

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210717 (CHEMBL397275 | N1-((2S,3R)-4-(3-ethylbenzylamino)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine--tRNA ligase alpha/beta subunit (Escherichia coli (Enterobacteria)) | BDBM231666 (N-(2-(1H-indol-3-yl)ethyl)-3-(3-thiazol-2-ylureido...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston | Assay Description Compounds were solubilized in DMSO. Serial 2-fold dilutions covering two concentration ranges, 10 mM to 19.5 μM and 100 μM to 195 nM, were ... | J Biol Chem 289: 21651-62 (2014) Article DOI: 10.1074/jbc.M114.574061 BindingDB Entry DOI: 10.7270/Q2BG2MVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine--tRNA ligase alpha/beta subunit (Escherichia coli (Enterobacteria)) | BDBM231665 (1-(3-(4-(Pyridin-2-yl)piperazin-1-ylsulfonyl)pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston | Assay Description Compounds were solubilized in DMSO. Serial 2-fold dilutions covering two concentration ranges, 10 mM to 19.5 μM and 100 μM to 195 nM, were ... | J Biol Chem 289: 21651-62 (2014) Article DOI: 10.1074/jbc.M114.574061 BindingDB Entry DOI: 10.7270/Q2BG2MVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210721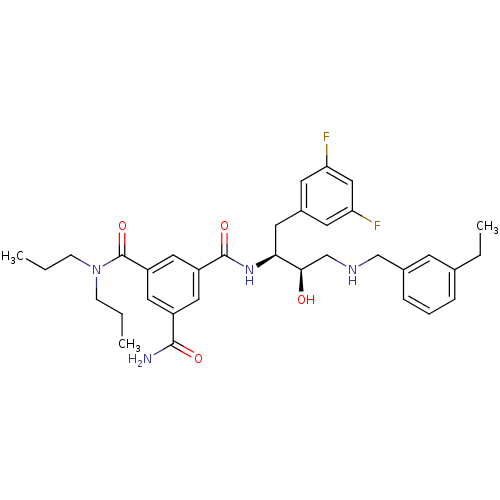 (CHEMBL397714 | N1-((2S,3R)-4-(3-ethylbenzylamino)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM15797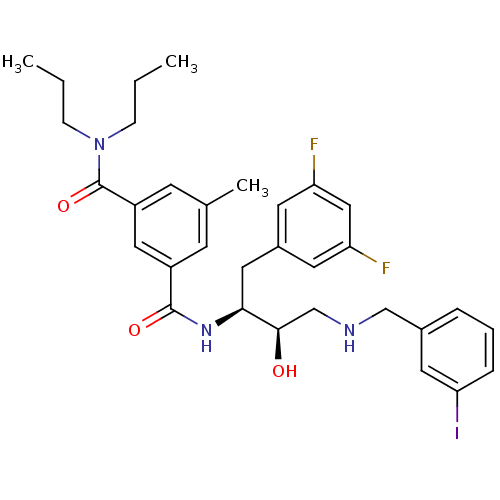 ((1S,2R)-N-[1-(3,5-Difluorobenzyl)-2-hydroxy-3-(3-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.8 | 37 |
Elan Pharmaceuticals | Assay Description Beta-cleavage ELISA assays were carried out in reaction buffer containing enzyme, substrate MBP-C125, and test compounds. Generated beta-cleaved prod... | J Med Chem 50: 776-81 (2007) Article DOI: 10.1021/jm061242y BindingDB Entry DOI: 10.7270/Q2K935TZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210709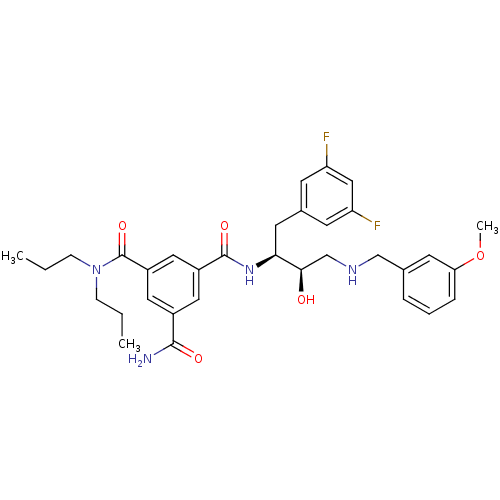 (CHEMBL396765 | N1-((2S,3R)-4-(3-methoxybenzylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phenylalanine--tRNA ligase alpha/beta subunit (Pseudomonas aeruginosa) | BDBM231666 (N-(2-(1H-indol-3-yl)ethyl)-3-(3-thiazol-2-ylureido...