 Found 265 hits with Last Name = 'preusser' and Initial = 'lc'
Found 265 hits with Last Name = 'preusser' and Initial = 'lc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM12656
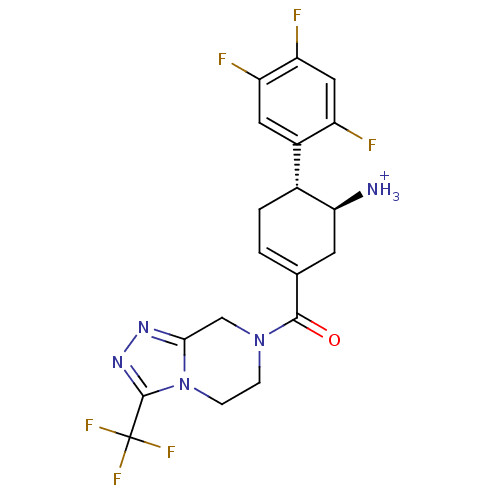
(((4R,5S)-5-Amino-4-(2,4,5-trifluorophenyl)cyclohex...)Show SMILES [NH3+][C@H]1CC(=CC[C@@H]1c1cc(F)c(F)cc1F)C(=O)N1CCn2c(C1)nnc2C(F)(F)F |r,c:3| Show InChI InChI=1S/C19H17F6N5O/c20-12-7-14(22)13(21)6-11(12)10-2-1-9(5-15(10)26)17(31)29-3-4-30-16(8-29)27-28-18(30)19(23,24)25/h1,6-7,10,15H,2-5,8,26H2/p+1/t10-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | -50.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
J Med Chem 49: 6439-42 (2006)
Article DOI: 10.1021/jm060955d
BindingDB Entry DOI: 10.7270/Q2G73BXR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM12654
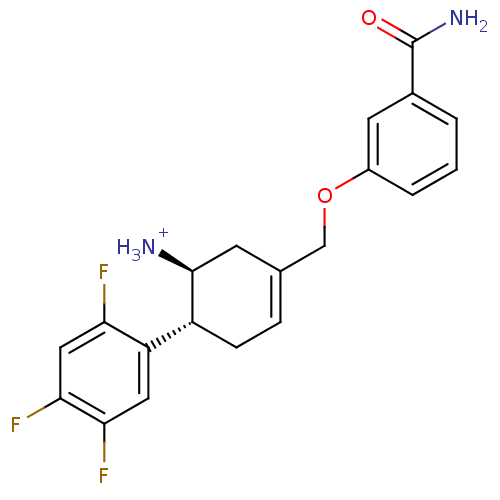
((1S,6R)-3-(3-carbamoylphenoxymethyl)-6-(2,4,5-trif...)Show SMILES NC(=O)c1cccc(OCC2=CC[C@@H]([C@@H]([NH3+])C2)c2cc(F)c(F)cc2F)c1 |r,t:10| Show InChI InChI=1S/C20H19F3N2O2/c21-16-9-18(23)17(22)8-15(16)14-5-4-11(6-19(14)24)10-27-13-3-1-2-12(7-13)20(25)26/h1-4,7-9,14,19H,5-6,10,24H2,(H2,25,26)/p+1/t14-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.60 | -47.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
J Med Chem 49: 6439-42 (2006)
Article DOI: 10.1021/jm060955d
BindingDB Entry DOI: 10.7270/Q2G73BXR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM12655
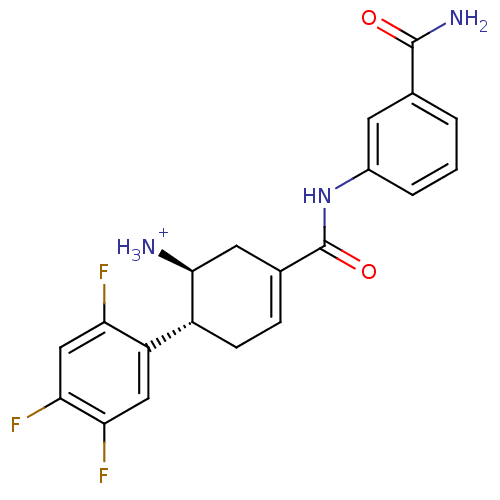
((1S,6R)-3-[(3-carbamoylphenyl)carbamoyl]-6-(2,4,5-...)Show SMILES NC(=O)c1cccc(NC(=O)C2=CC[C@@H]([C@@H]([NH3+])C2)c2cc(F)c(F)cc2F)c1 |r,t:11| Show InChI InChI=1S/C20H18F3N3O2/c21-15-9-17(23)16(22)8-14(15)13-5-4-11(7-18(13)24)20(28)26-12-3-1-2-10(6-12)19(25)27/h1-4,6,8-9,13,18H,5,7,24H2,(H2,25,27)(H,26,28)/p+1/t13-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | -47.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
J Med Chem 49: 6439-42 (2006)
Article DOI: 10.1021/jm060955d
BindingDB Entry DOI: 10.7270/Q2G73BXR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM12653
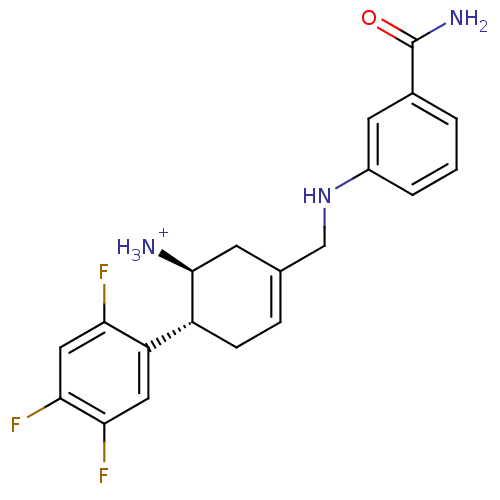
((1S,6R)-3-{[(3-carbamoylphenyl)amino]methyl}-6-(2,...)Show SMILES NC(=O)c1cccc(NCC2=CC[C@@H]([C@@H]([NH3+])C2)c2cc(F)c(F)cc2F)c1 |r,t:10| Show InChI InChI=1S/C20H20F3N3O/c21-16-9-18(23)17(22)8-15(16)14-5-4-11(6-19(14)24)10-26-13-3-1-2-12(7-13)20(25)27/h1-4,7-9,14,19,26H,5-6,10,24H2,(H2,25,27)/p+1/t14-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
J Med Chem 49: 6439-42 (2006)
Article DOI: 10.1021/jm060955d
BindingDB Entry DOI: 10.7270/Q2G73BXR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM12652
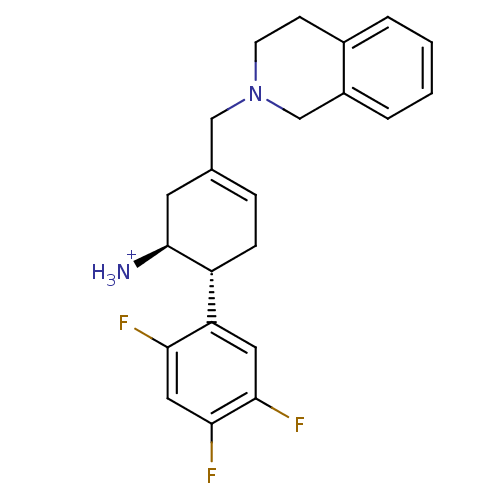
((1S,6R)-3-(1,2,3,4-tetrahydroisoquinolin-2-ylmethy...)Show SMILES [NH3+][C@H]1CC(CN2CCc3ccccc3C2)=CC[C@@H]1c1cc(F)c(F)cc1F |r,c:16| Show InChI InChI=1S/C22H23F3N2/c23-19-11-21(25)20(24)10-18(19)17-6-5-14(9-22(17)26)12-27-8-7-15-3-1-2-4-16(15)13-27/h1-5,10-11,17,22H,6-9,12-13,26H2/p+1/t17-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | -44.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
J Med Chem 49: 6439-42 (2006)
Article DOI: 10.1021/jm060955d
BindingDB Entry DOI: 10.7270/Q2G73BXR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM12651
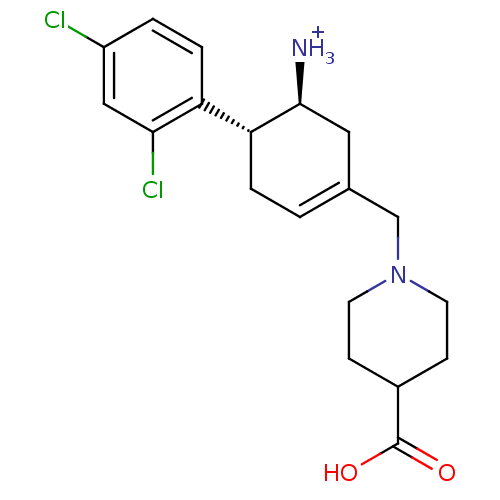
((1S,6R)-3-[(4-carboxypiperidin-1-yl)methyl]-6-(2,4...)Show SMILES [NH3+][C@H]1CC(CN2CCC(CC2)C(O)=O)=CC[C@@H]1c1ccc(Cl)cc1Cl |r,c:14| Show InChI InChI=1S/C19H24Cl2N2O2/c20-14-2-4-15(17(21)10-14)16-3-1-12(9-18(16)22)11-23-7-5-13(6-8-23)19(24)25/h1-2,4,10,13,16,18H,3,5-9,11,22H2,(H,24,25)/p+1/t16-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 45 | -41.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
J Med Chem 49: 6439-42 (2006)
Article DOI: 10.1021/jm060955d
BindingDB Entry DOI: 10.7270/Q2G73BXR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM12652

