

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150431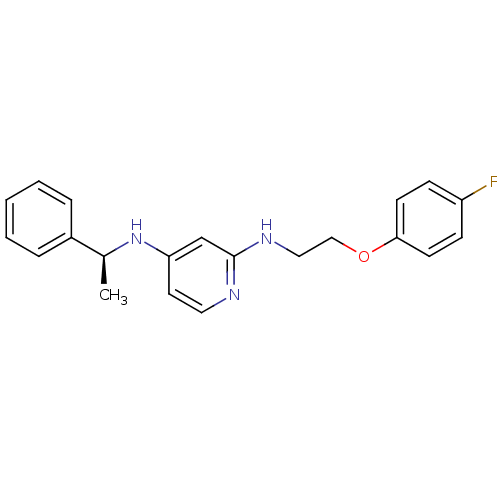 ((S)-N2-(2-(4-fluorophenoxy)ethyl)-N4-(1-phenylethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150439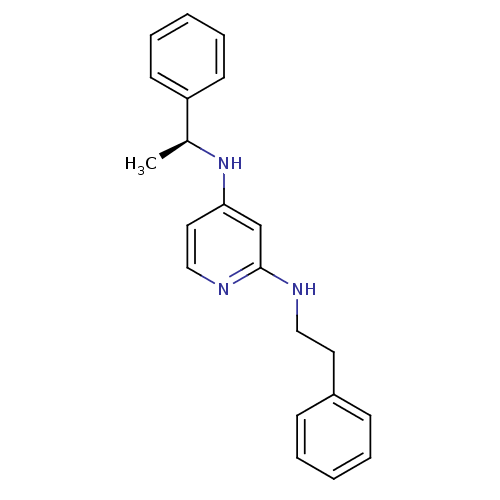 (CHEMBL182923 | N*2*-Phenethyl-N*4*-((S)-1-phenyl-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150430 (CHEMBL183277 | N*2*-[2-(4-Fluoro-phenoxy)-ethyl]-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150428 (CHEMBL183423 | N*4*-[2-(4-Fluoro-phenoxy)-ethyl]-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150425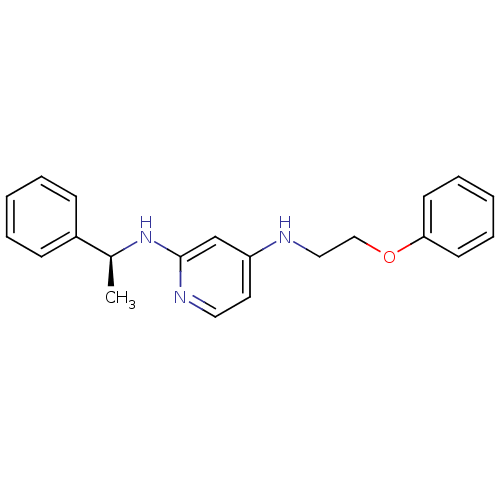 (CHEMBL184607 | N*4*-(2-Phenoxy-ethyl)-N*2*-((S)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150446 (CHEMBL181356 | N*2*-(2-Phenoxy-ethyl)-N*4*-((S)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150441 (CHEMBL181605 | N*2*-[2-(4-Fluoro-phenyl)-ethyl]-N*...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150422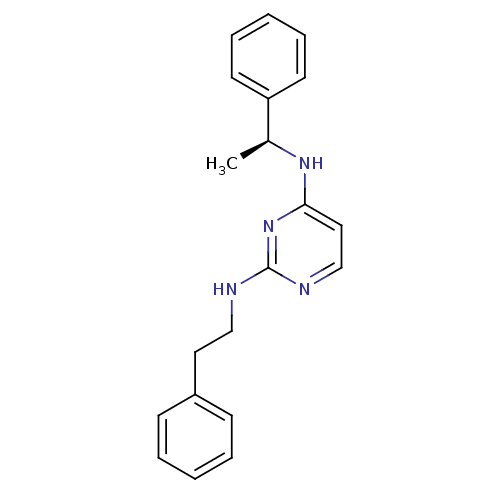 (CHEMBL185384 | N*2*-Phenethyl-N*4*-((S)-1-phenyl-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150438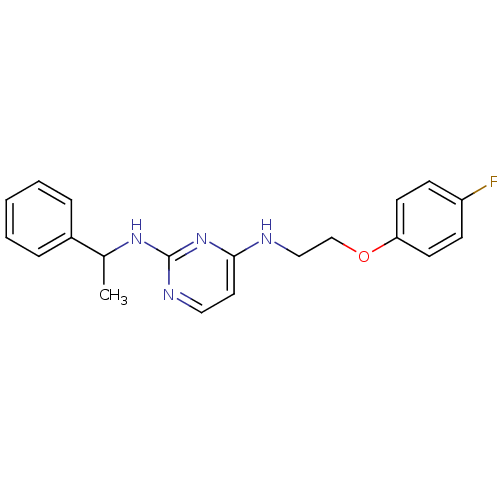 (CHEMBL182071 | N*4*-[2-(4-Fluoro-phenoxy)-ethyl]-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150433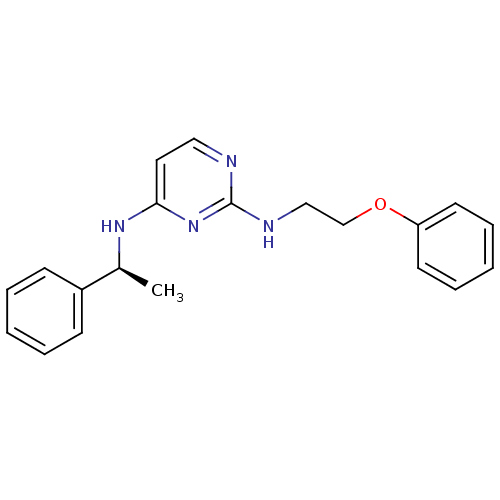 (CHEMBL185511 | N*2*-(2-Phenoxy-ethyl)-N*4*-((S)-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150436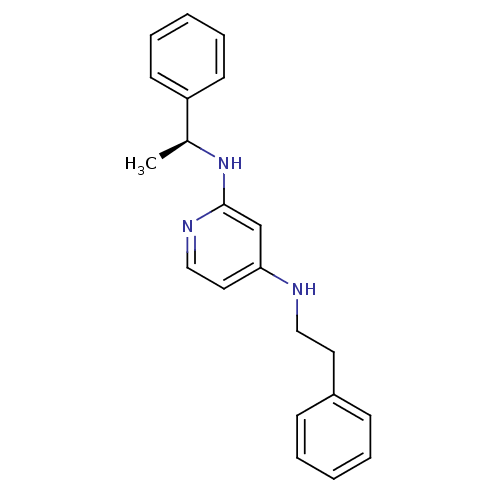 (CHEMBL181950 | N*4*-Phenethyl-N*2*-((S)-1-phenyl-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150432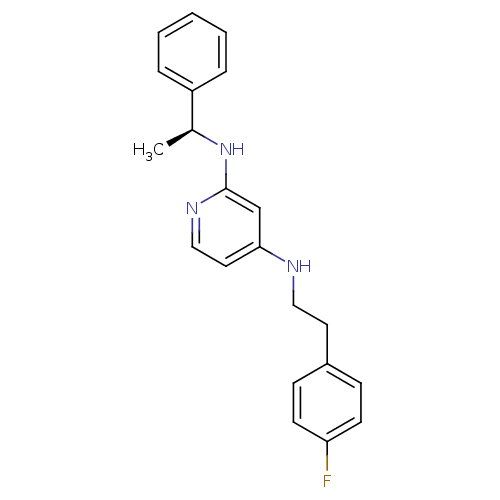 (CHEMBL185512 | N*4*-[2-(4-Fluoro-phenyl)-ethyl]-N*...