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston | Assay Description Compounds were solubilized in DMSO. Serial 2-fold dilutions covering two concentration ranges, 10 mM to 19.5 μM and 100 μM to 195 nM, were ... | J Biol Chem 289: 21651-62 (2014) Article DOI: 10.1074/jbc.M114.574061 BindingDB Entry DOI: 10.7270/Q2BG2MVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM15798 ((1S,2R)-N-[1-(3,5-Difluorobenzyl)-2-hydroxy-3-(3-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 4.8 | 37 |
Elan Pharmaceuticals | Assay Description Beta-cleavage ELISA assays were carried out in reaction buffer containing enzyme, substrate MBP-C125, and test compounds. Generated beta-cleaved prod... | J Med Chem 50: 776-81 (2007) Article DOI: 10.1021/jm061242y BindingDB Entry DOI: 10.7270/Q2K935TZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phenylalanine--tRNA ligase alpha/beta subunit (Pseudomonas aeruginosa) | BDBM231665 (1-(3-(4-(Pyridin-2-yl)piperazin-1-ylsulfonyl)pheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston | Assay Description Compounds were solubilized in DMSO. Serial 2-fold dilutions covering two concentration ranges, 10 mM to 19.5 μM and 100 μM to 195 nM, were ... | J Biol Chem 289: 21651-62 (2014) Article DOI: 10.1074/jbc.M114.574061 BindingDB Entry DOI: 10.7270/Q2BG2MVZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210719 (3-(((2S,3R)-4-(3-methoxybenzylamino)-3-hydroxy-1-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM15790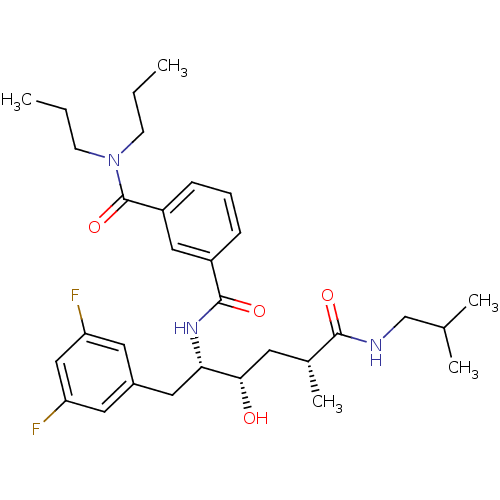 (3-N-[(2S,3S,5R)-1-(3,5-difluorophenyl)-3-hydroxy-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 4.8 | 37 |
Elan Pharmaceuticals | Assay Description Beta-cleavage ELISA assays were carried out in reaction buffer containing enzyme, substrate MBP-C125, and test compounds. Generated beta-cleaved prod... | J Med Chem 50: 776-81 (2007) Article DOI: 10.1021/jm061242y BindingDB Entry DOI: 10.7270/Q2K935TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine--tRNA