 Found 98 hits with Last Name = 'purcell' and Initial = 'm'
Found 98 hits with Last Name = 'purcell' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440788
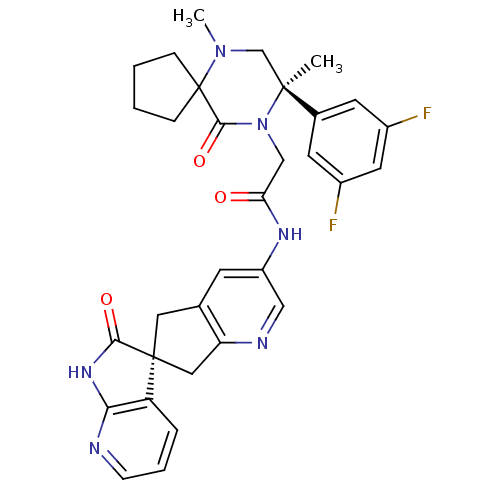
(CHEMBL2431249)Show SMILES CN1C[C@](C)(N(CC(=O)Nc2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C32H32F2N6O3/c1-30(20-11-21(33)13-22(34)12-20)18-39(2)32(7-3-4-8-32)29(43)40(30)17-26(41)37-23-10-19-14-31(15-25(19)36-16-23)24-6-5-9-35-27(24)38-28(31)42/h5-6,9-13,16H,3-4,7-8,14-15,17-18H2,1-2H3,(H,37,41)(H,35,38,42)/t30-,31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440782
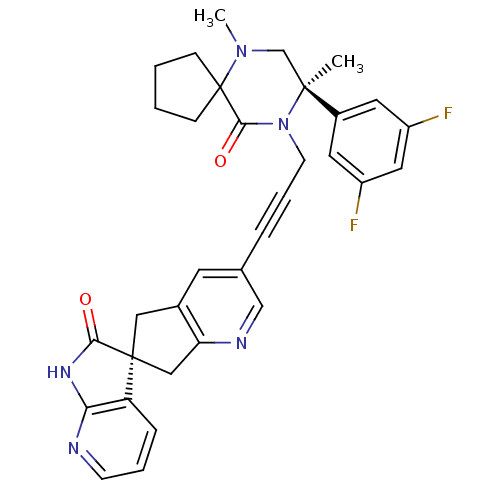
(CHEMBL2431246)Show SMILES CN1C[C@](C)(N(CC#Cc2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H31F2N5O2/c1-31(23-14-24(34)16-25(35)15-23)20-39(2)33(9-3-4-10-33)30(42)40(31)12-6-7-21-13-22-17-32(18-27(22)37-19-21)26-8-5-11-36-28(26)38-29(32)41/h5,8,11,13-16,19H,3-4,9-10,12,17-18,20H2,1-2H3,(H,36,38,41)/t31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440784
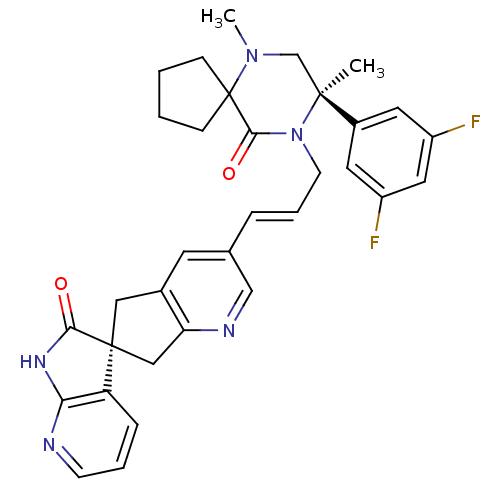
(CHEMBL2431253)Show SMILES CN1C[C@](C)(N(C\C=C\c2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H33F2N5O2/c1-31(23-14-24(34)16-25(35)15-23)20-39(2)33(9-3-4-10-33)30(42)40(31)12-6-7-21-13-22-17-32(18-27(22)37-19-21)26-8-5-11-36-28(26)38-29(32)41/h5-8,11,13-16,19H,3-4,9-10,12,17-18,20H2,1-2H3,(H,36,38,41)/b7-6+/t31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50356282
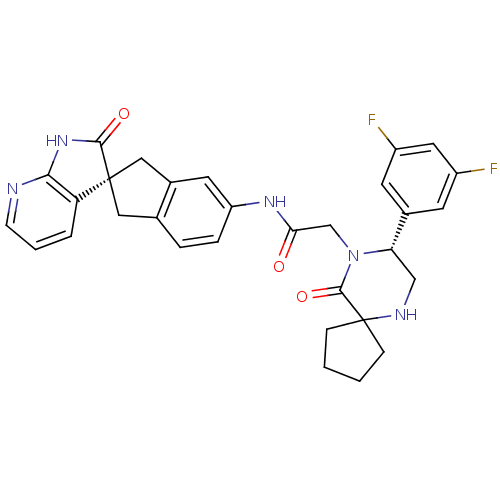
(CHEMBL1910936)Show SMILES Fc1cc(F)cc(c1)[C@@H]1CNC2(CCCC2)C(=O)N1CC(=O)Nc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31 |r| Show InChI InChI=1S/C31H29F2N5O3/c32-21-10-19(11-22(33)13-21)25-16-35-31(7-1-2-8-31)29(41)38(25)17-26(39)36-23-6-5-18-14-30(15-20(18)12-23)24-4-3-9-34-27(24)37-28(30)40/h3-6,9-13,25,35H,1-2,7-8,14-17H2,(H,36,39)(H,34,37,40)/t25-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440791
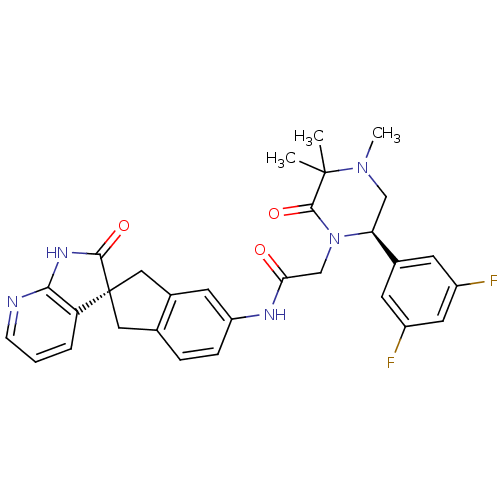
(CHEMBL2431256)Show SMILES CN1C[C@H](N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C1(C)C)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C30H29F2N5O3/c1-29(2)28(40)37(24(15-36(29)3)18-9-20(31)12-21(32)10-18)16-25(38)34-22-7-6-17-13-30(14-19(17)11-22)23-5-4-8-33-26(23)35-27(30)39/h4-12,24H,13-16H2,1-3H3,(H,34,38)(H,33,35,39)/t24-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125967

