 Found 1689 hits with Last Name = 'purkey' and Initial = 'he'
Found 1689 hits with Last Name = 'purkey' and Initial = 'he' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030752
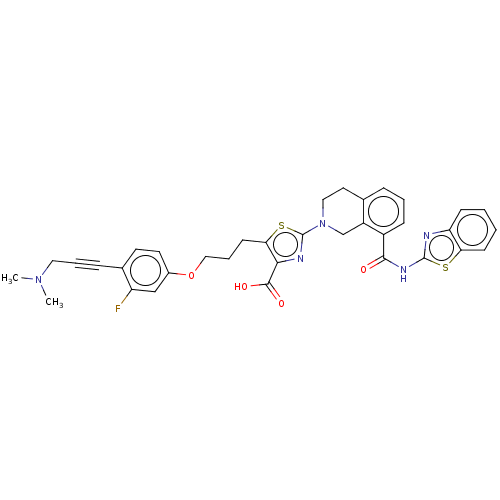
(CHEMBL3342333)Show SMILES CN(C)CC#Cc1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1F Show InChI InChI=1S/C35H32FN5O4S2/c1-40(2)17-6-9-23-14-15-24(20-27(23)36)45-19-7-13-30-31(33(43)44)38-35(47-30)41-18-16-22-8-5-10-25(26(22)21-41)32(42)39-34-37-28-11-3-4-12-29(28)46-34/h3-5,8,10-12,14-15,20H,7,13,16-19,21H2,1-2H3,(H,43,44)(H,37,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030754
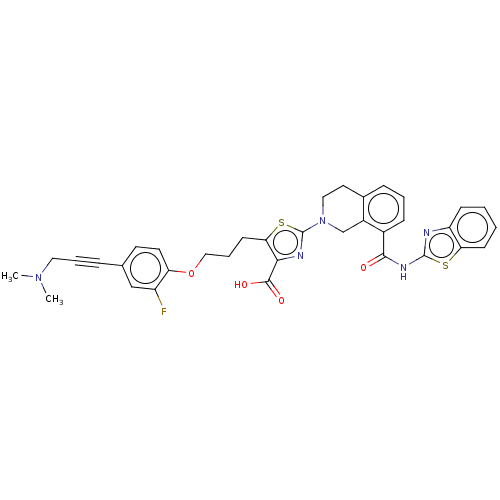
(CHEMBL3342332)Show SMILES CN(C)CC#Cc1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)c(F)c1 Show InChI InChI=1S/C35H32FN5O4S2/c1-40(2)17-6-8-22-14-15-28(26(36)20-22)45-19-7-13-30-31(33(43)44)38-35(47-30)41-18-16-23-9-5-10-24(25(23)21-41)32(42)39-34-37-27-11-3-4-12-29(27)46-34/h3-5,9-12,14-15,20H,7,13,16-19,21H2,1-2H3,(H,43,44)(H,37,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030757
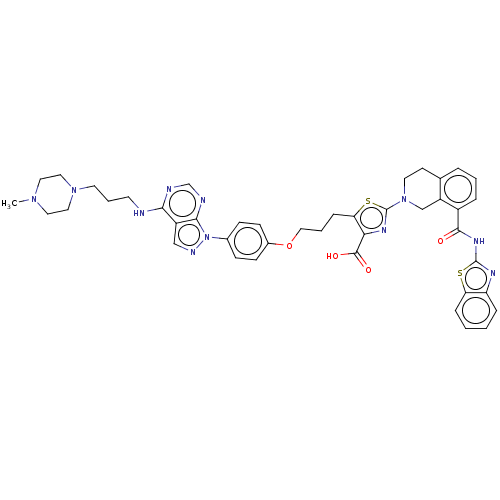