((1S,6R)-3-(1,2,3,4-tetrahydroisoquinolin-2-ylmethy...)Show SMILES [NH3+][C@H]1CC(CN2CCc3ccccc3C2)=CC[C@@H]1c1cc(F)c(F)cc1F |r,c:16| Show InChI InChI=1S/C22H23F3N2/c23-19-11-21(25)20(24)10-18(19)17-6-5-14(9-22(17)26)12-27-8-7-15-3-1-2-4-16(15)13-27/h1-5,10-11,17,22H,6-9,12-13,26H2/p+1/t17-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 73 | -40.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
J Med Chem 49: 6439-42 (2006)
Article DOI: 10.1021/jm060955d
BindingDB Entry DOI: 10.7270/Q2G73BXR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM12650
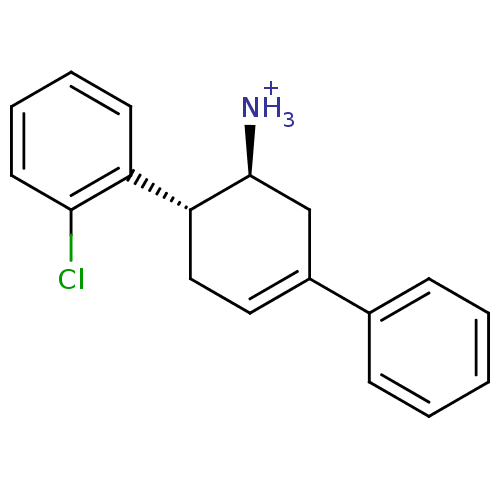
((1S,6R)-6-(2-chlorophenyl)-3-phenylcyclohex-3-en-1...)Show SMILES [NH3+][C@H]1CC(=CC[C@@H]1c1ccccc1Cl)c1ccccc1 |r,c:3| Show InChI InChI=1S/C18H18ClN/c19-17-9-5-4-8-15(17)16-11-10-14(12-18(16)20)13-6-2-1-3-7-13/h1-10,16,18H,11-12,20H2/p+1/t16-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <140 | <-38.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
J Med Chem 49: 6439-42 (2006)
Article DOI: 10.1021/jm060955d
BindingDB Entry DOI: 10.7270/Q2G73BXR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM12653

((1S,6R)-3-{[(3-carbamoylphenyl)amino]methyl}-6-(2,...)Show SMILES NC(=O)c1cccc(NCC2=CC[C@@H]([C@@H]([NH3+])C2)c2cc(F)c(F)cc2F)c1 |r,t:10| Show InChI InChI=1S/C20H20F3N3O/c21-16-9-18(23)17(22)8-15(16)14-5-4-11(6-19(14)24)10-26-13-3-1-2-12(7-13)20(25)27/h1-4,7-9,14,19,26H,5-6,10,24H2,(H2,25,27)/p+1/t14-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | -37.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
J Med Chem 49: 6439-42 (2006)
Article DOI: 10.1021/jm060955d
BindingDB Entry DOI: 10.7270/Q2G73BXR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM12652

((1S,6R)-3-(1,2,3,4-tetrahydroisoquinolin-2-ylmethy...)Show SMILES [NH3+][C@H]1CC(CN2CCc3ccccc3C2)=CC[C@@H]1c1cc(F)c(F)cc1F |r,c:16| Show InChI InChI=1S/C22H23F3N2/c23-19-11-21(25)20(24)10-18(19)17-6-5-14(9-22(17)26)12-27-8-7-15-3-1-2-4-16(15)13-27/h1-5,10-11,17,22H,6-9,12-13,26H2/p+1/t17-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 310 | -36.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
J Med Chem 49: 6439-42 (2006)
Article DOI: 10.1021/jm060955d
BindingDB Entry DOI: 10.7270/Q2G73BXR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM12654

((1S,6R)-3-(3-carbamoylphenoxymethyl)-6-(2,4,5-trif...)Show SMILES NC(=O)c1cccc(OCC2=CC[C@@H]([C@@H]([NH3+])C2)c2cc(F)c(F)cc2F)c1 |r,t:10| Show InChI InChI=1S/C20H19F3N2O2/c21-16-9-18(23)17(22)8-15(16)14-5-4-11(6-19(14)24)10-27-13-3-1-2-12(7-13)20(25)26/h1-4,7-9,14,19H,5-6,10,24H2,(H2,25,26)/p+1/t14-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 430 | -36.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
J Med Chem 49: 6439-42 (2006)
Article DOI: 10.1021/jm060955d
BindingDB Entry DOI: 10.7270/Q2G73BXR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM12653

((1S,6R)-3-{[(3-carbamoylphenyl)amino]methyl}-6-(2,...)Show SMILES NC(=O)c1cccc(NCC2=CC[C@@H]([C@@H]([NH3+])C2)c2cc(F)c(F)cc2F)c1 |r,t:10| Show InChI InChI=1S/C20H20F3N3O/c21-16-9-18(23)17(22)8-15(16)14-5-4-11(6-19(14)24)10-26-13-3-1-2-12(7-13)20(25)27/h1-4,7-9,14,19,26H,5-6,10,24H2,(H2,25,27)/p+1/t14-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | -35.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
J Med Chem 49: 6439-42 (2006)
Article DOI: 10.1021/jm060955d
BindingDB Entry DOI: 10.7270/Q2G73BXR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM12654