ligase alpha/beta subunit (Escherichia coli (Enterobacteria)) | BDBM231670 (5-(3-Methoxyphenyl)-3-(trifluoromethyl)-1H-pyrazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston | Assay Description Compounds were solubilized in DMSO. Serial 2-fold dilutions covering two concentration ranges, 10 mM to 19.5 μM and 100 μM to 195 nM, were ... | J Biol Chem 289: 21651-62 (2014) Article DOI: 10.1074/jbc.M114.574061 BindingDB Entry DOI: 10.7270/Q2BG2MVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM15794 ((1S,2R)-N-[1-(3,5-Difluoro-benzyl)-2-hydroxy-3-(S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | 4.8 | 37 |
Elan Pharmaceuticals | Assay Description Beta-cleavage ELISA assays were carried out in reaction buffer containing enzyme, substrate MBP-C125, and test compounds. Generated beta-cleaved prod... | J Med Chem 50: 776-81 (2007) Article DOI: 10.1021/jm061242y BindingDB Entry DOI: 10.7270/Q2K935TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine--tRNA ligase alpha/beta subunit (Escherichia coli (Enterobacteria)) | BDBM231674 (N-(2-(1-(methylsulfonyl)cyclopropyl)ethyl)-2-(3-(5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston | Assay Description Compounds were solubilized in DMSO. Serial 2-fold dilutions covering two concentration ranges, 10 mM to 19.5 μM and 100 μM to 195 nM, were ... | J Biol Chem 289: 21651-62 (2014) Article DOI: 10.1074/jbc.M114.574061 BindingDB Entry DOI: 10.7270/Q2BG2MVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210715 (CHEMBL230807 | N1-((2S,3R)-4-(3-methoxybenzylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239369 (CHEMBL4100303) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine--tRNA ligase alpha/beta subunit (Escherichia coli (Enterobacteria)) | BDBM231671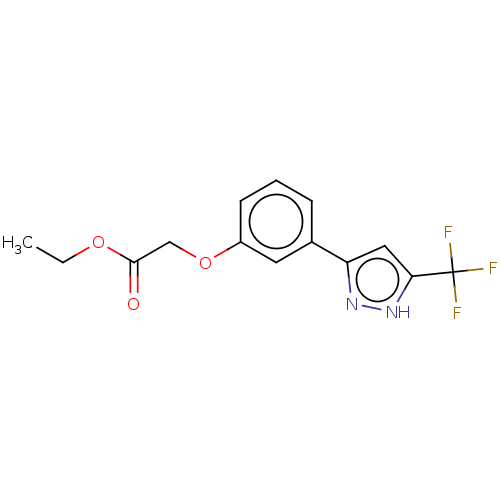 (Ethyl 2-(3-(5-(trifluoromethyl)-1H-pyrazol-3-yl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston | Assay Description Compounds were solubilized in DMSO. Serial 2-fold dilutions covering two concentration ranges, 10 mM to 19.5 μM