(CHEMBL3342196)Show SMILES CN1CCN(CCCNc2ncnc3n(ncc23)-c2ccc(OCCCc3sc(nc3C(O)=O)N3CCc4cccc(C(=O)Nc5nc6ccccc6s5)c4C3)cc2)CC1 Show InChI InChI=1S/C43H45N11O4S2/c1-51-20-22-52(23-21-51)18-6-17-44-38-32-25-47-54(39(32)46-27-45-38)29-12-14-30(15-13-29)58-24-5-11-36-37(41(56)57)49-43(60-36)53-19-16-28-7-4-8-31(33(28)26-53)40(55)50-42-48-34-9-2-3-10-35(34)59-42/h2-4,7-10,12-15,25,27H,5-6,11,16-24,26H2,1H3,(H,56,57)(H,44,45,46)(H,48,50,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030758
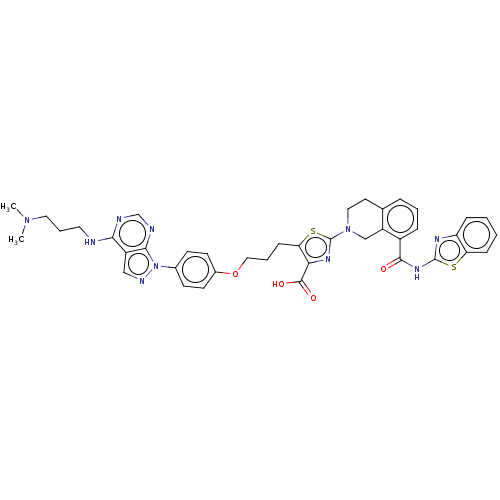
(CHEMBL3342195)Show SMILES CN(C)CCCNc1ncnc2n(ncc12)-c1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1 Show InChI InChI=1S/C40H40N10O4S2/c1-48(2)19-7-18-41-35-29-22-44-50(36(29)43-24-42-35)26-13-15-27(16-14-26)54-21-6-12-33-34(38(52)53)46-40(56-33)49-20-17-25-8-5-9-28(30(25)23-49)37(51)47-39-45-31-10-3-4-11-32(31)55-39/h3-5,8-11,13-16,22,24H,6-7,12,17-21,23H2,1-2H3,(H,52,53)(H,41,42,43)(H,45,47,51) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030759
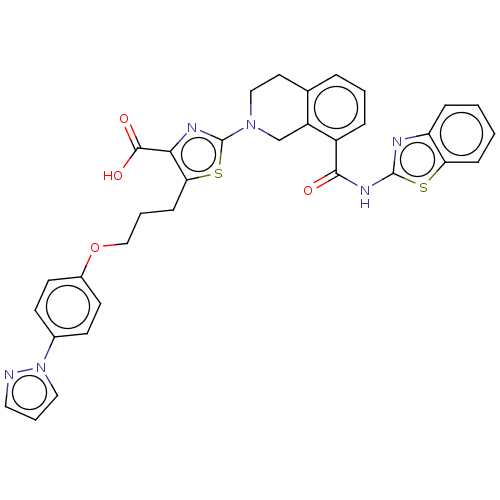
(CHEMBL3342194)Show SMILES OC(=O)c1nc(sc1CCCOc1ccc(cc1)-n1cccn1)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 Show InChI InChI=1S/C33H28N6O4S2/c40-30(37-32-35-26-8-1-2-9-27(26)44-32)24-7-3-6-21-15-18-38(20-25(21)24)33-36-29(31(41)42)28(45-33)10-4-19-43-23-13-11-22(12-14-23)39-17-5-16-34-39/h1-3,5-9,11-14,16-17H,4,10,15,18-20H2,(H,41,42)(H,35,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112421

