

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium tuberculosis) | BDBM50181153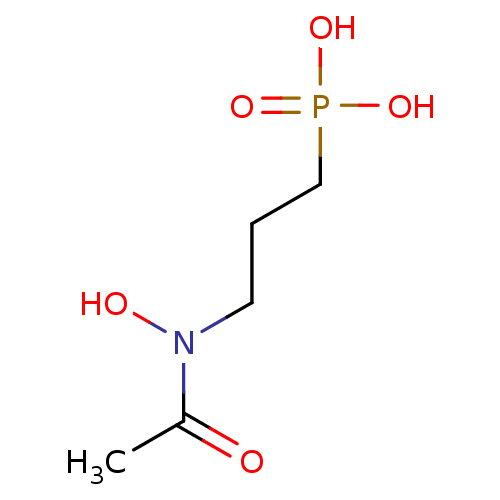 (3-(N-hydroxyacetamido)propylphosphonic acid | 3-(N...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant DXR expressed in Escherichia coli BL21 (DE3) using DXP as substrate and MgCl2 as cofactor preinc... | J Med Chem 54: 4721-34 (2011) Article DOI: 10.1021/jm200363d BindingDB Entry DOI: 10.7270/Q2NK3FCT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium tuberculosis) | BDBM50153713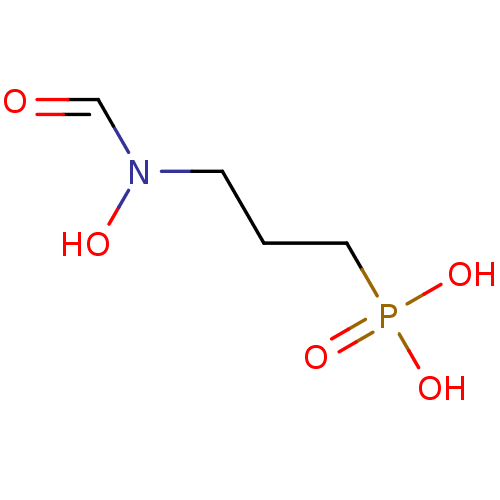 (3-(N-hydroxyformamido)propylphosphonic acid | 3-[F...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant DXR expressed in Escherichia coli BL21 (DE3) using DXP as substrate and MgCl2 as cofactor preinc... | J Med Chem 54: 4721-34 (2011) Article DOI: 10.1021/jm200363d BindingDB Entry DOI: 10.7270/Q2NK3FCT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium tuberculosis) | BDBM50181154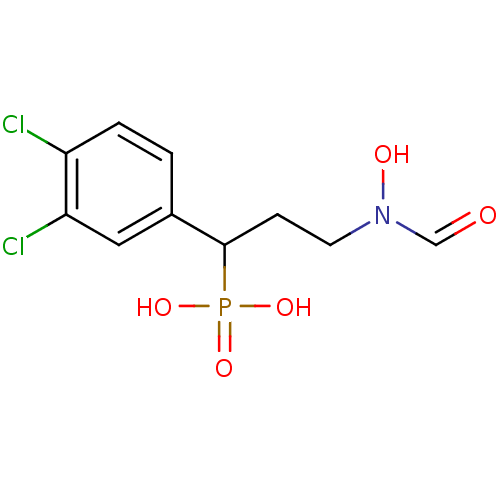 (1-(3,4-dichlorophenyl)-3-(N-hydroxyformamido)propy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant DXR expressed in Escherichia coli BL21 (DE3) using DXP as substrate and MgCl2 as cofactor preinc... | J Med Chem 54: 4721-34 (2011) Article DOI: 10.1021/jm200363d BindingDB Entry DOI: 10.7270/Q2NK3FCT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium tuberculosis) | BDBM50181148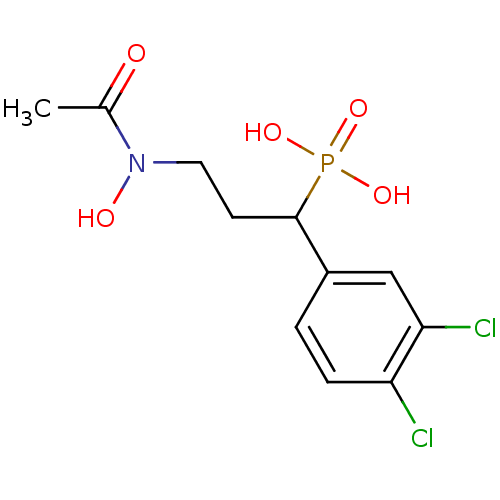 (1-(3,4-dichlorophenyl)-3-(N-hydroxyacetamido)propy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant DXR expressed in Escherichia coli BL21 (DE3) using DXP as substrate and MgCl2 as cofactor preinc... | J Med Chem 54: 4721-34 (2011) Article DOI: 10.1021/jm200363d BindingDB Entry DOI: 10.7270/Q2NK3FCT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium tuberculosis) | BDBM50335492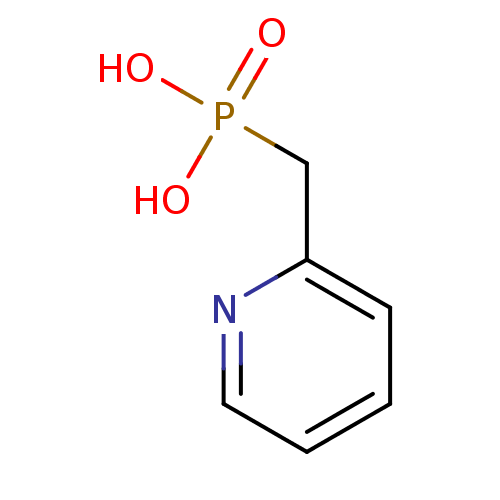 ((pyridin-2-ylmethyl)phosphonic acid | CHEMBL161524...