5,9b-hexahydro-...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound to rat alpha-1D adrenergic receptor expressed in LTK cells | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50100233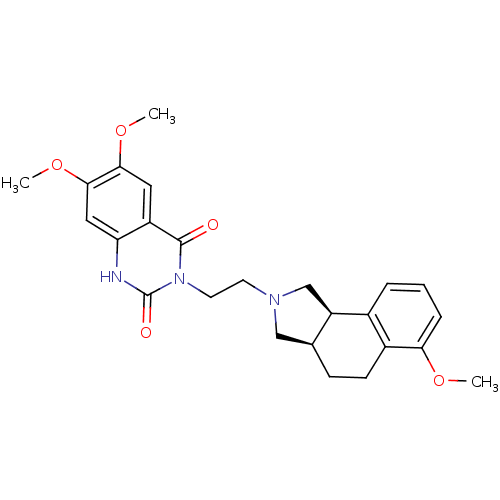 (6,7-Dimethoxy-3-[2-(6-methoxy-1,3,3a,4,5,9b-hexahy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to alpha-1A adrenergic receptor in rat submaxillary gland | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220432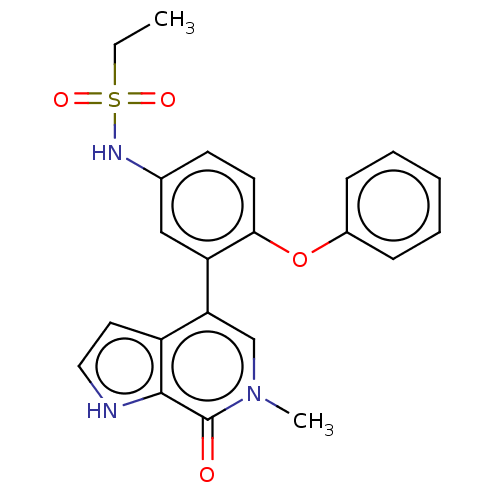 (US9296741, 21) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.25 | -54.8 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50100206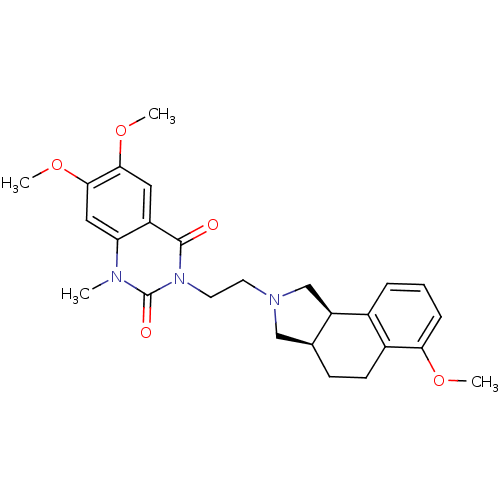 (6,7-Dimethoxy-3-[2-(6-methoxy-1,3,3a,4,5,9b-hexahy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to alpha-1A adrenergic receptor in rat submaxillary gland | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50100228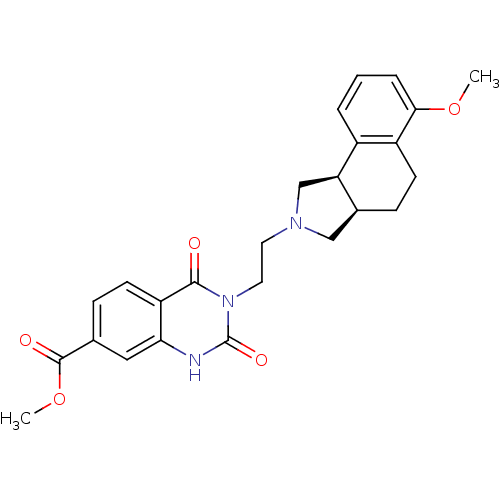 (3-[2-(6-Methoxy-1,3,3a,4,5,9b-hexahydro-benzo[e]is...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to alpha-1A adrenergic receptor in rat submaxillary gland | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50100222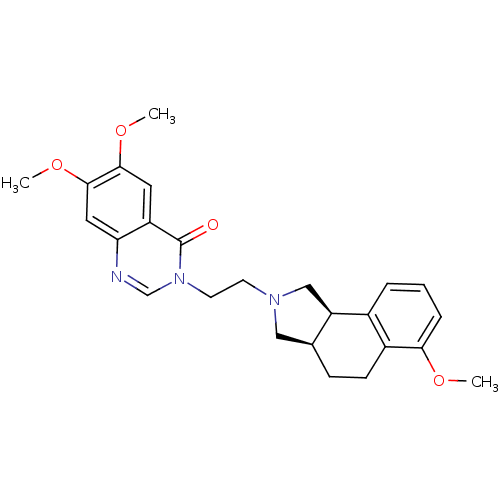 (6,7-Dimethoxy-3-[2-(6-methoxy-1,3,3a,4,5,9b-hexahy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to