and 100 μM to 195 nM, were ... | J Biol Chem 289: 21651-62 (2014) Article DOI: 10.1074/jbc.M114.574061 BindingDB Entry DOI: 10.7270/Q2BG2MVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239369 (CHEMBL4100303) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210718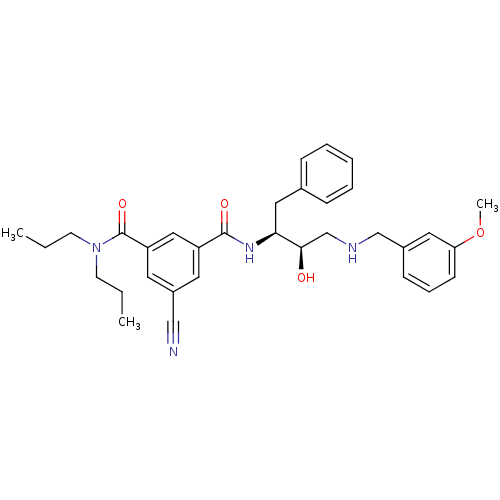 (CHEMBL231646 | N1-((2S,3R)-4-(3-methoxybenzylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine--tRNA ligase alpha/beta subunit (Escherichia coli (Enterobacteria)) | BDBM231676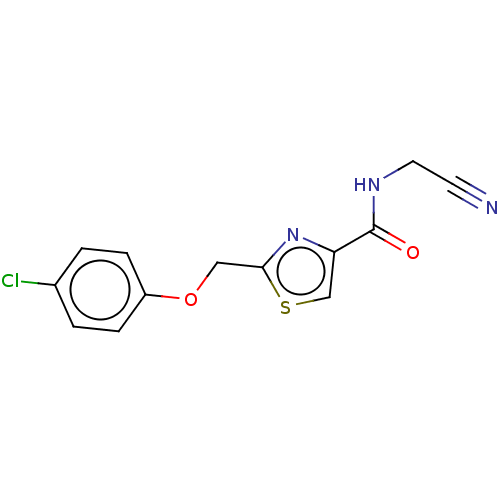 (2-((4-Chlorophenoxy)methyl)-N-(cyanomethyl)thiazol...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston | Assay Description Compounds were solubilized in DMSO. Serial 2-fold dilutions covering two concentration ranges, 10 mM to 19.5 μM and 100 μM to 195 nM, were ... | J Biol Chem 289: 21651-62 (2014) Article DOI: 10.1074/jbc.M114.574061 BindingDB Entry DOI: 10.7270/Q2BG2MVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239385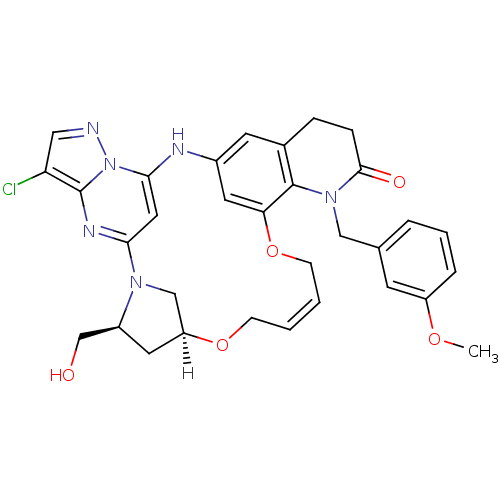 (CHEMBL4092565) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenergic receptor in guinea pig cerebral cortical membranes by displacement of [3H]- WB-4101 | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210722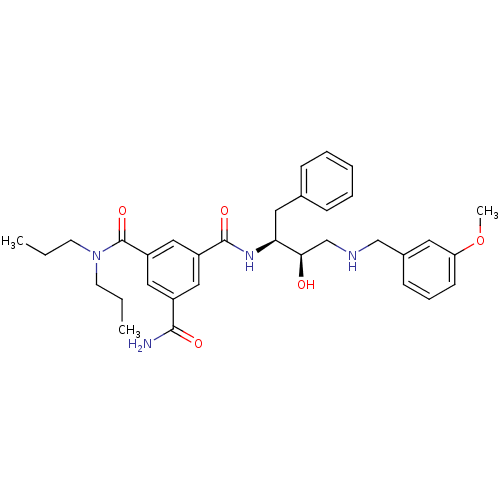 (CHEMBL231325 | N1-((2S,3R)-4-(3-methoxybenzylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210714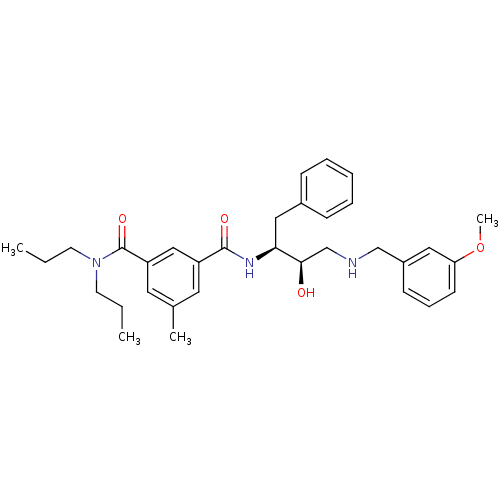 (CHEMBL231542 | N1-((2S,3R)-4-(3-methoxybenzylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine--tRNA ligase alpha/beta subunit (Escherichia coli (Enterobacteria)) | BDBM231669 (2-[3-[(4-Chloro-2-pyridyl)amino]phenoxy]-N-(1,1-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston | Assay Description Compounds were solubilized in DMSO. Serial 2-fold dilutions covering two concentration ranges, 10 mM to 19.5 μM and 100 μM to 195 nM, were ... | J Biol Chem 289: 21651-62 (2014) Article DOI: 10.1074/jbc.M114.574061 BindingDB Entry DOI: 10.7270/Q2BG2MVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239368 (CHEMBL4073463) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM15796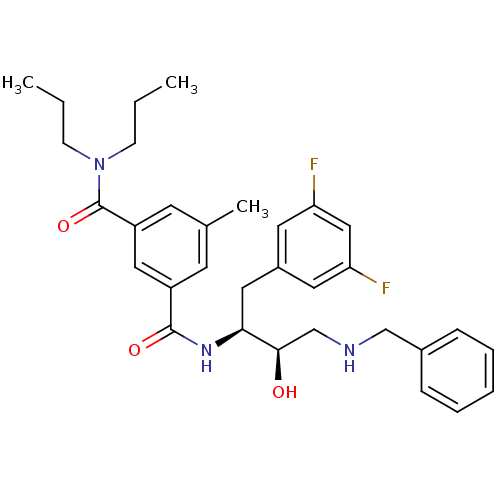 ((1S,2R)-N-[1-(3,5-Difluorobenzyl)-2-hydroxy-3-(ben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 4.8 | 37 |