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant DXR expressed in Escherichia coli BL21 (DE3) using DXP as substrate and MgCl2 as cofactor preinc... | J Med Chem 54: 4721-34 (2011) Article DOI: 10.1021/jm200363d BindingDB Entry DOI: 10.7270/Q2NK3FCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium tuberculosis) | BDBM50335493 (5-Phenylpyridin-2-ylmethylphosphonic acid | CHEMBL...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant DXR expressed in Escherichia coli BL21 (DE3) using DXP as substrate and MgCl2 as cofactor preinc... | J Med Chem 54: 4721-34 (2011) Article DOI: 10.1021/jm200363d BindingDB Entry DOI: 10.7270/Q2NK3FCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium tuberculosis) | BDBM50335490 (CHEMBL1651840 | Pyridin-4-ylmethylphosphonic acid) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant DXR expressed in Escherichia coli BL21 (DE3) using DXP as substrate and MgCl2 as cofactor preinc... | J Med Chem 54: 4721-34 (2011) Article DOI: 10.1021/jm200363d BindingDB Entry DOI: 10.7270/Q2NK3FCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium tuberculosis) | BDBM50347578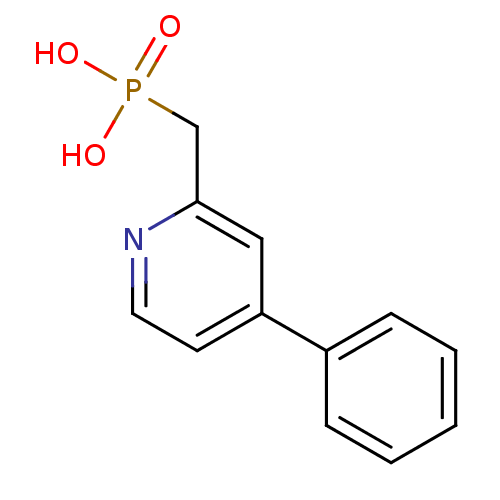 (CHEMBL1801648) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant DXR expressed in Escherichia coli BL21 (DE3) using DXP as substrate and MgCl2 as cofactor preinc... | J Med Chem 54: 4721-34 (2011) Article DOI: 10.1021/jm200363d BindingDB Entry DOI: 10.7270/Q2NK3FCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium tuberculosis) | BDBM50335494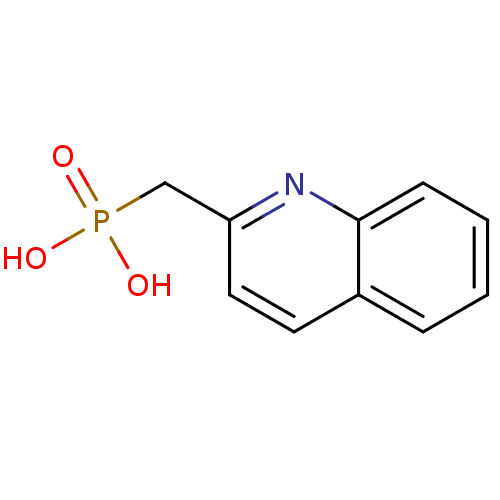 ((quinolin-2-ylmethyl)phosphonic acid | CHEMBL16152...