alpha-1A adrenergic receptor in rat submaxillary gland | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50100216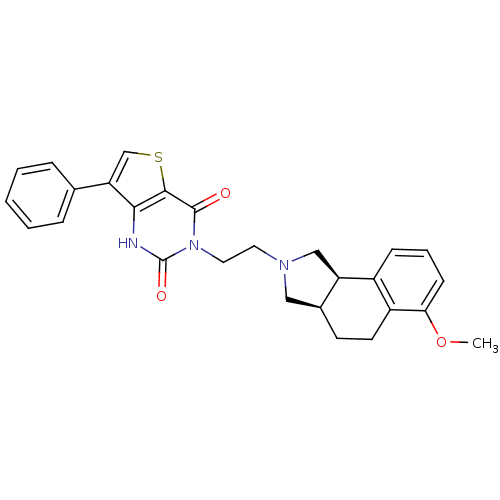 (3-[2-(6-Methoxy-1,3,3a,4,5,9b-hexahydro-benzo[e]is...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to hamster alpha-1B adrenergic receptor | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50100216 (3-[2-(6-Methoxy-1,3,3a,4,5,9b-hexahydro-benzo[e]is...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to alpha-1A adrenergic receptor in rat submaxillary gland | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220432 (US9296741, 21) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.390 | -53.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50100220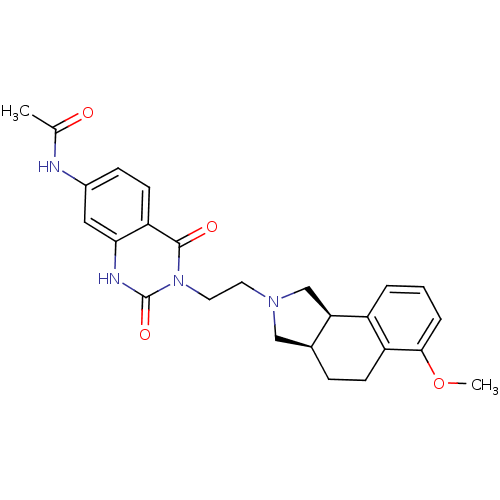 (CHEMBL61598 | N-{3-[2-(6-Methoxy-1,3,3a,4,5,9b-hex...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound to rat alpha-1D adrenergic receptor expressed in LTK cells | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50100217 (7-Isopropyl-6-methoxy-3-[2-(6-methoxy-1,3,3a,4,5,9...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to alpha-1A adrenergic receptor in rat submaxillary gland | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50100220 (CHEMBL61598 | N-{3-[2-(6-Methoxy-1,3,3a,4,5,9b-hex...