(CHEMBL3627846)Show SMILES Cc1ccc2c(c1)nc(CCN1C(=O)c3ccccc3C1=O)n(-c1ccc3n(C)ncc3c1)c2=O Show InChI InChI=1S/C27H21N5O3/c1-16-7-9-21-22(13-16)29-24(11-12-31-25(33)19-5-3-4-6-20(19)26(31)34)32(27(21)35)18-8-10-23-17(14-18)15-28-30(23)2/h3-10,13-15H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440793
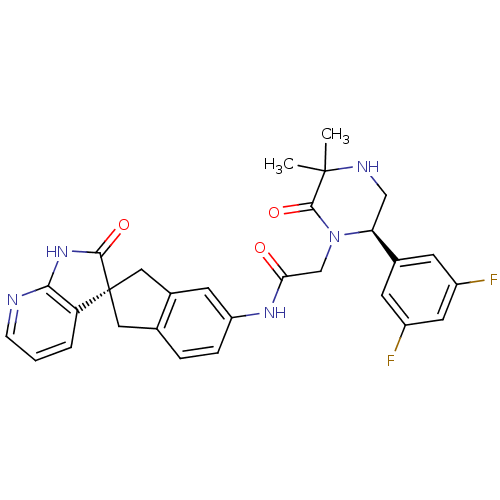
(CHEMBL2431254)Show SMILES CC1(C)NC[C@H](N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C1=O)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C29H27F2N5O3/c1-28(2)27(39)36(23(14-33-28)17-8-19(30)11-20(31)9-17)15-24(37)34-21-6-5-16-12-29(13-18(16)10-21)22-4-3-7-32-25(22)35-26(29)38/h3-11,23,33H,12-15H2,1-2H3,(H,34,37)(H,32,35,38)/t23-,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50385314
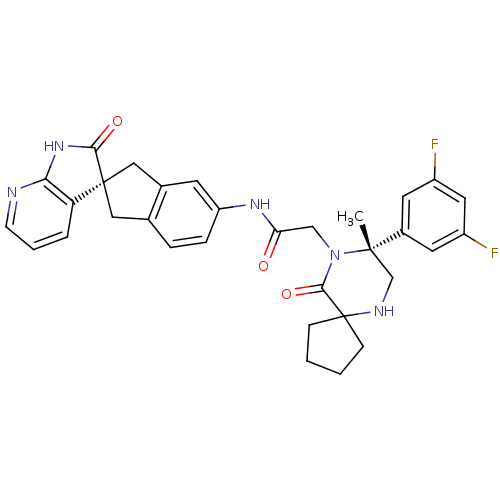
(CHEMBL2035981)Show SMILES C[C@]1(CNC2(CCCC2)C(=O)N1CC(=O)Nc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C32H31F2N5O3/c1-30(21-12-22(33)14-23(34)13-21)18-36-32(8-2-3-9-32)29(42)39(30)17-26(40)37-24-7-6-19-15-31(16-20(19)11-24)25-5-4-10-35-27(25)38-28(31)41/h4-7,10-14,36H,2-3,8-9,15-18H2,1H3,(H,37,40)(H,35,38,41)/t30-,31+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440786
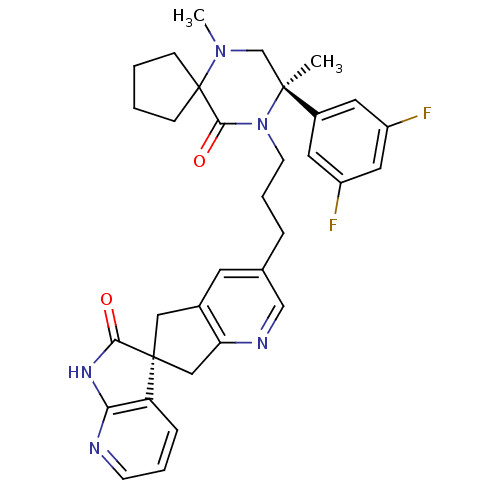
(CHEMBL2431251)Show SMILES CN1C[C@](C)(N(CCCc2cnc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H35F2N5O2/c1-31(23-14-24(34)16-25(35)15-23)20-39(2)33(9-3-4-10-33)30(42)40(31)12-6-7-21-13-22-17-32(18-27(22)37-19-21)26-8-5-11-36-28(26)38-29(32)41/h5,8,11,13-16,19H,3-4,6-7,9-10,12,17-18,20H2,1-2H3,(H,36,38,41)/t31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440789