((1S,6R)-3-(3-carbamoylphenoxymethyl)-6-(2,4,5-trif...)Show SMILES NC(=O)c1cccc(OCC2=CC[C@@H]([C@@H]([NH3+])C2)c2cc(F)c(F)cc2F)c1 |r,t:10| Show InChI InChI=1S/C20H19F3N2O2/c21-16-9-18(23)17(22)8-15(16)14-5-4-11(6-19(14)24)10-27-13-3-1-2-12(7-13)20(25)26/h1-4,7-9,14,19H,5-6,10,24H2,(H2,25,26)/p+1/t14-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 560 | -35.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
J Med Chem 49: 6439-42 (2006)
Article DOI: 10.1021/jm060955d
BindingDB Entry DOI: 10.7270/Q2G73BXR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM12649
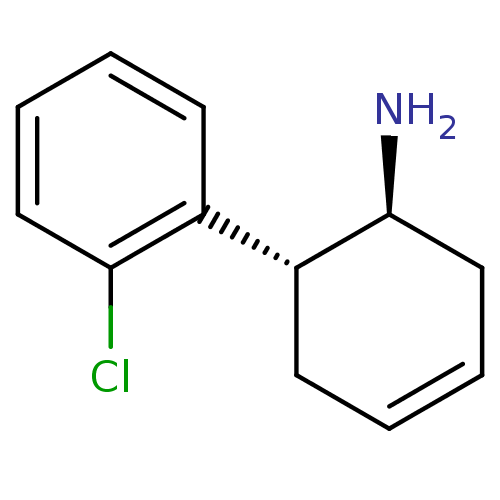
((1S,6R)-6-(2-chlorophenyl)cyclohex-3-en-1-amine | ...)Show InChI InChI=1S/C12H14ClN/c13-11-7-3-1-5-9(11)10-6-2-4-8-12(10)14/h1-5,7,10,12H,6,8,14H2/t10-,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 820 | -34.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
J Med Chem 49: 6439-42 (2006)
Article DOI: 10.1021/jm060955d
BindingDB Entry DOI: 10.7270/Q2G73BXR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM12656

(((4R,5S)-5-Amino-4-(2,4,5-trifluorophenyl)cyclohex...)Show SMILES [NH3+][C@H]1CC(=CC[C@@H]1c1cc(F)c(F)cc1F)C(=O)N1CCn2c(C1)nnc2C(F)(F)F |r,c:3| Show InChI InChI=1S/C19H17F6N5O/c20-12-7-14(22)13(21)6-11(12)10-2-1-9(5-15(10)26)17(31)29-3-4-30-16(8-29)27-28-18(30)19(23,24)25/h1,6-7,10,15H,2-5,8,26H2/p+1/t10-,15+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+3 | -30.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
J Med Chem 49: 6439-42 (2006)
Article DOI: 10.1021/jm060955d
BindingDB Entry DOI: 10.7270/Q2G73BXR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM12655

((1S,6R)-3-[(3-carbamoylphenyl)carbamoyl]-6-(2,4,5-...)Show SMILES NC(=O)c1cccc(NC(=O)C2=CC[C@@H]([C@@H]([NH3+])C2)c2cc(F)c(F)cc2F)c1 |r,t:11| Show InChI InChI=1S/C20H18F3N3O2/c21-15-9-17(23)16(22)8-14(15)13-5-4-11(7-18(13)24)20(28)26-12-3-1-2-10(6-12)19(25)27/h1-4,6,8-9,13,18H,5,7,24H2,(H2,25,27)(H,26,28)/p+1/t13-,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.80E+3 | -28.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
J Med Chem 49: 6439-42 (2006)
Article DOI: 10.1021/jm060955d
BindingDB Entry DOI: 10.7270/Q2G73BXR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM12655

((1S,6R)-3-[(3-carbamoylphenyl)carbamoyl]-6-(2,4,5-...)Show SMILES NC(=O)c1cccc(NC(=O)C2=CC[C@@H]([C@@H]([NH3+])C2)c2cc(F)c(F)cc2F)c1 |r,t:11| Show InChI InChI=1S/C20H18F3N3O2/c21-15-9-17(23)16(22)8-14(15)13-5-4-11(7-18(13)24)20(28)26-12-3-1-2-10(6-12)19(25)27/h1-4,6,8-9,13,18H,5,7,24H2,(H2,25,27)(H,26,28)/p+1/t13-,18+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+4 | -27.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
J Med Chem 49: 6439-42 (2006)
Article DOI: 10.1021/jm060955d
BindingDB Entry DOI: 10.7270/Q2G73BXR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM12656

(((4R,5S)-5-Amino-4-(2,4,5-trifluorophenyl)cyclohex...)Show SMILES [NH3+][C@H]1CC(=CC[C@@H]1c1cc(F)c(F)cc1F)C(=O)N1CCn2c(C1)nnc2C(F)(F)F |r,c:3| Show InChI InChI=1S/C19H17F6N5O/c20-12-7-14(22)13(21)6-11(12)10-2-1-9(5-15(10)26)17(31)29-3-4-30-16(8-29)27-28-18(30)19(23,24)25/h1,6-7,10,15H,2-5,8,26H2/p+1/t10-,15+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | >-25.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The DPP activity resulted in the formation of the fluorescent product amidomethylcoumarin (AMC), which was monitored by excitation at 355 nm and meas... |
J Med Chem 49: 6439-42 (2006)
Article DOI: 10.1021/jm060955d
BindingDB Entry DOI: 10.7270/Q2G73BXR |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50381696