Elan Pharmaceuticals | Assay Description Beta-cleavage ELISA assays were carried out in reaction buffer containing enzyme, substrate MBP-C125, and test compounds. Generated beta-cleaved prod... | J Med Chem 50: 776-81 (2007) Article DOI: 10.1021/jm061242y BindingDB Entry DOI: 10.7270/Q2K935TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM15792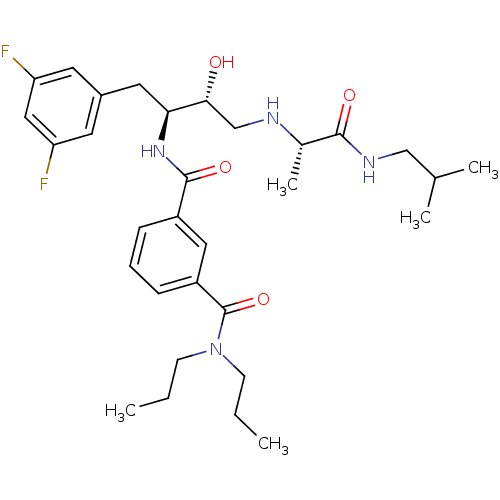 (3-N-[(2S,3R)-1-(3,5-difluorophenyl)-3-hydroxy-4-{[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 4.8 | 37 |
Elan Pharmaceuticals | Assay Description Beta-cleavage ELISA assays were carried out in reaction buffer containing enzyme, substrate MBP-C125, and test compounds. Generated beta-cleaved prod... | J Med Chem 50: 776-81 (2007) Article DOI: 10.1021/jm061242y BindingDB Entry DOI: 10.7270/Q2K935TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210713 (CHEMBL394596 | ethyl 3-(((2S,3R)-4-(3-methoxybenzy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239368 (CHEMBL4073463) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50210717 (CHEMBL397275 | N1-((2S,3R)-4-(3-ethylbenzylamino)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of cathepsin D | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine--tRNA ligase alpha/beta subunit (Escherichia coli (Enterobacteria)) | BDBM231677 (2-((4-Chlorophenoxy)methyl)-N-(2-sulfamoylethyl)th...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston | Assay Description Compounds were solubilized in DMSO. Serial 2-fold dilutions covering two concentration ranges, 10 mM to 19.5 μM and 100 μM to 195 nM, were ... | J Biol Chem 289: 21651-62 (2014) Article DOI: 10.1074/jbc.M114.574061 BindingDB Entry DOI: 10.7270/Q2BG2MVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM15795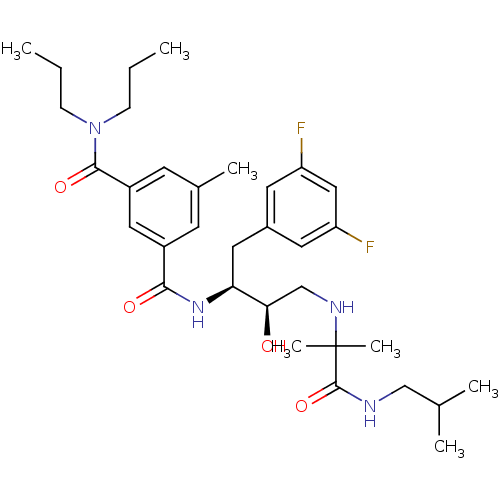 ((1S,2R)-N-[1-(3,5-Difluorobenzyl)-2-hydroxy-3-(1-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 4.8 | 37 |