(CHEMBL2431248)Show SMILES CN1C[C@](C)(N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H33F2N5O3/c1-31(22-13-23(34)15-24(35)14-22)19-39(2)33(9-3-4-10-33)30(43)40(31)18-27(41)37-25-8-7-20-16-32(17-21(20)12-25)26-6-5-11-36-28(26)38-29(32)42/h5-8,11-15H,3-4,9-10,16-19H2,1-2H3,(H,37,41)(H,36,38,42)/t31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440790
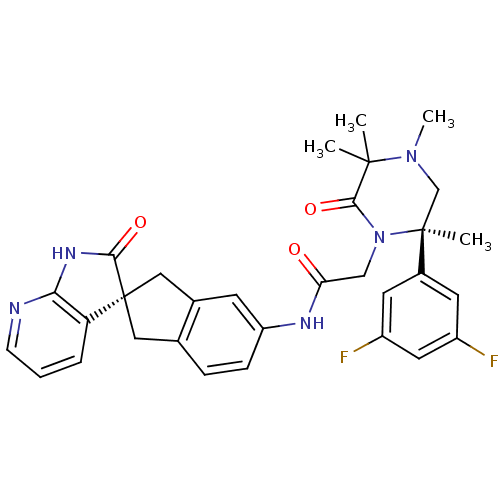
(CHEMBL2431247)Show SMILES CN1C[C@](C)(N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C1(C)C)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C31H31F2N5O3/c1-29(2)28(41)38(30(3,17-37(29)4)20-11-21(32)13-22(33)12-20)16-25(39)35-23-8-7-18-14-31(15-19(18)10-23)24-6-5-9-34-26(24)36-27(31)40/h5-13H,14-17H2,1-4H3,(H,35,39)(H,34,36,40)/t30-,31+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135600
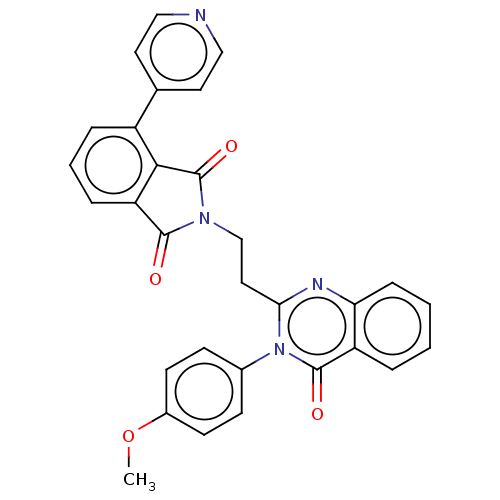
(US8846000, 1-16)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(c3C2=O)-c2ccncc2)nc2ccccc2c1=O Show InChI InChI=1S/C30H22N4O4/c1-38-21-11-9-20(10-12-21)34-26(32-25-8-3-2-5-23(25)29(34)36)15-18-33-28(35)24-7-4-6-22(27(24)30(33)37)19-13-16-31-17-14-19/h2-14,16-17H,15,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440785
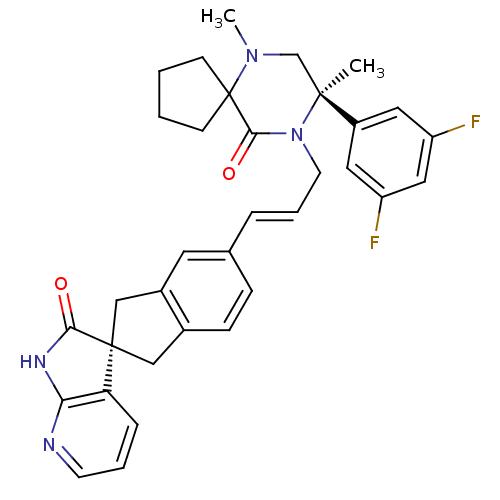
(CHEMBL2431252)Show SMILES CN1C[C@](C)(N(C\C=C\c2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C34H34F2N4O2/c1-32(25-16-26(35)18-27(36)17-25)21-39(2)34(11-3-4-12-34)31(42)40(32)14-6-7-22-9-10-23-19-33(20-24(23)15-22)28-8-5-13-37-29(28)38-30(33)41/h5-10,13,15-18H,3-4,11-12,14,19-21H2,1-2H3,(H,37,38,41)/b7-6+/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440792

(CHEMBL2431255)Show SMILES CC1(C)NC[C@](C)(N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C1=O)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C30H29F2N5O3/c1-28(2)27(40)37(29(3,16-34-28)19-10-20(31)12-21(32)11-19)15-24(38)35-22-7-6-17-13-30(14-18(17)9-22)23-5-4-8-33-25(23)36-26(30)39/h4-12,34H,13-16H2,1-3H3,(H,35,38)(H,33,36,39)/t29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440783
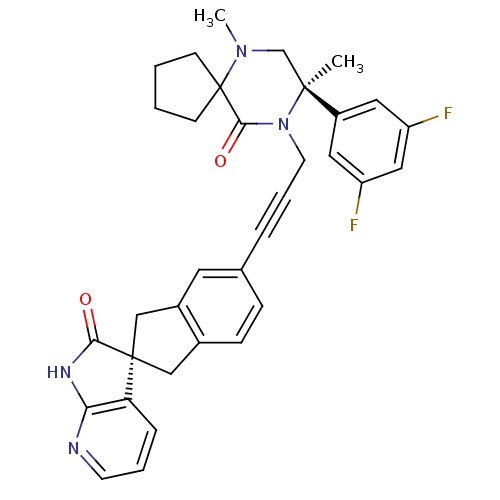
(CHEMBL2429882)Show SMILES CN1C[C@](C)(N(CC#Cc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C34H32F2N4O2/c1-32(25-16-26(35)18-27(36)17-25)21-39(2)34(11-3-4-12-34)31(42)40(32)14-6-7-22-9-10-23-19-33(20-24(23)15-22)28-8-5-13-37-29(28)38-30(33)41/h5,8-10,13,15-18H,3-4,11-12,14,19-21H2,1-2H3,(H,37,38,41)/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135614