(CHEMBL2021941)Show SMILES Cc1cccc(NC(=O)Nc2ccc(cc2)-c2csc3c(cnc(N)c23)-c2cc[nH]c2)c1 Show InChI InChI=1S/C25H21N5OS/c1-15-3-2-4-19(11-15)30-25(31)29-18-7-5-16(6-8-18)21-14-32-23-20(17-9-10-27-12-17)13-28-24(26)22(21)23/h2-14,27H,1H3,(H2,26,28)(H2,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human KDR phosphorylation expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 22: 3208-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.035
BindingDB Entry DOI: 10.7270/Q2000336 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50381719
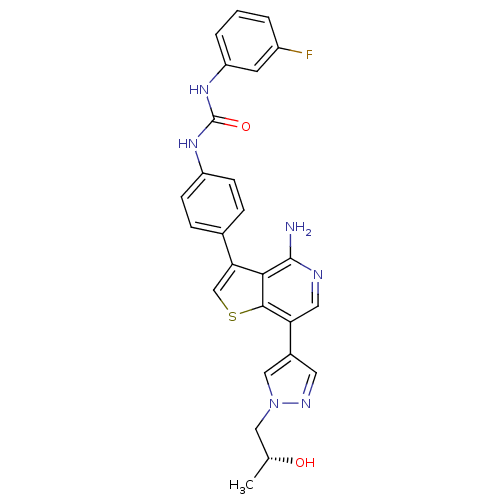
(CHEMBL2022856)Show SMILES C[C@@H](O)Cn1cc(cn1)-c1cnc(N)c2c(csc12)-c1ccc(NC(=O)Nc2cccc(F)c2)cc1 |r| Show InChI InChI=1S/C26H23FN6O2S/c1-15(34)12-33-13-17(10-30-33)21-11-29-25(28)23-22(14-36-24(21)23)16-5-7-19(8-6-16)31-26(35)32-20-4-2-3-18(27)9-20/h2-11,13-15,34H,12H2,1H3,(H2,28,29)(H2,31,32,35)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B using 1 mM ATP by HTRF assay |
Bioorg Med Chem Lett 22: 3208-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.035
BindingDB Entry DOI: 10.7270/Q2000336 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50381693
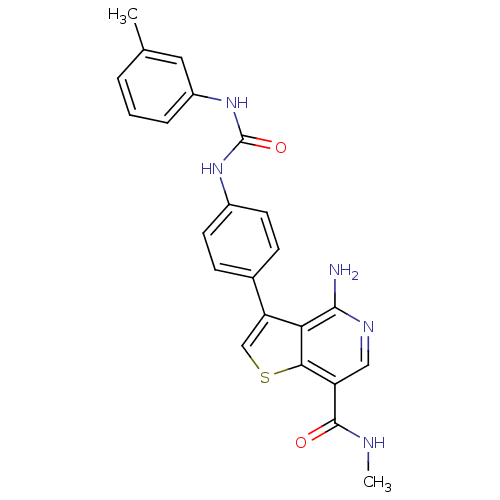
(CHEMBL2023221)Show SMILES CNC(=O)c1cnc(N)c2c(csc12)-c1ccc(NC(=O)Nc2cccc(C)c2)cc1 Show InChI InChI=1S/C23H21N5O2S/c1-13-4-3-5-16(10-13)28-23(30)27-15-8-6-14(7-9-15)18-12-31-20-17(22(29)25-2)11-26-21(24)19(18)20/h3-12H,1-2H3,(H2,24,26)(H,25,29)(H2,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human KDR phosphorylation expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 22: 3208-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.035
BindingDB Entry DOI: 10.7270/Q2000336 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50381713
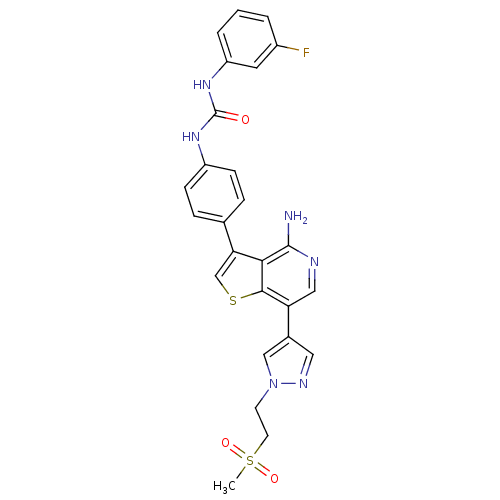
(CHEMBL2022853)Show SMILES CS(=O)(=O)CCn1cc(cn1)-c1cnc(N)c2c(csc12)-c1ccc(NC(=O)Nc2cccc(F)c2)cc1 Show InChI InChI=1S/C26H23FN6O3S2/c1-38(35,36)10-9-33-14-17(12-30-33)21-13-29-25(28)23-22(15-37-24(21)23)16-5-7-19(8-6-16)31-26(34)32-20-4-2-3-18(27)11-20/h2-8,11-15H,9-10H2,1H3,(H2,28,29)(H2,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human KDR phosphorylation expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 22: 3208-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.035
BindingDB Entry DOI: 10.7270/Q2000336 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50185040
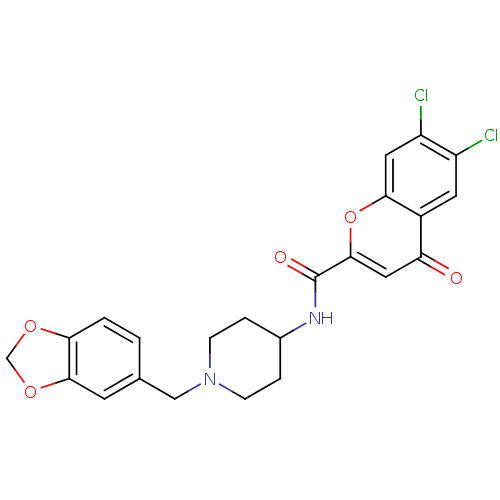
(CHEMBL204828 | N-[1-(1,3-benzodioxol-5-ylmethyl)pi...)Show SMILES Clc1cc2oc(cc(=O)c2cc1Cl)C(=O)NC1CCN(Cc2ccc3OCOc3c2)CC1 Show InChI InChI=1S/C23H20Cl2N2O5/c24-16-8-15-18(28)10-22(32-20(15)9-17(16)25)23(29)26-14-3-5-27(6-4-14)11-13-1-2-19-21(7-13)31-12-30-19/h1-2,7-10,14H,3-6,11-12H2,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG |
J Med Chem 49: 6569-84 (2006)
Article DOI: 10.1021/jm060683e
BindingDB Entry DOI: 10.7270/Q2NG4Q7P |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50162116
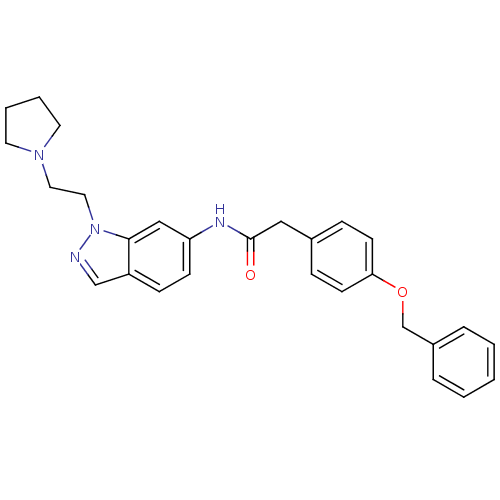
(2-(4-(benzyloxy)phenyl)-N-(1-(2-(pyrrolidin-1-yl)e...)Show SMILES O=C(Cc1ccc(OCc2ccccc2)cc1)Nc1ccc2cnn(CCN3CCCC3)c2c1 Show InChI InChI=1S/C28H30N4O2/c33-28(18-22-8-12-26(13-9-22)34-21-23-6-2-1-3-7-23)30-25-11-10-24-20-29-32(27(24)19-25)17-16-31-14-4-5-15-31/h1-3,6-13,19-20H,4-5,14-18,21H2,(H,30,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-MCH from MCHr1 expressed in IMR32 (I3.4.2) cells |
J Med Chem 49: 2339-52 (2006)
Article DOI: 10.1021/jm0512286
BindingDB Entry DOI: 10.7270/Q24F1Q9F |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50381716
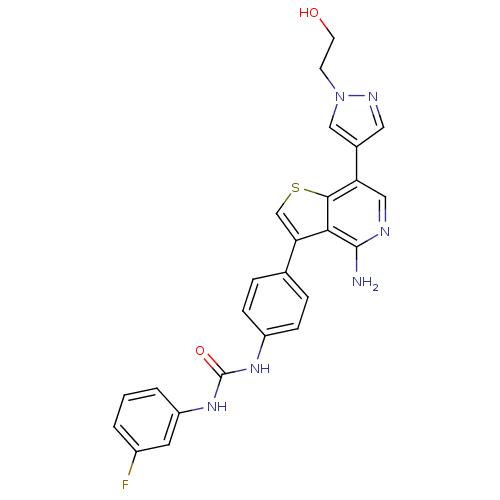
(ABT-348 | ILORASERTIB | US8722890, 1 | US8722890, ...)Show SMILES Nc1ncc(-c2cnn(CCO)c2)c2scc(-c3ccc(NC(=O)Nc4cccc(F)c4)cc3)c12 Show InChI InChI=1S/C25H21FN6O2S/c26-17-2-1-3-19(10-17)31-25(34)30-18-6-4-15(5-7-18)21-14-35-23-20(12-28-24(27)22(21)23)16-11-29-32(13-16)8-9-33/h1-7,10-14,33H,8-9H2,(H2,27,28)(H2,30,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human KDR phosphorylation expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 22: 3208-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.035
BindingDB Entry DOI: 10.7270/Q2000336 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50381709
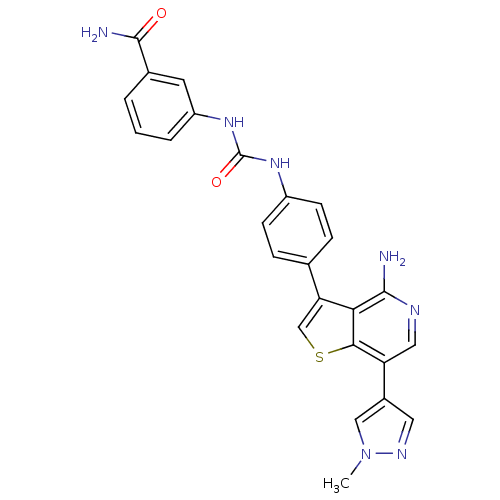
(CHEMBL2022850)Show SMILES Cn1cc(cn1)-c1cnc(N)c2c(csc12)-c1ccc(NC(=O)Nc2cccc(c2)C(N)=O)cc1 Show InChI InChI=1S/C25H21N7O2S/c1-32-12-16(10-29-32)19-11-28-23(26)21-20(13-35-22(19)21)14-5-7-17(8-6-14)30-25(34)31-18-4-2-3-15(9-18)24(27)33/h2-13H,1H3,(H2,26,28)(H2,27,33)(H2,30,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human KDR phosphorylation expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 22: 3208-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.035
BindingDB Entry DOI: 10.7270/Q2000336 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50197136