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150440 (CHEMBL359725 | N-(2-Phenoxy-ethyl)-N''-((S)-1-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150437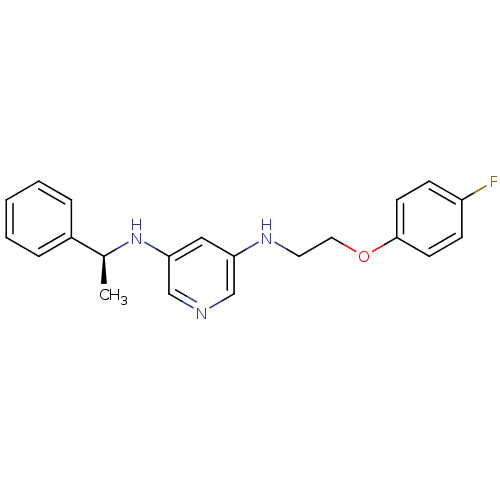 (CHEMBL184789 | N-[2-(4-Fluoro-phenoxy)-ethyl]-N''-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150424 (CHEMBL182943 | N-[2-(4-Fluoro-phenoxy)-ethyl]-N''-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150423 (CHEMBL182680 | N-[2-(4-Fluoro-phenyl)-ethyl]-N''-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150444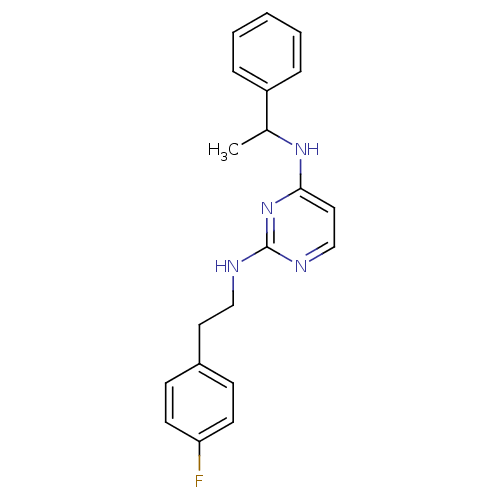 (CHEMBL185437 | N*2*-[2-(4-Fluoro-phenyl)-ethyl]-N*...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150442 (CHEMBL182070 | N*4*-Phenethyl-N*2*-(1-phenyl-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150427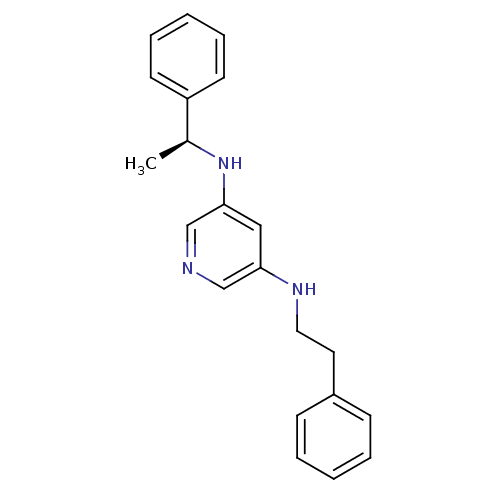 (CHEMBL182356 | N-Phenethyl-N''-((S)-1-phenyl-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50150428 (CHEMBL183423 | N*4*-[2-(4-Fluoro-phenoxy)-ethyl]-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 2C receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50150431 ((S)-N2-(2-(4-fluorophenoxy)ethyl)-N4-(1-phenylethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 2C receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150426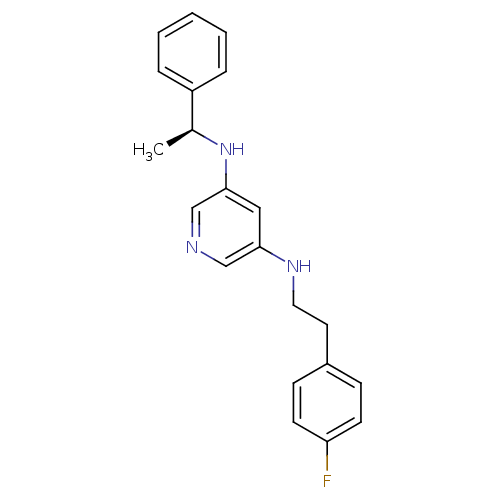 (CHEMBL182006 | N-[2-(4-Fluoro-phenyl)-ethyl]-N''-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150445 (CHEMBL180087 | N-Phenethyl-N''-((S)-1-phenyl-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50150438 (CHEMBL182071 | N*4*-[2-(4-Fluoro-phenoxy)-ethyl]-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 2C receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50150431 ((S)-N2-(2-(4-fluorophenoxy)ethyl)-N4-(1-phenylethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against D2L receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50150437 (CHEMBL184789 | N-[2-(4-Fluoro-phenoxy)-ethyl]-N''-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 476 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 2C receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150443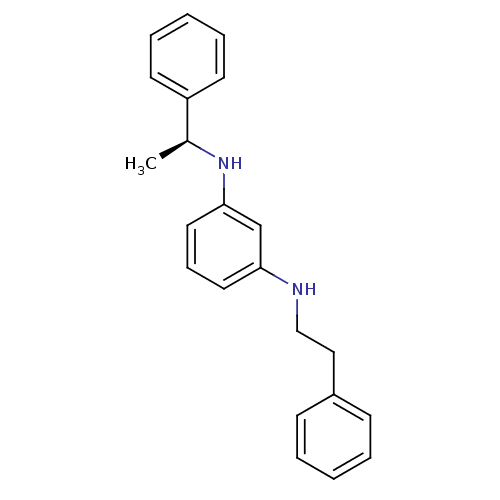 (CHEMBL182938 | N-Phenethyl-N''-((S)-1-phenyl-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50150437 (CHEMBL184789 | N-[2-(4-Fluoro-phenoxy)-ethyl]-N''-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against D2L receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50150428 (CHEMBL183423 | N*4*-[2-(4-Fluoro-phenoxy)-ethyl]-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against D2L receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50150438 (CHEMBL182071 | N*4*-[2-(4-Fluoro-phenoxy)-ethyl]-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against D2L receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150429 (CHEMBL182860 | N-(2-Phenoxy-ethyl)-N''-((S)-1-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50150430 (CHEMBL183277 | N*2*-[2-(4-Fluoro-phenoxy)-ethyl]-N...