(US8846000, D-7)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC(C)C)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C28H25N3O5/c1-17(2)36-23-10-6-8-21-25(23)28(34)30(26(21)32)16-15-24-29-22-9-5-4-7-20(22)27(33)31(24)18-11-13-19(35-3)14-12-18/h4-14,17H,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135595
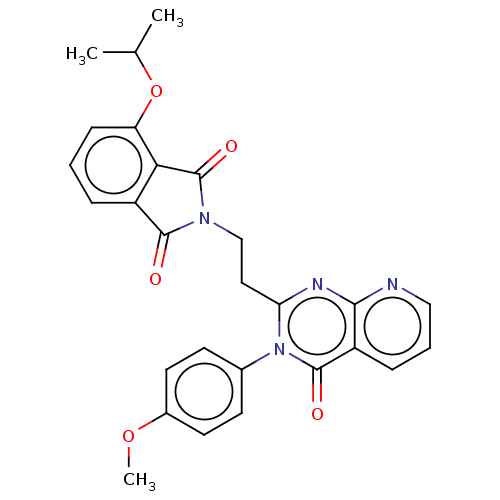
(US8846000, 1-11)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC(C)C)c3C2=O)nc2ncccc2c1=O Show InChI InChI=1S/C27H24N4O5/c1-16(2)36-21-8-4-6-19-23(21)27(34)30(25(19)32)15-13-22-29-24-20(7-5-14-28-24)26(33)31(22)17-9-11-18(35-3)12-10-17/h4-12,14,16H,13,15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440787
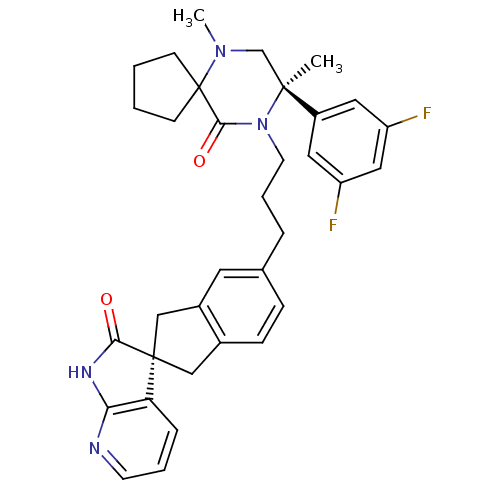
(CHEMBL2431250)Show SMILES CN1C[C@](C)(N(CCCc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C34H36F2N4O2/c1-32(25-16-26(35)18-27(36)17-25)21-39(2)34(11-3-4-12-34)31(42)40(32)14-6-7-22-9-10-23-19-33(20-24(23)15-22)28-8-5-13-37-29(28)38-30(33)41/h5,8-10,13,15-18H,3-4,6-7,11-12,14,19-21H2,1-2H3,(H,37,38,41)/t32-,33+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50224431
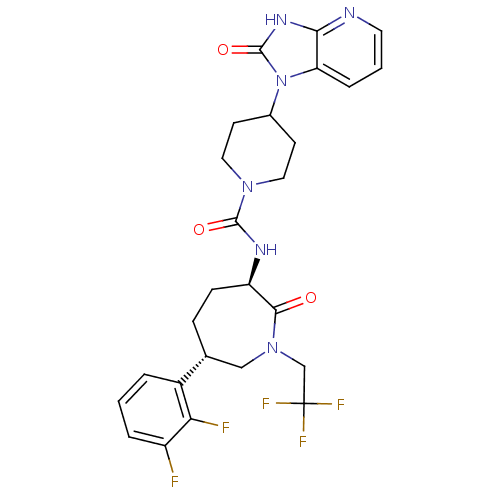
(CHEMBL236593 | MK-0974 | N-[(3R,6S)-6-(2,3-difluor...)Show SMILES Fc1cccc([C@@H]2CC[C@@H](NC(=O)N3CCC(CC3)n3c4cccnc4[nH]c3=O)C(=O)N(CC(F)(F)F)C2)c1F Show InChI InChI=1S/C26H27F5N6O3/c27-18-4-1-3-17(21(18)28)15-6-7-19(23(38)36(13-15)14-26(29,30)31)33-24(39)35-11-8-16(9-12-35)37-20-5-2-10-32-22(20)34-25(37)40/h1-5,10,15-16,19H,6-9,11-14H2,(H,33,39)(H,32,34,40)/t15-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CGRP receptor expressed in HEK293 cells |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50126108
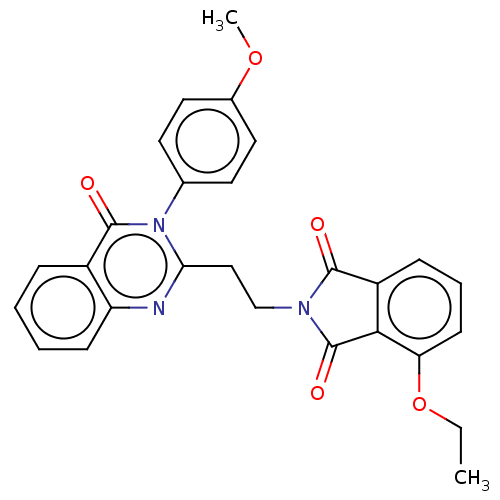
(CHEMBL3627840)Show SMILES CCOc1cccc2C(=O)N(CCc3nc4ccccc4c(=O)n3-c3ccc(OC)cc3)C(=O)c12 Show InChI InChI=1S/C27H23N3O5/c1-3-35-22-10-6-8-20-24(22)27(33)29(25(20)31)16-15-23-28-21-9-5-4-7-19(21)26(32)30(23)17-11-13-18(34-2)14-12-17/h4-14H,3,15-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50126110
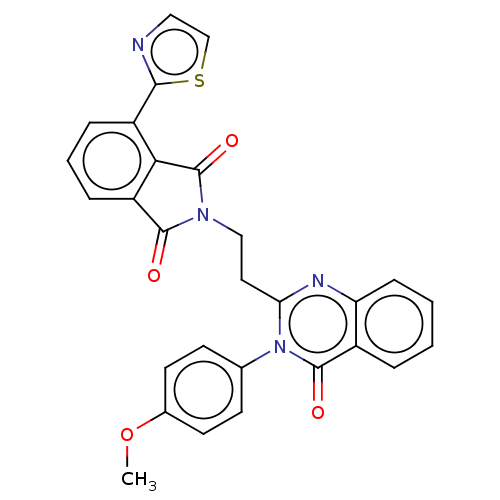
(CHEMBL3627843)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(-c4nccs4)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C28H20N4O4S/c1-36-18-11-9-17(10-12-18)32-23(30-22-8-3-2-5-19(22)27(32)34)13-15-31-26(33)21-7-4-6-20(24(21)28(31)35)25-29-14-16-37-25/h2-12,14,16H,13,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398008
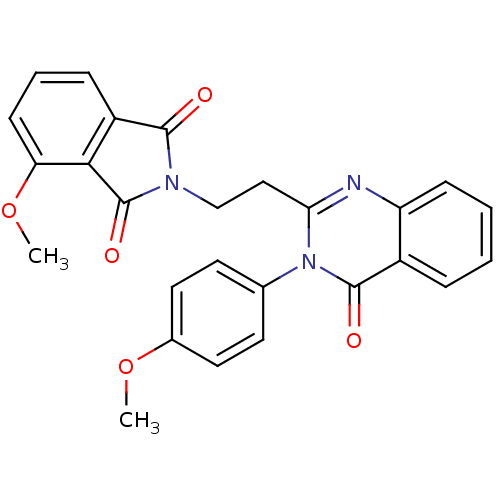
(CHEMBL2180426)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C26H21N3O5/c1-33-17-12-10-16(11-13-17)29-22(27-20-8-4-3-6-18(20)25(29)31)14-15-28-24(30)19-7-5-9-21(34-2)23(19)26(28)32/h3-13H,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135588
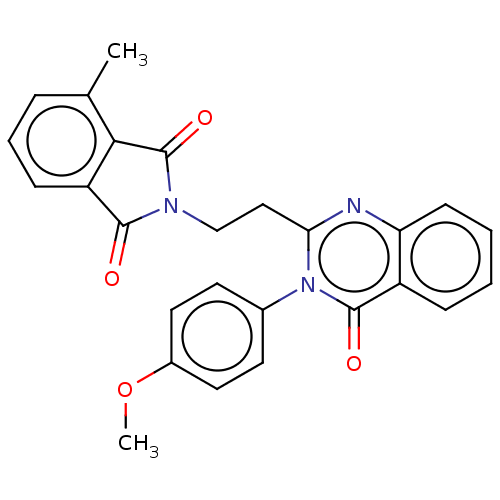
(US8846000, 1-4)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(C)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C26H21N3O4/c1-16-6-5-8-20-23(16)26(32)28(24(20)30)15-14-22-27-21-9-4-3-7-19(21)25(31)29(22)17-10-12-18(33-2)13-11-17/h3-13H,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50126107