Elan Pharmaceuticals | Assay Description Beta-cleavage ELISA assays were carried out in reaction buffer containing enzyme, substrate MBP-C125, and test compounds. Generated beta-cleaved prod... | J Med Chem 50: 776-81 (2007) Article DOI: 10.1021/jm061242y BindingDB Entry DOI: 10.7270/Q2K935TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239362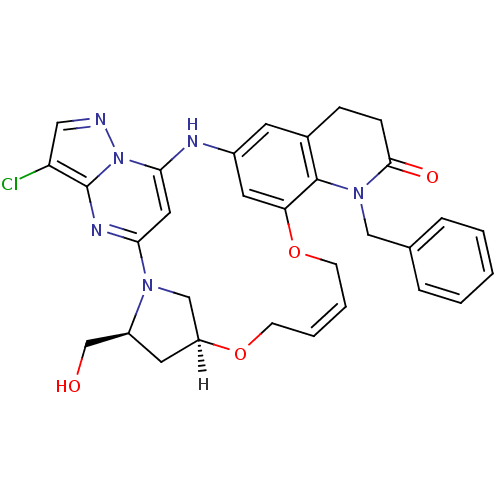 (CHEMBL4064865) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239362 (CHEMBL4064865) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210712 (CHEMBL124380 | N1-((2S,3R)-4-(3-methoxybenzylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210720 (CHEMBL397715 | N1-((2S,3R)-4-(3-methoxybenzylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210710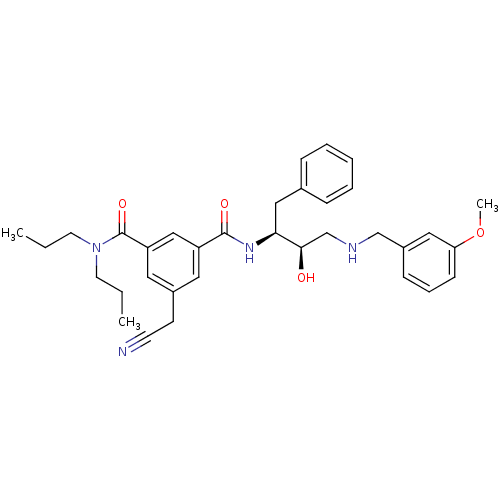 (CHEMBL230190 | N1-((2S,3R)-4-(3-methoxybenzylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239380 (CHEMBL4062105) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase (Staphylococcus aureus) | BDBM50444676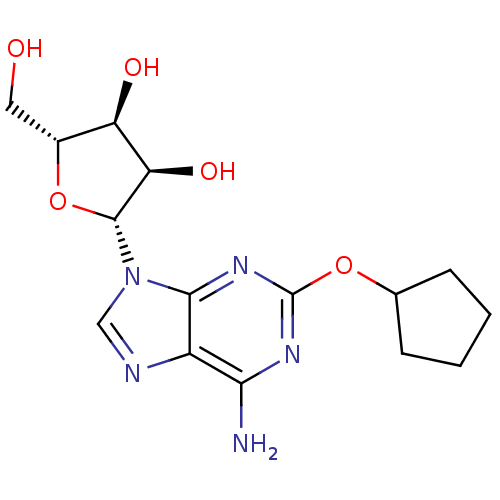 (CHEMBL1807815) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus LigA | Bioorg Med Chem Lett 24: 360-6 (2013) Article DOI: 10.1016/j.bmcl.2013.11.007 BindingDB Entry DOI: 10.7270/Q20866R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239380 (CHEMBL4062105) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50210714 (CHEMBL231542 | N1-((2S,3R)-4-(3-methoxybenzylamino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of cathepsin D | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239383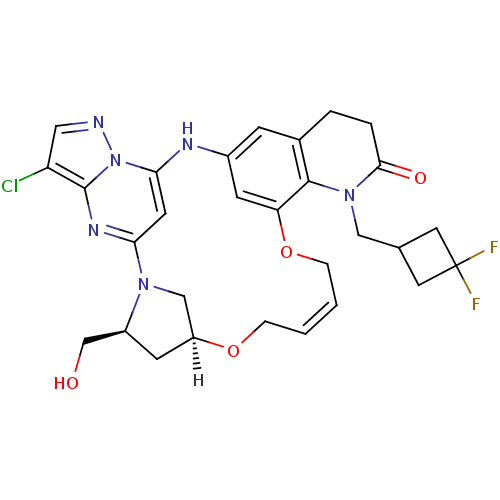 (CHEMBL4096773) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM15793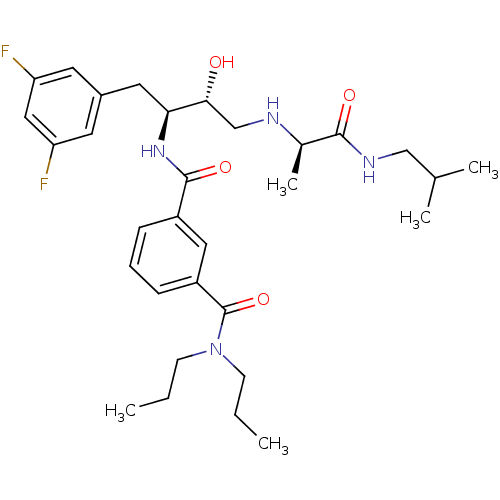 ((1S,2R)-N-[1-(3,5-Difluorobenzyl)-2-hydroxy-3-(R)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | 4.8 | 37 |