(CHEMBL387178 | N-[1-(1,3-benzodioxol-5-ylmethyl)pi...)Show SMILES Fc1cc2oc(cc(=O)c2cc1F)C(=O)NC1CCN(Cc2ccc3OCOc3c2)CC1 Show InChI InChI=1S/C23H20F2N2O5/c24-16-8-15-18(28)10-22(32-20(15)9-17(16)25)23(29)26-14-3-5-27(6-4-14)11-13-1-2-19-21(7-13)31-12-30-19/h1-2,7-10,14H,3-6,11-12H2,(H,26,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHr1 expressed in IMR32 cells |
J Med Chem 49: 6569-84 (2006)
Article DOI: 10.1021/jm060683e
BindingDB Entry DOI: 10.7270/Q2NG4Q7P |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50197143

(CHEMBL277531 | N-[1-(1,3-benzodioxol-5-ylmethyl)pi...)Show SMILES Cc1c(oc2cc(Cl)ccc2c1=O)C(=O)NC1CCN(Cc2ccc3OCOc3c2)CC1 Show InChI InChI=1S/C24H23ClN2O5/c1-14-22(28)18-4-3-16(25)11-20(18)32-23(14)24(29)26-17-6-8-27(9-7-17)12-15-2-5-19-21(10-15)31-13-30-19/h2-5,10-11,17H,6-9,12-13H2,1H3,(H,26,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHr1 expressed in IMR32 cells |
J Med Chem 49: 6569-84 (2006)
Article DOI: 10.1021/jm060683e
BindingDB Entry DOI: 10.7270/Q2NG4Q7P |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50197135
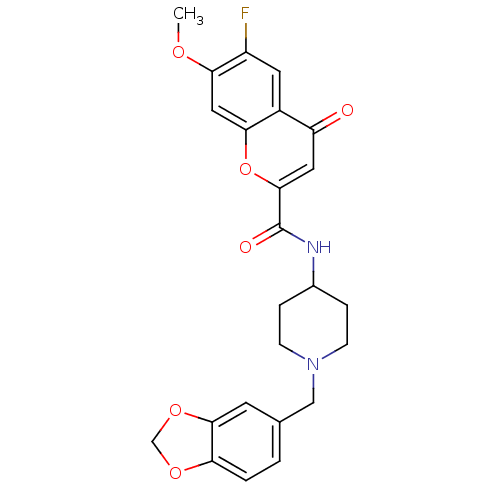
(CHEMBL217442 | N-[1-(1,3-benzodioxol-5-ylmethyl)pi...)Show SMILES COc1cc2oc(cc(=O)c2cc1F)C(=O)NC1CCN(Cc2ccc3OCOc3c2)CC1 Show InChI InChI=1S/C24H23FN2O6/c1-30-21-11-20-16(9-17(21)25)18(28)10-23(33-20)24(29)26-15-4-6-27(7-5-15)12-14-2-3-19-22(8-14)32-13-31-19/h2-3,8-11,15H,4-7,12-13H2,1H3,(H,26,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHr1 expressed in IMR32 cells |
J Med Chem 49: 6569-84 (2006)
Article DOI: 10.1021/jm060683e
BindingDB Entry DOI: 10.7270/Q2NG4Q7P |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50173460
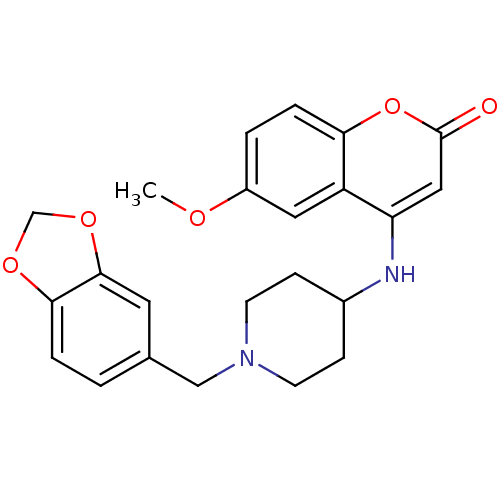
(4-(1-(benzo[d][1,3]dioxol-5-ylmethyl)piperidin-4-y...)Show SMILES COc1ccc2oc(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C23H24N2O5/c1-27-17-3-5-20-18(11-17)19(12-23(26)30-20)24-16-6-8-25(9-7-16)13-15-2-4-21-22(10-15)29-14-28-21/h2-5,10-12,16,24H,6-9,13-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-MCH from MCHr1 expressed in IMR32 (I3.4.2) cells |
J Med Chem 49: 2339-52 (2006)
Article DOI: 10.1021/jm0512286
BindingDB Entry DOI: 10.7270/Q24F1Q9F |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50171590
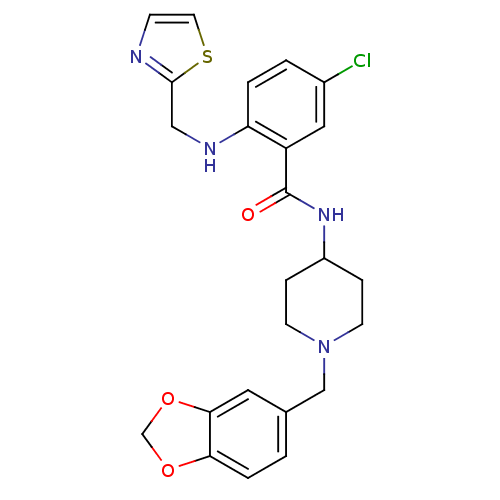
(CHEMBL194697 | N-(1-Benzo[1,3]dioxol-5-ylmethyl-pi...)Show SMILES Clc1ccc(NCc2nccs2)c(c1)C(=O)NC1CCN(Cc2ccc3OCOc3c2)CC1 Show InChI InChI=1S/C24H25ClN4O3S/c25-17-2-3-20(27-13-23-26-7-10-33-23)19(12-17)24(30)28-18-5-8-29(9-6-18)14-16-1-4-21-22(11-16)32-15-31-21/h1-4,7,10-12,18,27H,5-6,8-9,13-15H2,(H,28,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-MCH from MCHr1 expressed in IMR32 (I3.4.2) cells |
J Med Chem 49: 2339-52 (2006)
Article DOI: 10.1021/jm0512286
BindingDB Entry DOI: 10.7270/Q24F1Q9F |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50381693