(CHEMBL3627818)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3c(C2=O)c(Cl)ccc3Cl)nc2ccccc2c1=O Show InChI InChI=1S/C25H17Cl2N3O4/c1-34-15-8-6-14(7-9-15)30-20(28-19-5-3-2-4-16(19)23(30)31)12-13-29-24(32)21-17(26)10-11-18(27)22(21)25(29)33/h2-11H,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125995
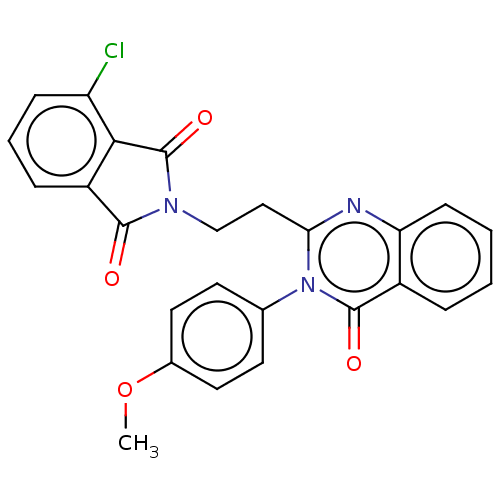
(CHEMBL3627816)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(Cl)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C25H18ClN3O4/c1-33-16-11-9-15(10-12-16)29-21(27-20-8-3-2-5-17(20)24(29)31)13-14-28-23(30)18-6-4-7-19(26)22(18)25(28)32/h2-12H,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50126109

(CHEMBL3627842)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(Br)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C25H18BrN3O4/c1-33-16-11-9-15(10-12-16)29-21(27-20-8-3-2-5-17(20)24(29)31)13-14-28-23(30)18-6-4-7-19(26)22(18)25(28)32/h2-12H,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125966

(CHEMBL3627814)Show SMILES O=C1N(CCc2nc3ccccc3c(=O)n2-c2ccc3[nH]ccc3c2)C(=O)c2ccccc12 Show InChI InChI=1S/C26H18N4O3/c31-24-18-5-1-2-6-19(18)25(32)29(24)14-12-23-28-22-8-4-3-7-20(22)26(33)30(23)17-9-10-21-16(15-17)11-13-27-21/h1-11,13,15,27H,12,14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125994
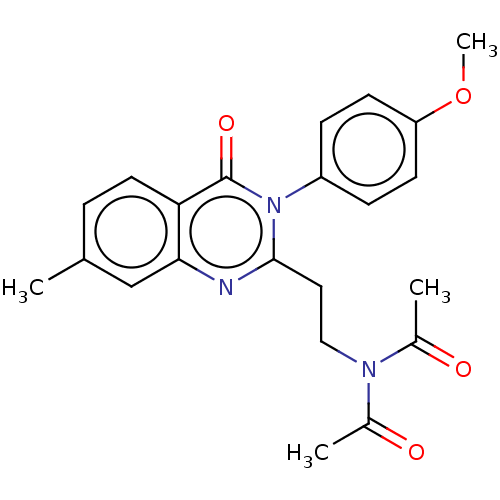
(CHEMBL3627813)Show SMILES COc1ccc(cc1)-n1c(CCN(C(C)=O)C(C)=O)nc2cc(C)ccc2c1=O Show InChI InChI=1S/C22H23N3O4/c1-14-5-10-19-20(13-14)23-21(11-12-24(15(2)26)16(3)27)25(22(19)28)17-6-8-18(29-4)9-7-17/h5-10,13H,11-12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135610

(US8846000, B-2)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(O)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C25H19N3O5/c1-33-16-11-9-15(10-12-16)28-21(26-19-7-3-2-5-17(19)24(28)31)13-14-27-23(30)18-6-4-8-20(29)22(18)25(27)32/h2-12,29H,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50126100