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against D2L receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150435 (CHEMBL182561 | N-(2-Phenoxy-ethyl)-N''-((S)-1-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50150434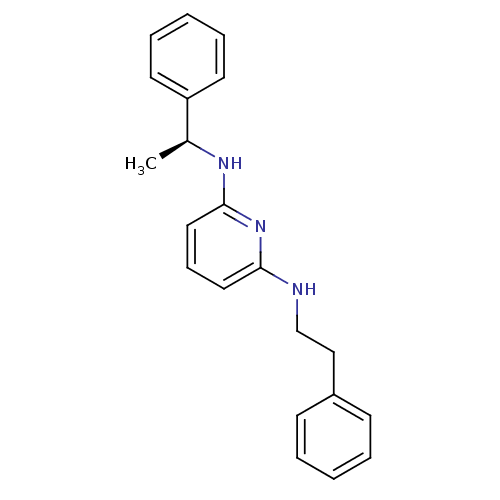 (CHEMBL360658 | N-Phenethyl-N''-((S)-1-phenyl-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against 5-Hydroxy tryptamine 7 receptor | Bioorg Med Chem Lett 14: 4249-52 (2004) Article DOI: 10.1016/j.bmcl.2004.06.007 BindingDB Entry DOI: 10.7270/Q2BV7G34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142829 (US8940736, 391) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50258790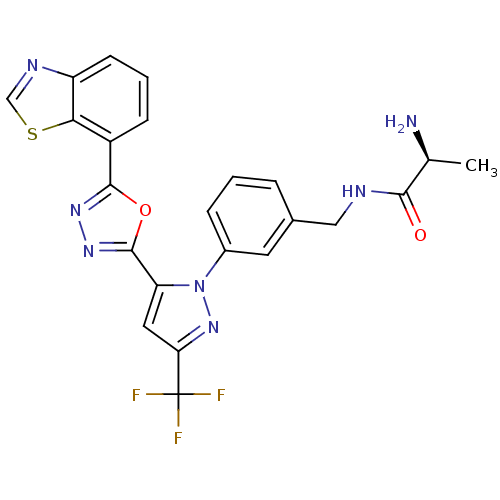 ((S)-2-amino-N-(3-(5-(5-(benzo[d]thiazol-7-yl)-1,3,...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CARM1 assessed as inhibition of histone3 methylation | Bioorg Med Chem Lett 19: 5063-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.040 BindingDB Entry DOI: 10.7270/Q2KW5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142775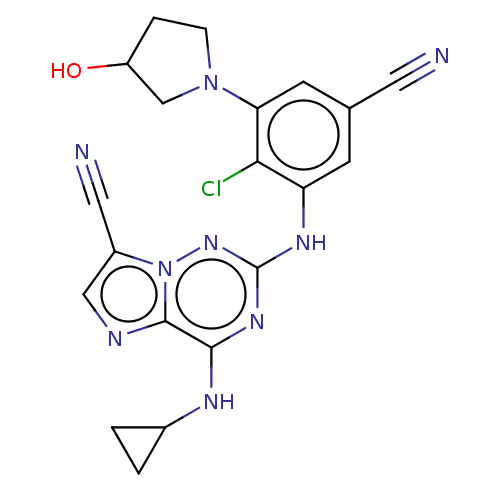 (US8940736, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50301077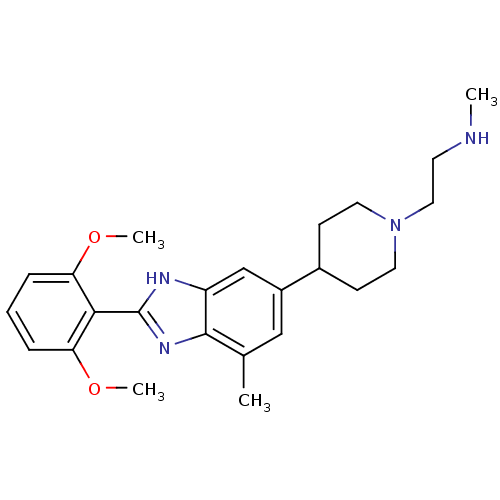 (2-(4-(2-(2,6-dimethoxyphenyl)-7-methyl-1H-benzo[d]...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CARM1 assessed as inhibition of histone3 methylation | Bioorg Med Chem Lett 19: 5063-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.040 BindingDB Entry DOI: 10.7270/Q2KW5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142774 (US8940736, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142816 (US8940736, 281) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142792 (US8940736, 160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142777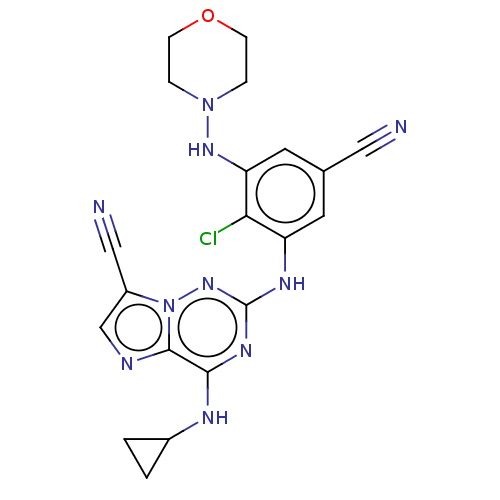 (US8940736, 49) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142835 (US8940736, 402) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50301079 (2-(4-(2-(2-fluoro-6-methoxyphenyl)-7-methyl-1H-ben...