(CHEMBL2023221)Show SMILES CNC(=O)c1cnc(N)c2c(csc12)-c1ccc(NC(=O)Nc2cccc(C)c2)cc1 Show InChI InChI=1S/C23H21N5O2S/c1-13-4-3-5-16(10-13)28-23(30)27-15-8-6-14(7-9-15)18-12-31-20-17(22(29)25-2)11-26-21(24)19(18)20/h3-12H,1-2H3,(H2,24,26)(H,25,29)(H2,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human KDR phosphorylation expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 22: 3208-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.035
BindingDB Entry DOI: 10.7270/Q2000336 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50173461

(4-(1-Benzo[1,3]dioxol-5-ylmethyl-piperidin-4-ylami...)Show SMILES Clc1ccc2oc(=O)cc(NC3CCN(Cc4ccc5OCOc5c4)CC3)c2c1 Show InChI InChI=1S/C22H21ClN2O4/c23-15-2-4-19-17(10-15)18(11-22(26)29-19)24-16-5-7-25(8-6-16)12-14-1-3-20-21(9-14)28-13-27-20/h1-4,9-11,16,24H,5-8,12-13H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration aganist melanin concentrating hormone receptor 1 expressed in IMR-32 cells using [125I]-MCH |
J Med Chem 48: 5888-91 (2005)
Article DOI: 10.1021/jm050598r
BindingDB Entry DOI: 10.7270/Q2QJ7GTV |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50381717
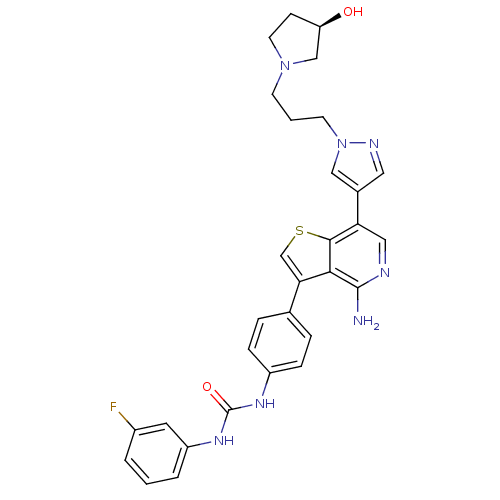
(CHEMBL2022857)Show SMILES Nc1ncc(-c2cnn(CCCN3CC[C@@H](O)C3)c2)c2scc(-c3ccc(NC(=O)Nc4cccc(F)c4)cc3)c12 |r| Show InChI InChI=1S/C30H30FN7O2S/c31-21-3-1-4-23(13-21)36-30(40)35-22-7-5-19(6-8-22)26-18-41-28-25(15-33-29(32)27(26)28)20-14-34-38(16-20)11-2-10-37-12-9-24(39)17-37/h1,3-8,13-16,18,24,39H,2,9-12,17H2,(H2,32,33)(H2,35,36,40)/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B using 1 mM ATP by HTRF assay |
Bioorg Med Chem Lett 22: 3208-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.035
BindingDB Entry DOI: 10.7270/Q2000336 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50381699
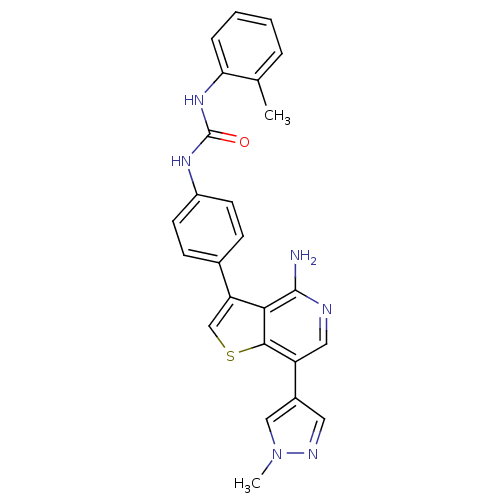
(CHEMBL2023225)Show SMILES Cc1ccccc1NC(=O)Nc1ccc(cc1)-c1csc2c(cnc(N)c12)-c1cnn(C)c1 Show InChI InChI=1S/C25H22N6OS/c1-15-5-3-4-6-21(15)30-25(32)29-18-9-7-16(8-10-18)20-14-33-23-19(12-27-24(26)22(20)23)17-11-28-31(2)13-17/h3-14H,1-2H3,(H2,26,27)(H2,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human KDR phosphorylation expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 22: 3208-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.035
BindingDB Entry DOI: 10.7270/Q2000336 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50381716

(ABT-348 | ILORASERTIB | US8722890, 1 | US8722890, ...)Show SMILES Nc1ncc(-c2cnn(CCO)c2)c2scc(-c3ccc(NC(=O)Nc4cccc(F)c4)cc3)c12 Show InChI InChI=1S/C25H21FN6O2S/c26-17-2-1-3-19(10-17)31-25(34)30-18-6-4-15(5-7-18)21-14-35-23-20(12-28-24(27)22(21)23)16-11-29-32(13-16)8-9-33/h1-7,10-14,33H,8-9H2,(H2,27,28)(H2,30,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Aurora B by TR-FRET assay |
Bioorg Med Chem Lett 22: 3208-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.035
BindingDB Entry DOI: 10.7270/Q2000336 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50381698
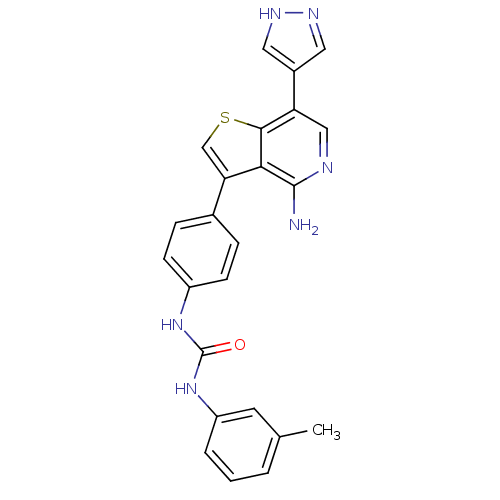
(CHEMBL1969102 | US8722890, 6)Show SMILES Cc1cccc(NC(=O)Nc2ccc(cc2)-c2csc3c(cnc(N)c23)-c2cn[nH]c2)c1 Show InChI InChI=1S/C24H20N6OS/c1-14-3-2-4-18(9-14)30-24(31)29-17-7-5-15(6-8-17)20-13-32-22-19(16-10-27-28-11-16)12-26-23(25)21(20)22/h2-13H,1H3,(H2,25,26)(H,27,28)(H2,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of KDR using 1 mM ATP by HTRF assay |
Bioorg Med Chem Lett 22: 3208-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.035
BindingDB Entry DOI: 10.7270/Q2000336 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50381717