(CHEMBL3627817)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3ccc(Cl)cc3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C25H18ClN3O4/c1-33-17-9-7-16(8-10-17)29-22(27-21-5-3-2-4-19(21)25(29)32)12-13-28-23(30)18-11-6-15(26)14-20(18)24(28)31/h2-11,14H,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125993
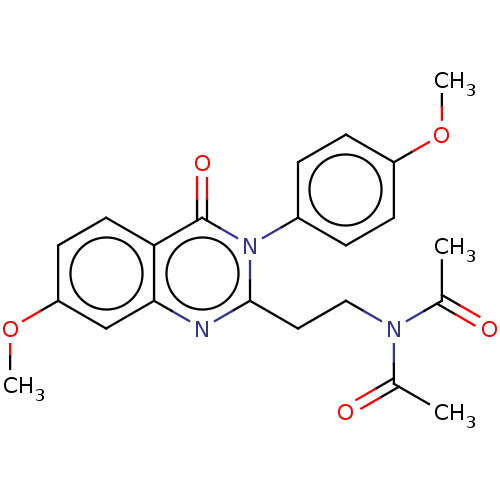
(CHEMBL3627812)Show SMILES COc1ccc(cc1)-n1c(CCN(C(C)=O)C(C)=O)nc2cc(OC)ccc2c1=O Show InChI InChI=1S/C22H23N3O5/c1-14(26)24(15(2)27)12-11-21-23-20-13-18(30-4)9-10-19(20)22(28)25(21)16-5-7-17(29-3)8-6-16/h5-10,13H,11-12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125991
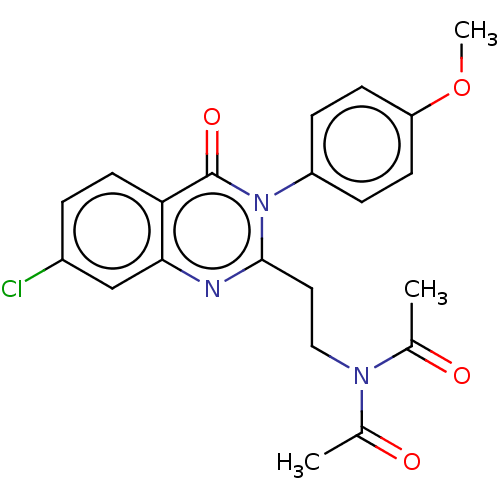
(CHEMBL3627810)Show SMILES COc1ccc(cc1)-n1c(CCN(C(C)=O)C(C)=O)nc2cc(Cl)ccc2c1=O Show InChI InChI=1S/C21H20ClN3O4/c1-13(26)24(14(2)27)11-10-20-23-19-12-15(22)4-9-18(19)21(28)25(20)16-5-7-17(29-3)8-6-16/h4-9,12H,10-11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125986
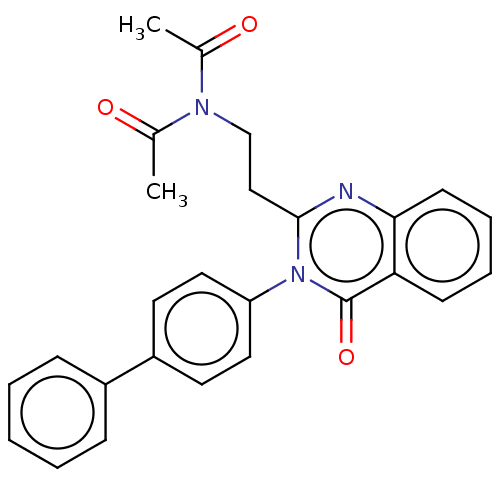
(CHEMBL3627805)Show SMILES CC(=O)N(CCc1nc2ccccc2c(=O)n1-c1ccc(cc1)-c1ccccc1)C(C)=O Show InChI InChI=1S/C26H23N3O3/c1-18(30)28(19(2)31)17-16-25-27-24-11-7-6-10-23(24)26(32)29(25)22-14-12-21(13-15-22)20-8-4-3-5-9-20/h3-15H,16-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125987
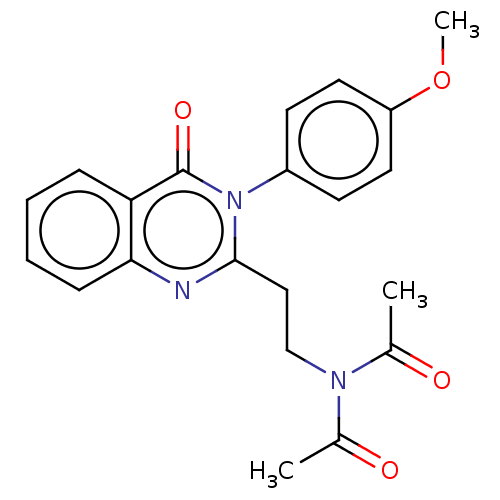
(CHEMBL3627806)Show SMILES COc1ccc(cc1)-n1c(CCN(C(C)=O)C(C)=O)nc2ccccc2c1=O Show InChI InChI=1S/C21H21N3O4/c1-14(25)23(15(2)26)13-12-20-22-19-7-5-4-6-18(19)21(27)24(20)16-8-10-17(28-3)11-9-16/h4-11H,12-13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125988

(CHEMBL3627807)Show SMILES COc1ccc(cc1OC)-n1c(CCN(C(C)=O)C(C)=O)nc2ccccc2c1=O Show InChI InChI=1S/C22H23N3O5/c1-14(26)24(15(2)27)12-11-21-23-18-8-6-5-7-17(18)22(28)25(21)16-9-10-19(29-3)20(13-16)30-4/h5-10,13H,11-12H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125982
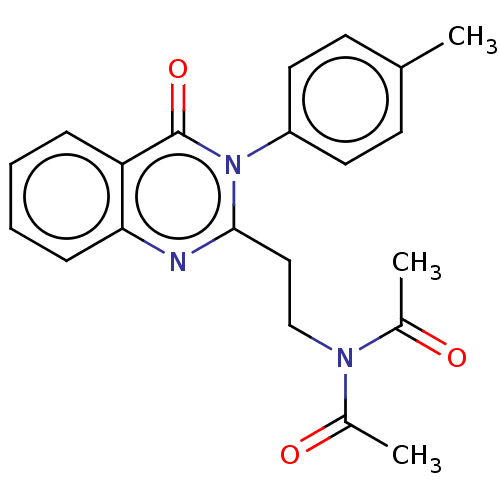
(CHEMBL3627801)Show SMILES CC(=O)N(CCc1nc2ccccc2c(=O)n1-c1ccc(C)cc1)C(C)=O Show InChI InChI=1S/C21H21N3O3/c1-14-8-10-17(11-9-14)24-20(12-13-23(15(2)25)16(3)26)22-19-7-5-4-6-18(19)21(24)27/h4-11H,12-13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125969
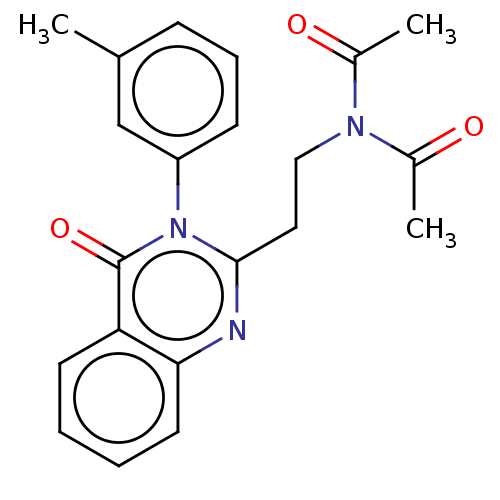
(CHEMBL3627924)Show SMILES CC(=O)N(CCc1nc2ccccc2c(=O)n1-c1cccc(C)c1)C(C)=O Show InChI InChI=1S/C21H21N3O3/c1-14-7-6-8-17(13-14)24-20(11-12-23(15(2)25)16(3)26)22-19-10-5-4-9-18(19)21(24)27/h4-10,13H,11-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125984
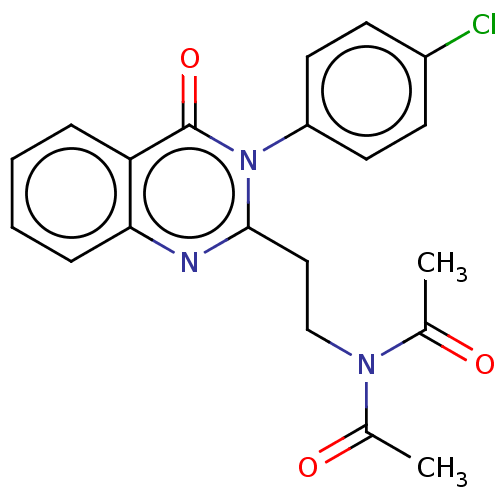
(CHEMBL3627803)Show SMILES CC(=O)N(CCc1nc2ccccc2c(=O)n1-c1ccc(Cl)cc1)C(C)=O Show InChI InChI=1S/C20H18ClN3O3/c1-13(25)23(14(2)26)12-11-19-22-18-6-4-3-5-17(18)20(27)24(19)16-9-7-15(21)8-10-16/h3-10H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125990