Elan Pharmaceuticals | Assay Description Beta-cleavage ELISA assays were carried out in reaction buffer containing enzyme, substrate MBP-C125, and test compounds. Generated beta-cleaved prod... | J Med Chem 50: 776-81 (2007) Article DOI: 10.1021/jm061242y BindingDB Entry DOI: 10.7270/Q2K935TZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239383 (CHEMBL4096773) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine--tRNA ligase alpha/beta subunit (Escherichia coli (Enterobacteria)) | BDBM231668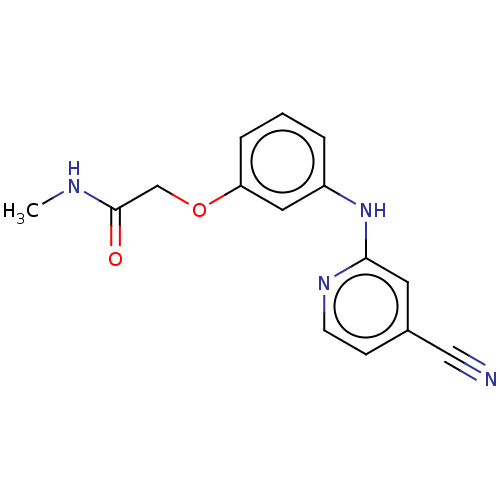 (2-(3-(4-Cyanopyridin-2-ylamino)phenoxy)-N-methylac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | 8.0 | n/a |
AstraZeneca R&D Boston | Assay Description Compounds were solubilized in DMSO. Serial 2-fold dilutions covering two concentration ranges, 10 mM to 19.5 μM and 100 μM to 195 nM, were ... | J Biol Chem 289: 21651-62 (2014) Article DOI: 10.1074/jbc.M114.574061 BindingDB Entry DOI: 10.7270/Q2BG2MVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50210710 (CHEMBL230190 | N1-((2S,3R)-4-(3-methoxybenzylamino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of cathepsin D | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50210718 (CHEMBL231646 | N1-((2S,3R)-4-(3-methoxybenzylamino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of cathepsin D | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50210711 (CHEMBL230314 | N1-((2S,3R)-4-(3-methoxybenzylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 17: 3378-83 (2007) Article DOI: 10.1016/j.bmcl.2007.03.096 BindingDB Entry DOI: 10.7270/Q2RB748S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239379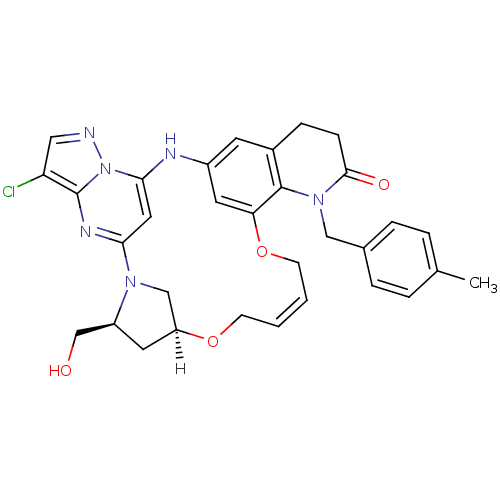 (CHEMBL4083842) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B-cell lymphoma 6 protein (Homo sapiens) | BDBM50239379 (CHEMBL4083842) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GAL4 DNA binding domain fused BCL6 BTB domain (unknown origin) expressed in HEK 293T/17 cells after 24 hrs by Bright-Glo luciferase cel... | J Med Chem 60: 4386-4402 (2017) Article DOI: 10.1021/acs.jmedchem.7b00359 BindingDB Entry DOI: 10.7270/Q2ZW1P1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 127 total ) | Next | Last >> |