(CHEMBL2022857)Show SMILES Nc1ncc(-c2cnn(CCCN3CC[C@@H](O)C3)c2)c2scc(-c3ccc(NC(=O)Nc4cccc(F)c4)cc3)c12 |r| Show InChI InChI=1S/C30H30FN7O2S/c31-21-3-1-4-23(13-21)36-30(40)35-22-7-5-19(6-8-22)26-18-41-28-25(15-33-29(32)27(26)28)20-14-34-38(16-20)11-2-10-37-12-9-24(39)17-37/h1,3-8,13-16,18,24,39H,2,9-12,17H2,(H2,32,33)(H2,35,36,40)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human KDR phosphorylation expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 22: 3208-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.035
BindingDB Entry DOI: 10.7270/Q2000336 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50197116
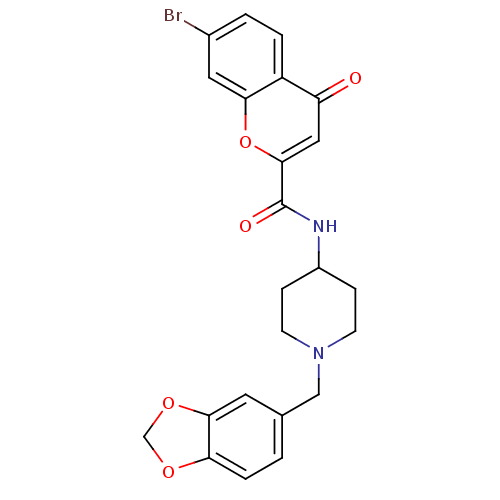
(CHEMBL217706 | N-[1-(1,3-benzodioxol-5-ylmethyl)pi...)Show SMILES Brc1ccc2c(c1)oc(cc2=O)C(=O)NC1CCN(Cc2ccc3OCOc3c2)CC1 Show InChI InChI=1S/C23H21BrN2O5/c24-15-2-3-17-18(27)11-22(31-20(17)10-15)23(28)25-16-5-7-26(8-6-16)12-14-1-4-19-21(9-14)30-13-29-19/h1-4,9-11,16H,5-8,12-13H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG |
J Med Chem 49: 6569-84 (2006)
Article DOI: 10.1021/jm060683e
BindingDB Entry DOI: 10.7270/Q2NG4Q7P |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50185040

(CHEMBL204828 | N-[1-(1,3-benzodioxol-5-ylmethyl)pi...)Show SMILES Clc1cc2oc(cc(=O)c2cc1Cl)C(=O)NC1CCN(Cc2ccc3OCOc3c2)CC1 Show InChI InChI=1S/C23H20Cl2N2O5/c24-16-8-15-18(28)10-22(32-20(15)9-17(16)25)23(29)26-14-3-5-27(6-4-14)11-13-1-2-19-21(7-13)31-12-30-19/h1-2,7-10,14H,3-6,11-12H2,(H,26,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-MCH from MCHr1 expressed in IMR32 (I3.4.2) cells |
J Med Chem 49: 2339-52 (2006)
Article DOI: 10.1021/jm0512286
BindingDB Entry DOI: 10.7270/Q24F1Q9F |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
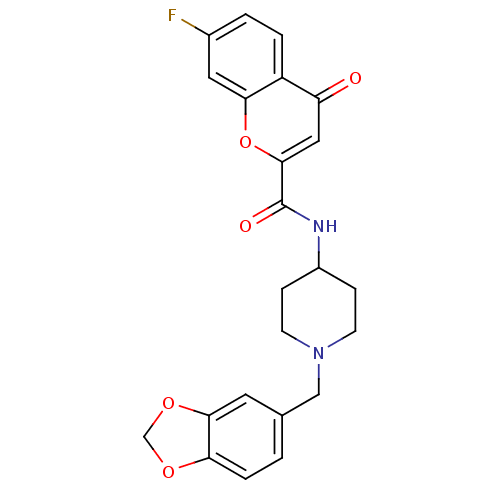
(Homo sapiens (Human)) | BDBM50197139
(CHEMBL214021 | N-(1-(benzo[d][1,3]dioxol-5-ylmethy...)Show SMILES Fc1ccc2c(c1)oc(cc2=O)C(=O)NC1CCN(Cc2ccc3OCOc3c2)CC1 Show InChI InChI=1S/C23H21FN2O5/c24-15-2-3-17-18(27)11-22(31-20(17)10-15)23(28)25-16-5-7-26(8-6-16)12-14-1-4-19-21(9-14)30-13-29-19/h1-4,9-11,16H,5-8,12-13H2,(H,25,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHr1 expressed in IMR32 cells |
J Med Chem 49: 6569-84 (2006)
Article DOI: 10.1021/jm060683e
BindingDB Entry DOI: 10.7270/Q2NG4Q7P |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50173454

(4-(1-Benzooxazol-5-ylmethyl-piperidin-4-ylamino)-6...)Show SMILES Clc1ccc2oc(=O)cc(NC3CCN(Cc4ccc5ocnc5c4)CC3)c2c1 Show InChI InChI=1S/C22H20ClN3O3/c23-15-2-4-20-17(10-15)18(11-22(27)29-20)25-16-5-7-26(8-6-16)12-14-1-3-21-19(9-14)24-13-28-21/h1-4,9-11,13,16,25H,5-8,12H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration aganist melanin concentrating hormone receptor 1 expressed in IMR-32 cells using [125I]-MCH |
J Med Chem 48: 5888-91 (2005)
Article DOI: 10.1021/jm050598r
BindingDB Entry DOI: 10.7270/Q2QJ7GTV |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50197144