(CHEMBL3627809)Show SMILES COc1ccc(cc1)-n1c(CCN(C(C)=O)C(C)=O)nc2ccc(Cl)cc2c1=O Show InChI InChI=1S/C21H20ClN3O4/c1-13(26)24(14(2)27)11-10-20-23-19-9-4-15(22)12-18(19)21(28)25(20)16-5-7-17(29-3)8-6-16/h4-9,12H,10-11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125989
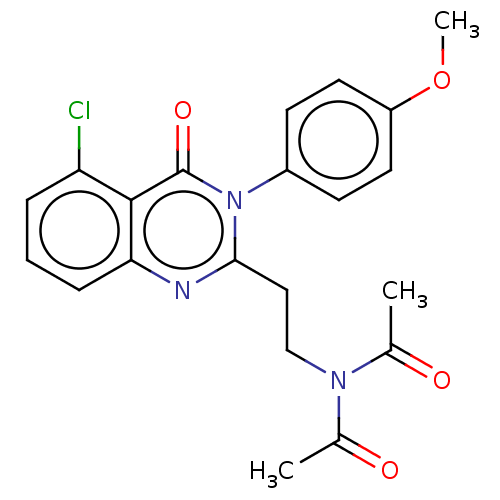
(CHEMBL3627808)Show SMILES COc1ccc(cc1)-n1c(CCN(C(C)=O)C(C)=O)nc2cccc(Cl)c2c1=O Show InChI InChI=1S/C21H20ClN3O4/c1-13(26)24(14(2)27)12-11-19-23-18-6-4-5-17(22)20(18)21(28)25(19)15-7-9-16(29-3)10-8-15/h4-10H,11-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 171 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135601

(US8846000, 1-18)Show SMILES COc1ccc(cc1)-n1c(CCCN2C(=O)c3ccccc3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C26H21N3O4/c1-33-18-14-12-17(13-15-18)29-23(27-22-10-5-4-9-21(22)26(29)32)11-6-16-28-24(30)19-7-2-3-8-20(19)25(28)31/h2-5,7-10,12-15H,6,11,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 219 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125985
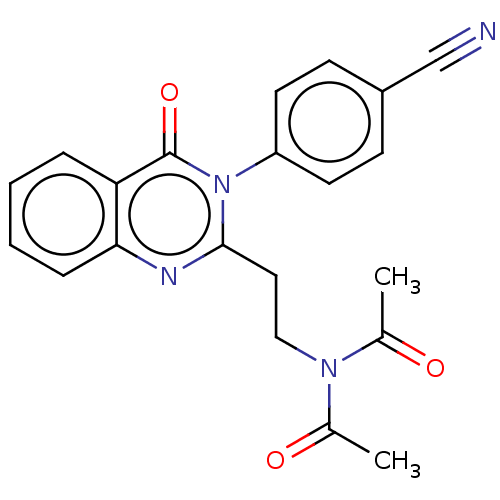
(CHEMBL3627804)Show SMILES CC(=O)N(CCc1nc2ccccc2c(=O)n1-c1ccc(cc1)C#N)C(C)=O Show InChI InChI=1S/C21H18N4O3/c1-14(26)24(15(2)27)12-11-20-23-19-6-4-3-5-18(19)21(28)25(20)17-9-7-16(13-22)8-10-17/h3-10H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 265 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125983

(CHEMBL3627802)Show SMILES CC(=O)N(CCc1nc2ccccc2c(=O)n1-c1ccc(F)cc1)C(C)=O Show InChI InChI=1S/C20H18FN3O3/c1-13(25)23(14(2)26)12-11-19-22-18-6-4-3-5-17(18)20(27)24(19)16-9-7-15(21)8-10-16/h3-10H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125992
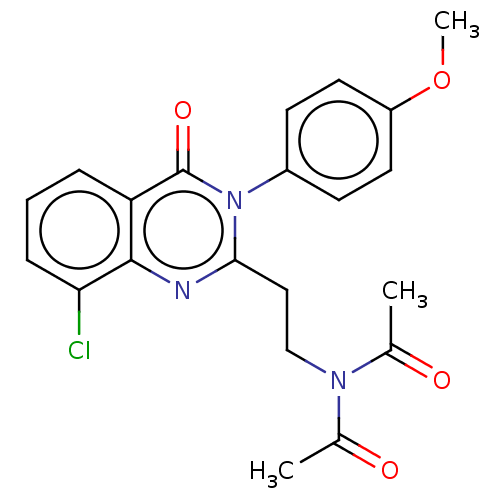
(CHEMBL3627811)Show SMILES COc1ccc(cc1)-n1c(CCN(C(C)=O)C(C)=O)nc2c(Cl)cccc2c1=O Show InChI InChI=1S/C21H20ClN3O4/c1-13(26)24(14(2)27)12-11-19-23-20-17(5-4-6-18(20)22)21(28)25(19)15-7-9-16(29-3)10-8-15/h4-10H,11-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50398009