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant DXR expressed in Escherichia coli BL21 (DE3) using DXP as substrate and MgCl2 as cofactor preinc... | J Med Chem 54: 4721-34 (2011) Article DOI: 10.1021/jm200363d BindingDB Entry DOI: 10.7270/Q2NK3FCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium tuberculosis) | BDBM50347582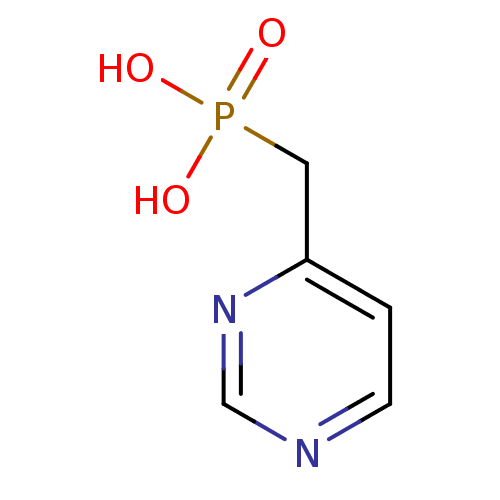 (CHEMBL1801642) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant DXR expressed in Escherichia coli BL21 (DE3) using DXP as substrate and MgCl2 as cofactor preinc... | J Med Chem 54: 4721-34 (2011) Article DOI: 10.1021/jm200363d BindingDB Entry DOI: 10.7270/Q2NK3FCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium tuberculosis) | BDBM50347580 (CHEMBL1801651) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant DXR expressed in Escherichia coli BL21 (DE3) using DXP as substrate and MgCl2 as cofactor preinc... | J Med Chem 54: 4721-34 (2011) Article DOI: 10.1021/jm200363d BindingDB Entry DOI: 10.7270/Q2NK3FCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium tuberculosis) | BDBM50335486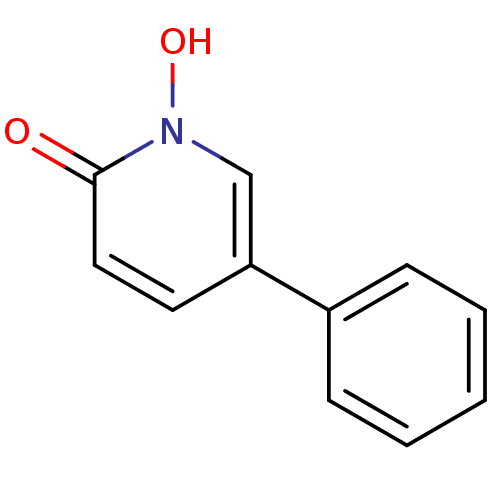 (1-Hydroxy-5-phenylpyridin-2-one | 1-hydroxy-5-phen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant DXR expressed in Escherichia coli BL21 (DE3) using DXP as substrate and MgCl2 as cofactor preinc... | J Med Chem 54: 4721-34 (2011) Article DOI: 10.1021/jm200363d BindingDB Entry DOI: 10.7270/Q2NK3FCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium tuberculosis) | BDBM50135835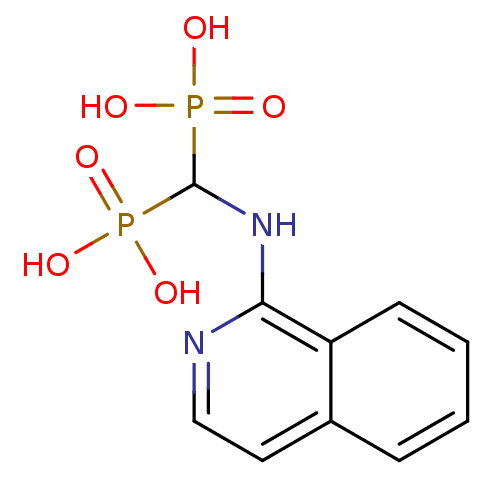 ((isoquinolin-1-ylamino)methylenediphosphonic acid ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 3.