(CHEMBL387418 | N-[1-(1,3-benzodioxol-5-ylmethyl)pi...)Show SMILES Fc1cc2c(cc1Cl)oc(cc2=O)C(=O)NC1CCN(Cc2ccc3OCOc3c2)CC1 Show InChI InChI=1S/C23H20ClFN2O5/c24-16-9-20-15(8-17(16)25)18(28)10-22(32-20)23(29)26-14-3-5-27(6-4-14)11-13-1-2-19-21(7-13)31-12-30-19/h1-2,7-10,14H,3-6,11-12H2,(H,26,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]MCH from MCHr1 expressed in IMR32 cells |
J Med Chem 49: 6569-84 (2006)
Article DOI: 10.1021/jm060683e
BindingDB Entry DOI: 10.7270/Q2NG4Q7P |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50381708
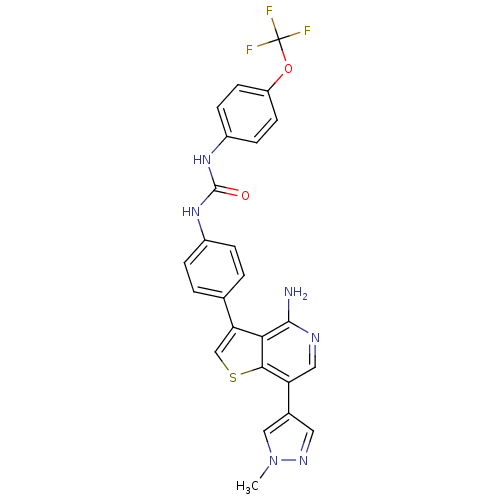
(CHEMBL2023232)Show SMILES Cn1cc(cn1)-c1cnc(N)c2c(csc12)-c1ccc(NC(=O)Nc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C25H19F3N6O2S/c1-34-12-15(10-31-34)19-11-30-23(29)21-20(13-37-22(19)21)14-2-4-16(5-3-14)32-24(35)33-17-6-8-18(9-7-17)36-25(26,27)28/h2-13H,1H3,(H2,29,30)(H2,32,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human KDR phosphorylation expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 22: 3208-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.035
BindingDB Entry DOI: 10.7270/Q2000336 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
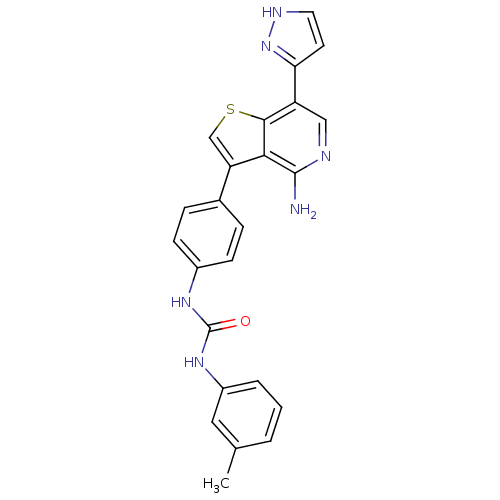
(Homo sapiens (Human)) | BDBM50381697
(CHEMBL2023224)Show SMILES Cc1cccc(NC(=O)Nc2ccc(cc2)-c2csc3c(cnc(N)c23)-c2cc[nH]n2)c1 Show InChI InChI=1S/C24H20N6OS/c1-14-3-2-4-17(11-14)29-24(31)28-16-7-5-15(6-8-16)19-13-32-22-18(20-9-10-27-30-20)12-26-23(25)21(19)22/h2-13H,1H3,(H2,25,26)(H,27,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human KDR phosphorylation expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 22: 3208-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.035
BindingDB Entry DOI: 10.7270/Q2000336 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
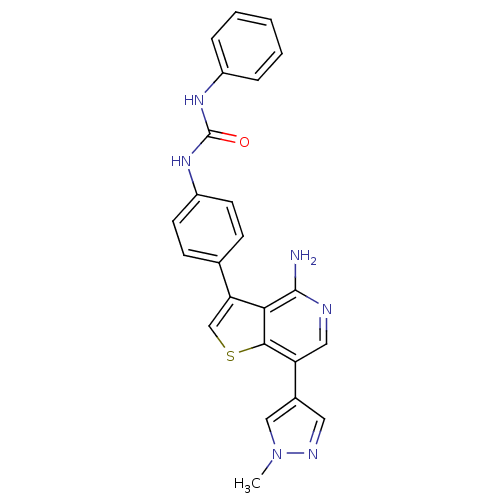
(Homo sapiens (Human)) | BDBM50381701
(CHEMBL2023226)Show SMILES Cn1cc(cn1)-c1cnc(N)c2c(csc12)-c1ccc(NC(=O)Nc2ccccc2)cc1 Show InChI InChI=1S/C24H20N6OS/c1-30-13-16(11-27-30)19-12-26-23(25)21-20(14-32-22(19)21)15-7-9-18(10-8-15)29-24(31)28-17-5-3-2-4-6-17/h2-14H,1H3,(H2,25,26)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human KDR phosphorylation expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 22: 3208-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.035
BindingDB Entry DOI: 10.7270/Q2000336 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
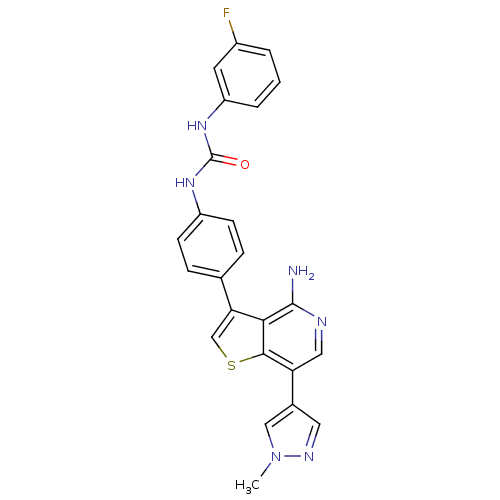
(Homo sapiens (Human)) | BDBM50381703
(CHEMBL1970317)Show SMILES Cn1cc(cn1)-c1cnc(N)c2c(csc12)-c1ccc(NC(=O)Nc2cccc(F)c2)cc1 Show InChI InChI=1S/C24H19FN6OS/c1-31-12-15(10-28-31)19-11-27-23(26)21-20(13-33-22(19)21)14-5-7-17(8-6-14)29-24(32)30-18-4-2-3-16(25)9-18/h2-13H,1H3,(H2,26,27)(H2,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human KDR phosphorylation expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 22: 3208-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.035
BindingDB Entry DOI: 10.7270/Q2000336 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50381702

(CHEMBL2023227)Show SMILES Cn1cc(cn1)-c1cnc(N)c2c(csc12)-c1ccc(NC(=O)Nc2ccccc2F)cc1 Show InChI InChI=1S/C24H19FN6OS/c1-31-12-15(10-28-31)17-11-27-23(26)21-18(13-33-22(17)21)14-6-8-16(9-7-14)29-24(32)30-20-5-3-2-4-19(20)25/h2-13H,1H3,(H2,26,27)(H2,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human KDR phosphorylation expressed in mouse NIH3T3 cells |
Bioorg Med Chem Lett 22: 3208-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.035
BindingDB Entry DOI: 10.7270/Q2000336 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50381716

(ABT-348 | ILORASERTIB | US8722890, 1 | US8722890, ...)Show SMILES Nc1ncc(-c2cnn(CCO)c2)c2scc(-c3ccc(NC(=O)Nc4cccc(F)c4)cc3)c12 Show InChI InChI=1S/C25H21FN6O2S/c26-17-2-1-3-19(10-17)31-25(34)30-18-6-4-15(5-7-18)21-14-35-23-20(12-28-24(27)22(21)23)16-11-29-32(13-16)8-9-33/h1-7,10-14,33H,8-9H2,(H2,27,28)(H2,30,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of LCK by TR-FRET assay |
Bioorg Med Chem Lett 22: 3208-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.035
BindingDB Entry DOI: 10.7270/Q2000336 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50381716

(ABT-348 | ILORASERTIB | US8722890, 1 | US8722890, ...)Show SMILES Nc1ncc(-c2cnn(CCO)c2)c2scc(-c3ccc(NC(=O)Nc4cccc(F)c4)cc3)c12 Show InChI InChI=1S/C25H21FN6O2S/c26-17-2-1-3-19(10-17)31-25(34)30-18-6-4-15(5-7-18)21-14-35-23-20(12-28-24(27)22(21)23)16-11-29-32(13-16)8-9-33/h1-7,10-14,33H,8-9H2,(H2,27,28)(H2,30,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta by TR-FRET assay |
Bioorg Med Chem Lett 22: 3208-12 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.035
BindingDB Entry DOI: 10.7270/Q2000336 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data