(CHEMBL2180425)Show SMILES COc1ccc(cc1)-n1c(CN2C(=O)c3ccccc3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C24H17N3O4/c1-31-16-12-10-15(11-13-16)27-21(25-20-9-5-4-8-19(20)24(27)30)14-26-22(28)17-6-2-3-7-18(17)23(26)29/h2-13H,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125968
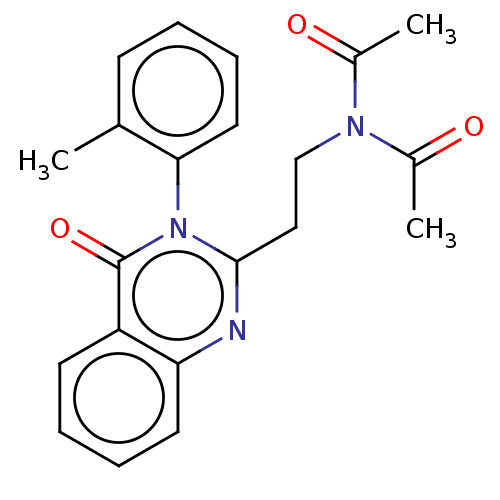
(CHEMBL3627923)Show SMILES CC(=O)N(CCc1nc2ccccc2c(=O)n1-c1ccccc1C)C(C)=O |(4.26,-6.01,;5.32,-5.4,;6.39,-6.02,;5.33,-3.86,;3.99,-3.08,;4,-1.54,;2.66,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.33,2.77,;2.66,.77,;4,1.54,;5.33,.77,;6.66,1.53,;6.67,3.07,;5.33,3.85,;4,3.08,;2.93,3.7,;6.66,-3.09,;7.73,-3.7,;6.66,-1.85,)| Show InChI InChI=1S/C21H21N3O3/c1-14-8-4-7-11-19(14)24-20(12-13-23(15(2)25)16(3)26)22-18-10-6-5-9-17(18)21(24)27/h4-11H,12-13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50403370
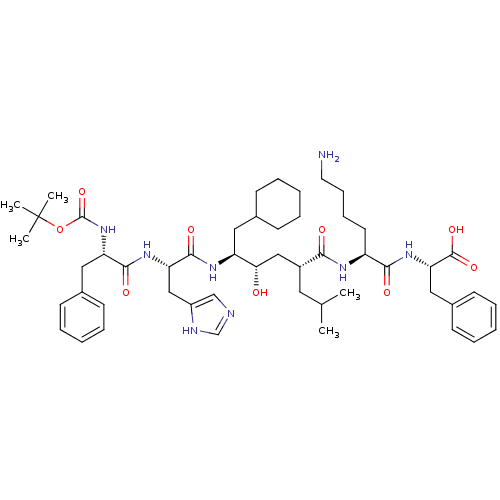
(CHEMBL2052021 | CP-71362)Show SMILES CC(C)C[C@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C51H76N8O9/c1-33(2)25-37(45(61)55-39(23-15-16-24-52)46(62)58-43(49(65)66)28-36-21-13-8-14-22-36)29-44(60)40(26-34-17-9-6-10-18-34)56-48(64)42(30-38-31-53-32-54-38)57-47(63)41(27-35-19-11-7-12-20-35)59-50(67)68-51(3,4)5/h7-8,11-14,19-22,31-34,37,39-44,60H,6,9-10,15-18,23-30,52H2,1-5H3,(H,53,54)(H,55,61)(H,56,64)(H,57,63)(H,58,62)(H,59,67)(H,65,66)/t37-,39+,40+,41+,42+,43+,44+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against dog plasma renin |
Bioorg Med Chem Lett 4: 589-592 (1994)
Article DOI: 10.1016/S0960-894X(01)80160-8
BindingDB Entry DOI: 10.7270/Q2KD1XVS |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50437205

(CHEMBL2402572)Show SMILES Cc1ccccc1[C@H]1[C@@H]2CN(C[C@H]2CC[C@@H]1O[C@H](CO)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1nc(O)co1 |r| Show InChI InChI=1S/C28H28F6N2O4/c1-15-4-2-3-5-20(15)25-21-12-36(26-35-24(38)14-39-26)11-16(21)6-7-22(25)40-23(13-37)17-8-18(27(29,30)31)10-19(9-17)28(32,33)34/h2-5,8-10,14,16,21-23,25,37-38H,6-7,11-13H2,1H3/t16-,21-,22+,23-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P from human NK1 receptor expressed in CHO cells |
J Med Chem 56: 5940-8 (2014)
Article DOI: 10.1021/jm400751p
BindingDB Entry DOI: 10.7270/Q2ZW1N9J |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50277511

(3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromet...)Show SMILES C[C@@H](O[C@H]1CC[C@@H]2CN(C[C@H]2[C@@H]1c1ccc(F)cc1)C1=CC(=O)CC1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,t:22| Show InChI InChI=1S/C29H28F7NO2/c1-16(19-10-20(28(31,32)33)12-21(11-19)29(34,35)36)39-26-9-4-18-14-37(23-7-8-24(38)13-23)15-25(18)27(26)17-2-5-22(30)6-3-17/h2-3,5-6,10-13,16,18,25-27H,4,7-9,14-15H2,1H3/t16-,18-,25-,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]substance P from human NK1 receptor expressed in CHO cells |
J Med Chem 56: 5940-8 (2014)
Article DOI: 10.1021/jm400751p
BindingDB Entry DOI: 10.7270/Q2ZW1N9J |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50440789

(CHEMBL2431248)Show SMILES CN1C[C@](C)(N(CC(=O)Nc2ccc3C[C@]4(Cc3c2)C(=O)Nc2ncccc42)C(=O)C11CCCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C33H33F2N5O3/c1-31(22-13-23(34)15-24(35)14-22)19-39(2)33(9-3-4-10-33)30(43)40(31)18-27(41)37-25-8-7-20-16-32(17-21(20)12-25)26-6-5-11-36-28(26)38-29(32)42/h5-8,11-15H,3-4,9-10,16-19H2,1-2H3,(H,37,41)(H,36,38,42)/t31-,32+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Labortories
Curated by ChEMBL
| Assay Description
Antagonist activity at CGRP receptor (unknown origin) by cell based cAMP assay |
ACS Med Chem Lett 4: 863-8 (2013)
Article DOI: 10.1021/ml400199p
BindingDB Entry DOI: 10.7270/Q25D8T8V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data