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to alpha-1A adrenergic receptor in rat submaxillary gland | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220626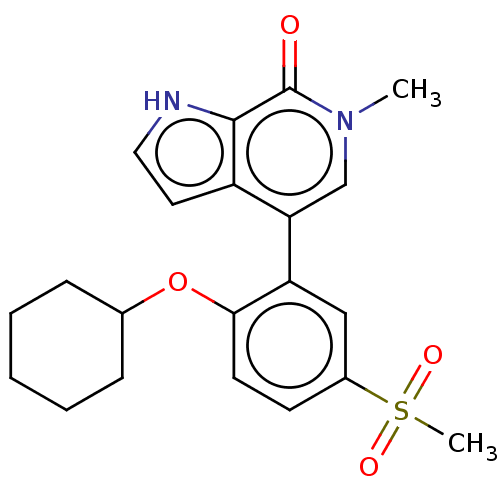 (US9296741, 215) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.460 | -53.3 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM50457489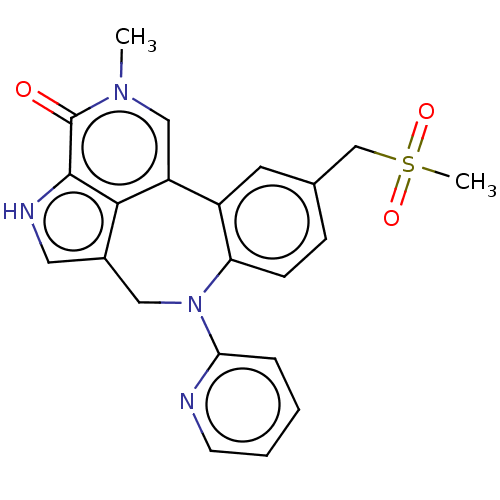 (CHEMBL4208129) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to BRD2 bromodomain 1 to 2 (G73 to A560 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent probe by b... | Bioorg Med Chem Lett 28: 1804-1810 (2018) Article DOI: 10.1016/j.bmcl.2018.04.020 BindingDB Entry DOI: 10.7270/Q2542R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50100215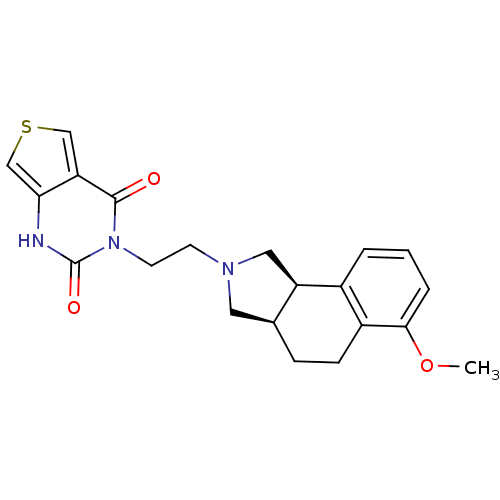 (3-[2-(6-Methoxy-1,3,3a,4,5,9b-hexahydro-benzo[e]is...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to alpha-1A adrenergic receptor in rat submaxillary gland | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM439590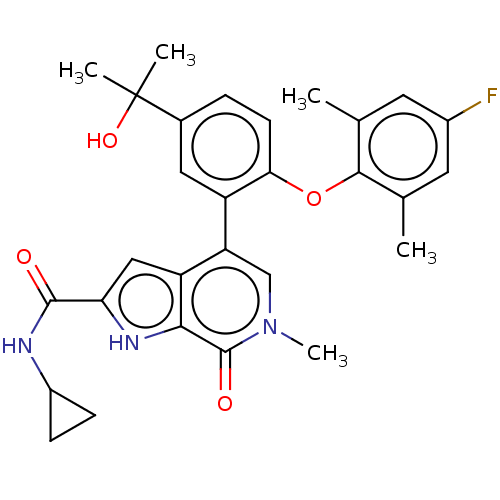 (US10633379, Example 121) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511849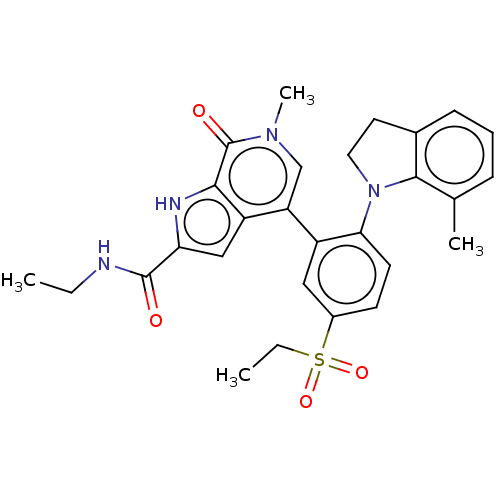 (CHEMBL4465299) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511850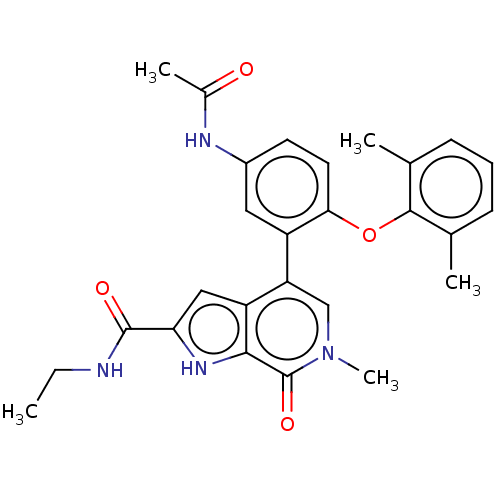 (CHEMBL4435166) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511875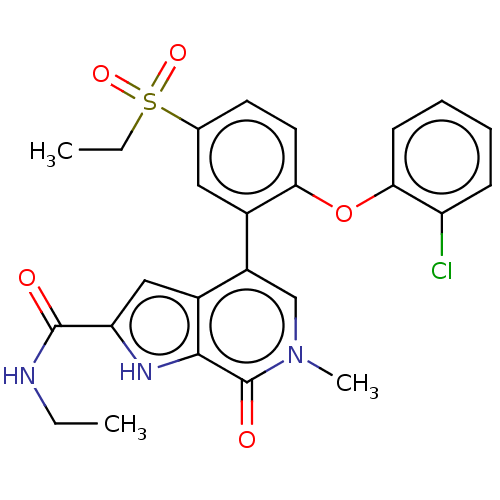 (CHEMBL4548794) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220518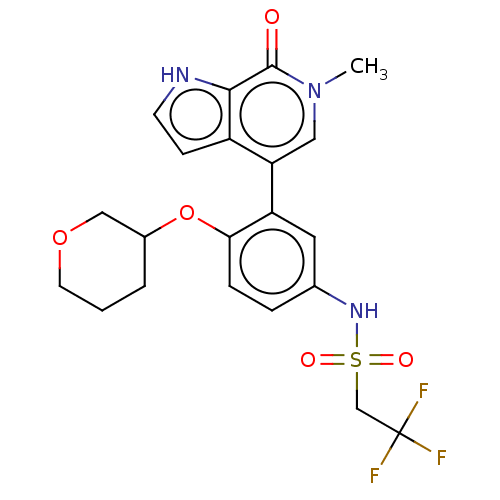 (US9296741, 107) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220484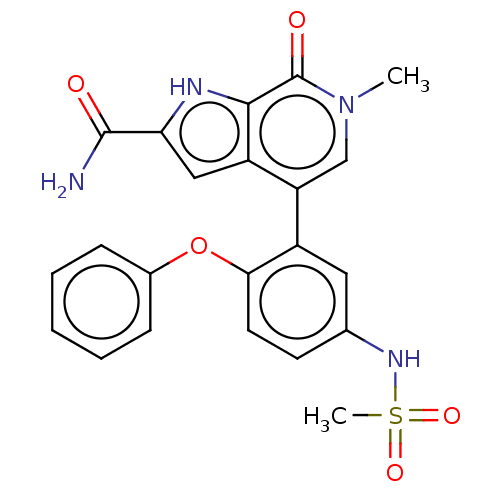 (US9296741, 73) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220452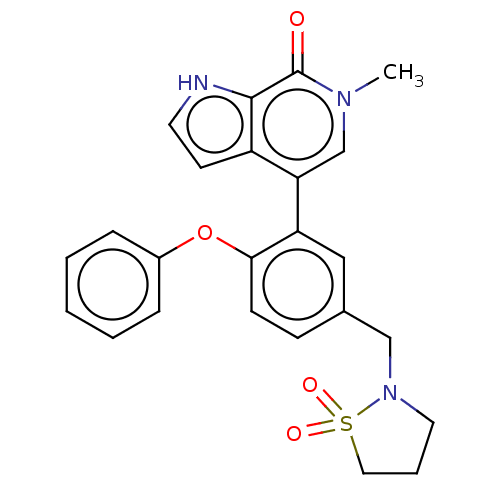 (US9296741, 41) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.510 | -53.0 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220516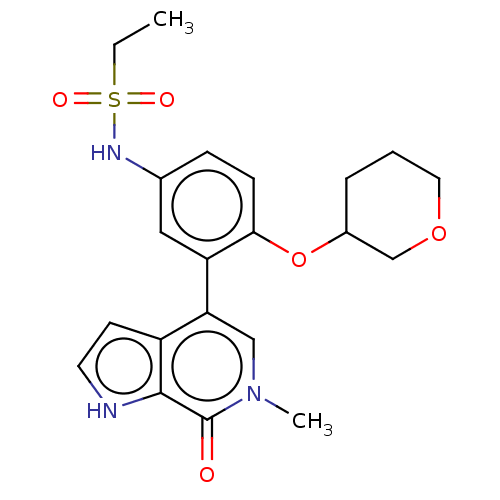 (US9296741, 105) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.520 | -53.0 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50100215 (3-[2-(6-Methoxy-1,3,3a,4,5,9b-hexahydro-benzo[e]is...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound to rat alpha-1D adrenergic receptor expressed in LTK cells | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139374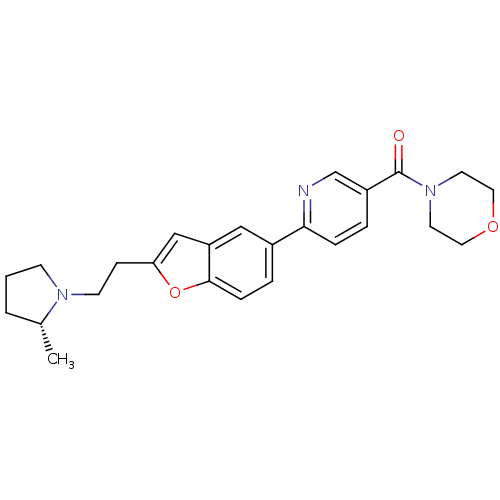 ((6-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139374 ((6-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50100204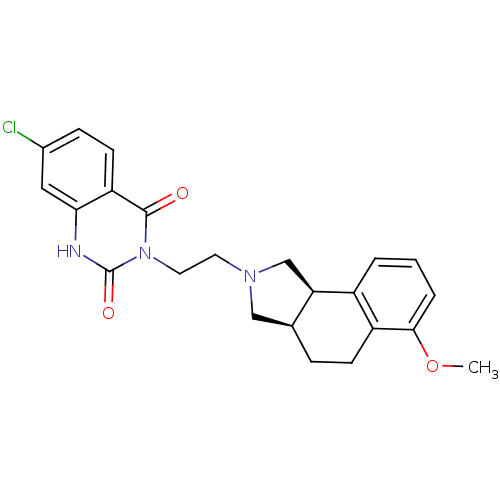 (7-Chloro-3-[2-(6-methoxy-1,3,3a,4,5,9b-hexahydro-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to alpha-1A adrenergic receptor in rat submaxillary gland | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220679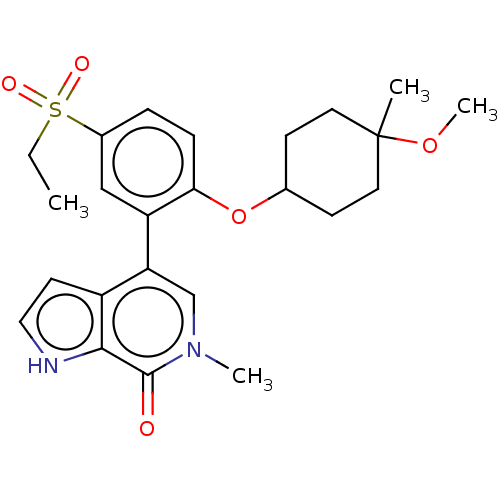 (US9296741, 268) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.570 | -52.8 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50100204 (7-Chloro-3-[2-(6-methoxy-1,3,3a,4,5,9b-hexahydro-b...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound to rat alpha-1D adrenergic receptor expressed in LTK cells | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50124595 (5-Allyl-10-chloro-2,2,4-trimethyl-2,5-dihydro-1H-6...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human glucocorticoid receptor (GR) was determined using [3H]-Dexamethasone as radioligand in SF-1 cells | J Med Chem 46: 1016-30 (2003) Article DOI: 10.1021/jm020335m BindingDB Entry DOI: 10.7270/Q2MP52NB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50511864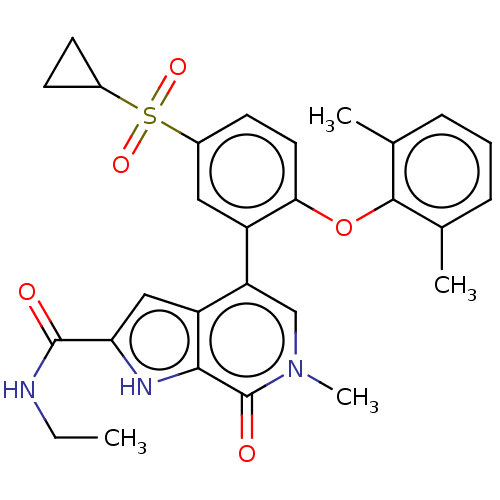 (CHEMBL4564879) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM439461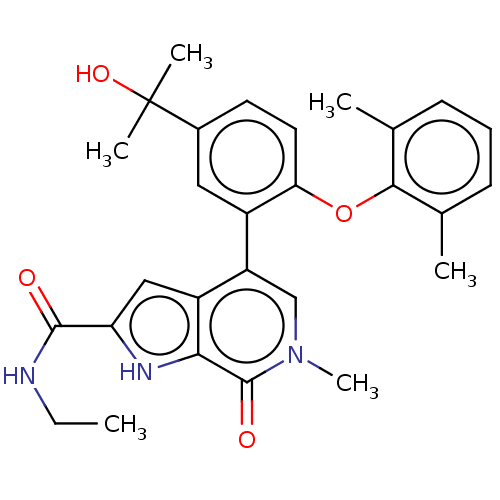 (US10633379, Example 3 | US10633379, Example 68) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6 tagged BRD4 BD2 (352 to 457) residues expressed in Escherichia coli BL21(DE3) cells