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant DXR expressed in Escherichia coli BL21 (DE3) using DXP as substrate and MgCl2 as cofactor preinc... | J Med Chem 54: 4721-34 (2011) Article DOI: 10.1021/jm200363d BindingDB Entry DOI: 10.7270/Q2NK3FCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium tuberculosis) | BDBM50347577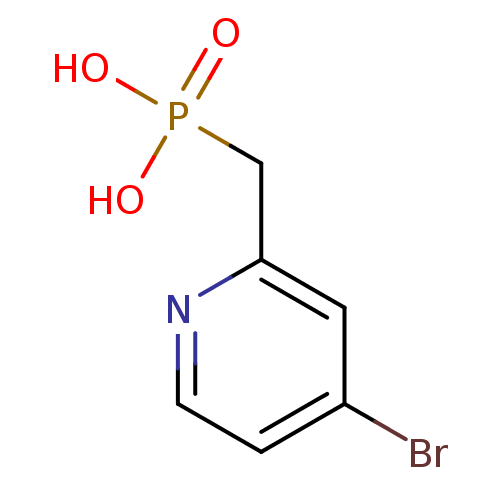 (CHEMBL1801647) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant DXR expressed in Escherichia coli BL21 (DE3) using DXP as substrate and MgCl2 as cofactor preinc... | J Med Chem 54: 4721-34 (2011) Article DOI: 10.1021/jm200363d BindingDB Entry DOI: 10.7270/Q2NK3FCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium tuberculosis) | BDBM50347579 (CHEMBL1801650) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant DXR expressed in Escherichia coli BL21 (DE3) using DXP as substrate and MgCl2 as cofactor preinc... | J Med Chem 54: 4721-34 (2011) Article DOI: 10.1021/jm200363d BindingDB Entry DOI: 10.7270/Q2NK3FCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium tuberculosis) | BDBM50347581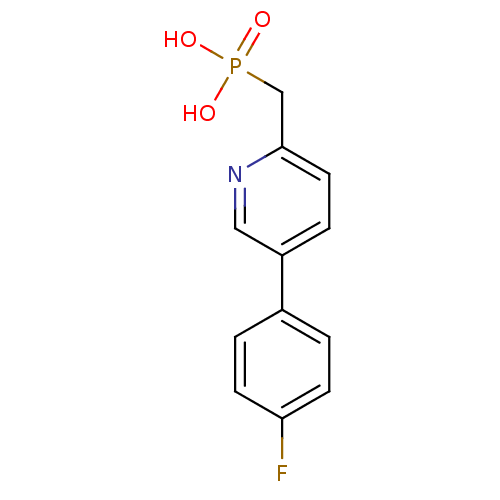 (CHEMBL1801655) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant DXR expressed in Escherichia coli BL21 (DE3) using DXP as substrate and MgCl2 as cofactor preinc... | J Med Chem 54: 4721-34 (2011) Article DOI: 10.1021/jm200363d BindingDB Entry DOI: 10.7270/Q2NK3FCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Mycobacterium tuberculosis) | BDBM50153713 (3-(N-hydroxyformamido)propylphosphonic acid | 3-[F...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor College of Medicine Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant DXR expressed in Escherichia coli BL21 (DE3) using DXP as substrate and Mn2+ as cofactor preincu... | J Med Chem 54: 4721-34 (2011) Article DOI: 10.1021/jm200363d BindingDB Entry DOI: 10.7270/Q2NK3FCT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50388398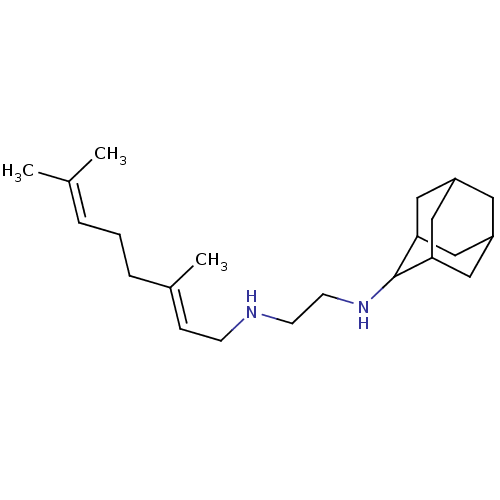 (CHEMBL561057 | SQ-109) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human geranylgeranyl diphosphate synthase | J Med Chem 57: 3126-39 (2014) Article DOI: 10.1021/jm500131s BindingDB Entry DOI: 10.7270/Q27P90ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50011479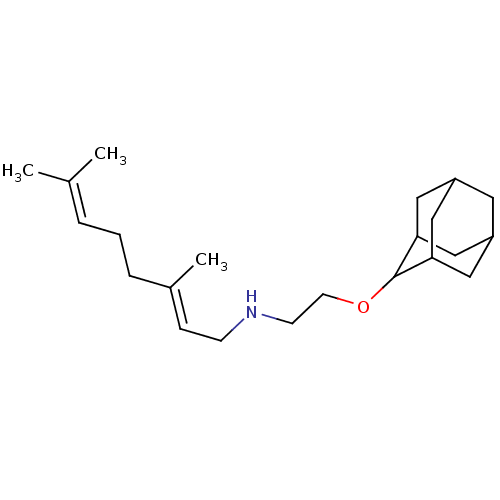 (CHEMBL3261880) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human SQS | J Med Chem 57: 3126-39 (2014) Article DOI: 10.1021/jm500131s BindingDB Entry DOI: 10.7270/Q27P90ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50388398 (CHEMBL561057 | SQ-109) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human SQS | J Med Chem 57: 3126-39 (2014) Article DOI: 10.1021/jm500131s BindingDB Entry DOI: 10.7270/Q27P90ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50011480 (CHEMBL3261881) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus CrtM | J Med Chem 57: 3126-39 (2014) Article DOI: 10.1021/jm500131s BindingDB Entry DOI: 10.7270/Q27P90ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50011479 (CHEMBL3261880) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus CrtM | J Med Chem 57: 3126-39 (2014) Article DOI: 10.1021/jm500131s BindingDB Entry DOI: 10.7270/Q27P90ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4,4'-diapophytoene synthase (Staphylococcus aureus) | BDBM50388398 (CHEMBL561057 | SQ-109) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus CrtM | J Med Chem 57: 3126-39 (2014) Article DOI: 10.1021/jm500131s BindingDB Entry DOI: 10.7270/Q27P90ZN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50011480 (CHEMBL3261881) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Inhibition of human SQS | J Med Chem 57: 3126-39 (2014) Article DOI: 10.1021/jm500131s BindingDB Entry DOI: 10.7270/Q27P90ZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||