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CARM1 assessed as inhibition of histone3 methylation | Bioorg Med Chem Lett 19: 5063-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.040 BindingDB Entry DOI: 10.7270/Q2KW5G3W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142822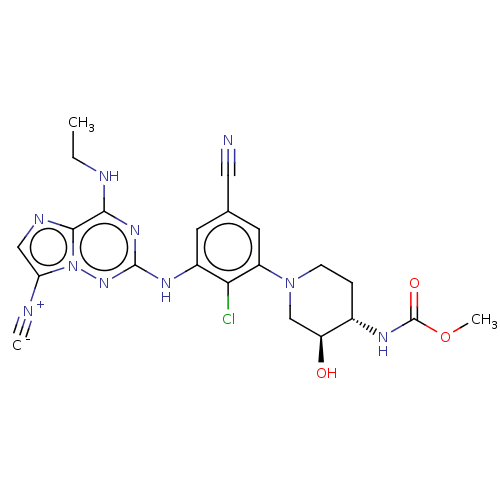 (US8940736, 328) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha (Homo sapiens (Human)) | BDBM142829 (US8940736, 391) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha (Homo sapiens (Human)) | BDBM142817 (US8940736, 285) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha (Homo sapiens (Human)) | BDBM142774 (US8940736, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha' (Homo sapiens (Human)) | BDBM142784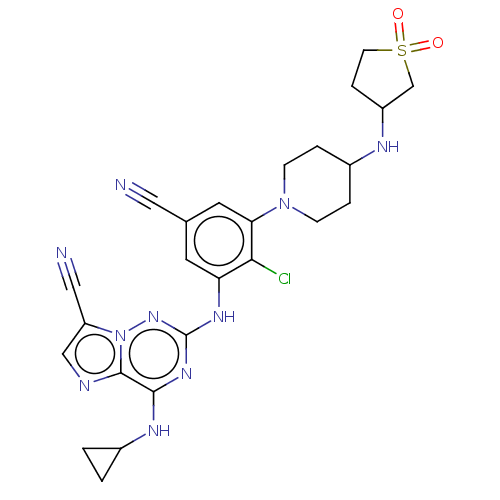 (US8940736, 117) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Casein kinase II subunit alpha (Homo sapiens (Human)) | BDBM142835 (US8940736, 402) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Bristol-Myers Squibb Company US Patent | Assay Description The effectiveness of compounds of the present invention as inhibitors of protein kinases can be readily tested by assays known to those skilled in th... | US Patent US8940736 (2015) BindingDB Entry DOI: 10.7270/Q2QV3K72 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1027 total ) | Next | Last >> |