after 1 hr by TR-FRET assay | J Med Chem 63: 5585-5623 (2020) Article DOI: 10.1021/acs.jmedchem.0c00628 BindingDB Entry DOI: 10.7270/Q2057K8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50100209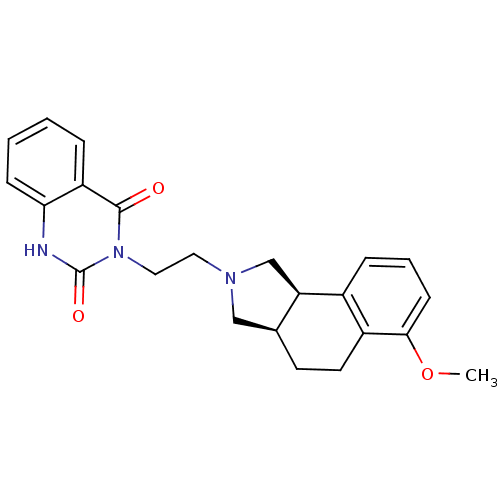 (3-[2-(6-Methoxy-1,3,3a,4,5,9b-hexahydro-benzo[e]is...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound to rat alpha-1D adrenergic receptor expressed in LTK cells | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50457489 (CHEMBL4208129) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Binding affinity to His-tagged BRD4 bromodomain 1 to 2 (K57 to K550 amino acids) (unknown origin) using Alexa647-labeled BET-inhibitor as fluorescent... | Bioorg Med Chem Lett 28: 1804-1810 (2018) Article DOI: 10.1016/j.bmcl.2018.04.020 BindingDB Entry DOI: 10.7270/Q2542R63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [57-168] (Homo sapiens (Human)) | BDBM220507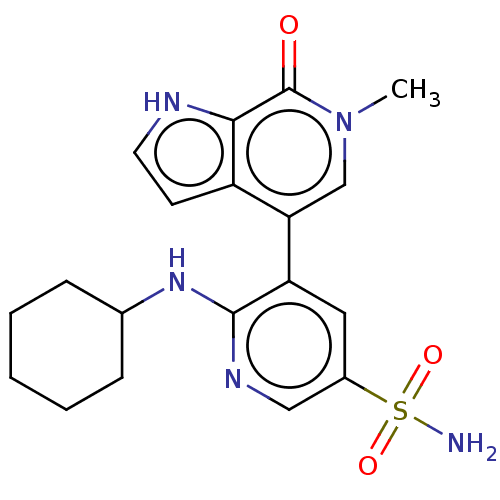 (US9296741, 96) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.630 | -52.5 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [352-457] (Homo sapiens (Human)) | BDBM220495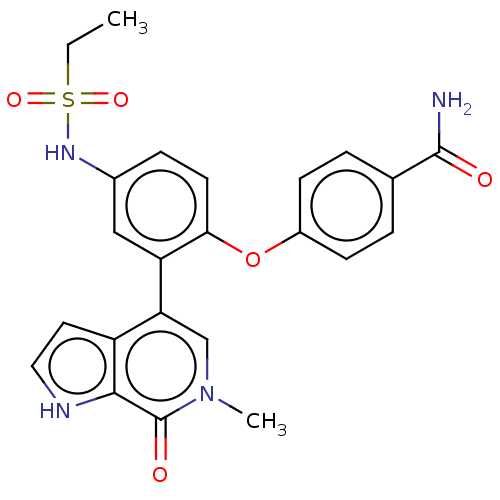 (US9296741, 84) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.650 | -52.4 | n/a | n/a | n/a | n/a | n/a | 6.0 | 25 |
AbbVie Inc. US Patent | Assay Description A time-resolved fluorescence resonance energy transfer (TR-FRET) assay was used to determine the affinities of compounds of the Examples listed in Ta... | US Patent US9296741 (2016) BindingDB Entry DOI: 10.7270/Q26W98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50100216 (3-[2-(6-Methoxy-1,3,3a,4,5,9b-hexahydro-benzo[e]is...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound to rat alpha-1D adrenergic receptor expressed in LTK cells | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50100238 (7-Methoxy-3-[2-(6-methoxy-1,3,3a,4,5,9b-hexahydro-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to hamster alpha-1B adrenergic receptor | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50100221 (7,8-Dimethoxy-3-[2-(6-methoxy-1,3,3a,4,5,9b-hexahy...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to hamster alpha-1B adrenergic receptor | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM50100209 (3-[2-(6-Methoxy-1,3,3a,4,5,9b-hexahydro-benzo[e]is...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to alpha-1A adrenergic receptor in rat submaxillary gland | J Med Chem 44: 1971-85 (2001) BindingDB Entry DOI: 10.7270/Q2KP81GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2509 total ) | Next | Last >> |