(US8623889, 420)Show SMILES Cc1c(F)cncc1-c1ccc2cc(NC(=O)[C@@H]3C[C@@H]3F)ncc2c1 |r| Show InChI InChI=1S/C19H15F2N3O/c1-10-15(8-22-9-17(10)21)12-3-2-11-5-18(23-7-13(11)4-12)24-19(25)14-6-16(14)20/h2-5,7-9,14,16H,6H2,1H3,(H,23,24,25)/t14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112161

(US8623889, 159)Show InChI InChI=1S/C19H16FN3O/c1-11-16(9-21-10-17(11)20)14-5-4-13-7-18(22-8-15(13)6-14)23-19(24)12-2-3-12/h4-10,12H,2-3H2,1H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112230
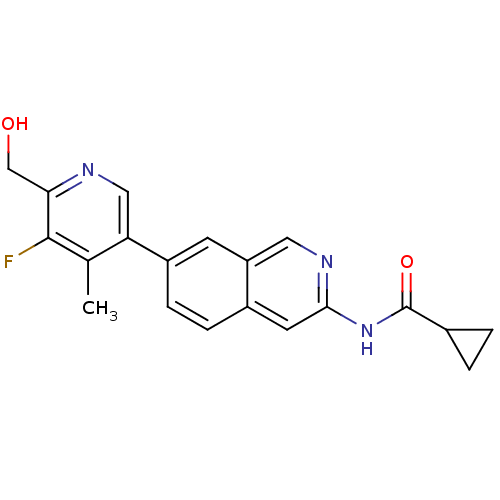
(US8623889, 228)Show SMILES Cc1c(F)c(CO)ncc1-c1ccc2cc(NC(=O)C3CC3)ncc2c1 Show InChI InChI=1S/C20H18FN3O2/c1-11-16(9-22-17(10-25)19(11)21)14-5-4-13-7-18(23-8-15(13)6-14)24-20(26)12-2-3-12/h4-9,12,25H,2-3,10H2,1H3,(H,23,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030756
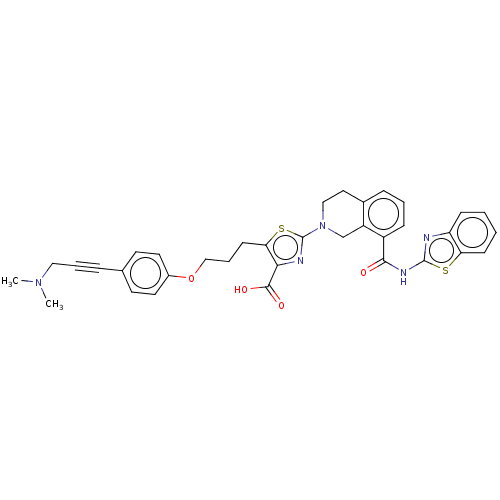
(CHEMBL3342197)Show SMILES CN(C)CC#Cc1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1 Show InChI InChI=1S/C35H33N5O4S2/c1-39(2)19-6-8-23-14-16-25(17-15-23)44-21-7-13-30-31(33(42)43)37-35(46-30)40-20-18-24-9-5-10-26(27(24)22-40)32(41)38-34-36-28-11-3-4-12-29(28)45-34/h3-5,9-12,14-17H,7,13,18-22H2,1-2H3,(H,42,43)(H,36,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112111
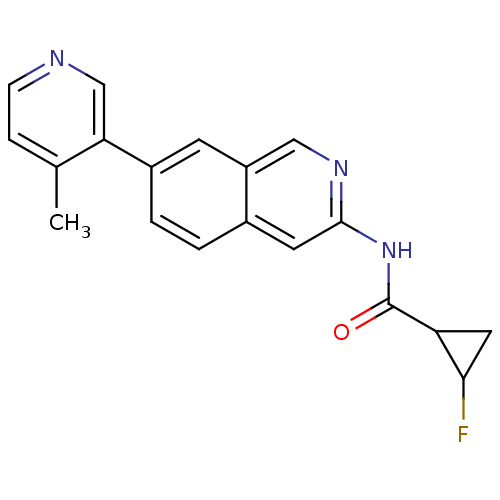
(US8623889, 109)Show InChI InChI=1S/C19H16FN3O/c1-11-4-5-21-10-16(11)13-3-2-12-7-18(22-9-14(12)6-13)23-19(24)15-8-17(15)20/h2-7,9-10,15,17H,8H2,1H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112359

(US8623889, 358)Show SMILES Cc1ccc2[nH]ncc2c1-c1cc2cnc(NC(=O)[C@@H]3C[C@@H]3F)cc2cn1 |r| Show InChI InChI=1S/C20H16FN5O/c1-10-2-3-16-14(9-24-26-16)19(10)17-4-11-8-23-18(5-12(11)7-22-17)25-20(27)13-6-15(13)21/h2-5,7-9,13,15H,6H2,1H3,(H,24,26)(H,23,25,27)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112193
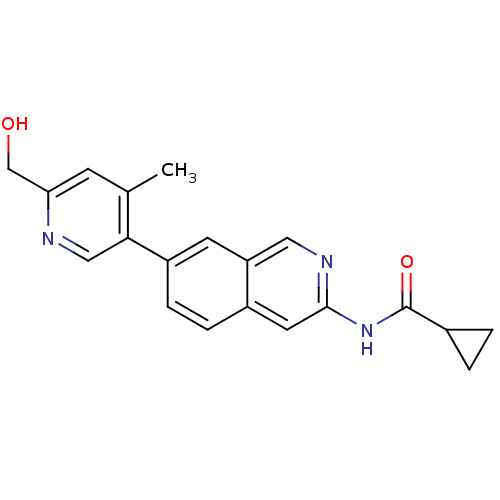
(US8623889, 191)Show InChI InChI=1S/C20H19N3O2/c1-12-6-17(11-24)21-10-18(12)15-5-4-14-8-19(22-9-16(14)7-15)23-20(25)13-2-3-13/h4-10,13,24H,2-3,11H2,1H3,(H,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112066

(US8623889, 64)Show InChI InChI=1S/C20H18N2O2/c1-12-2-7-17(23)10-18(12)15-6-5-14-9-19(21-11-16(14)8-15)22-20(24)13-3-4-13/h2,5-11,13,23H,3-4H2,1H3,(H,21,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112312

(US8623889, 311)Show SMILES Cc1cc(ncc1-c1ccc2cc(NC(=O)C3C[C@@H]3F)ncc2c1)[C@H](O)C(F)(F)F |r| Show InChI InChI=1S/C21H17F4N3O2/c1-10-4-17(19(29)21(23,24)25)26-9-15(10)12-3-2-11-6-18(27-8-13(11)5-12)28-20(30)14-7-16(14)22/h2-6,8-9,14,16,19,29H,7H2,1H3,(H,27,28,30)/t14?,16-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602306
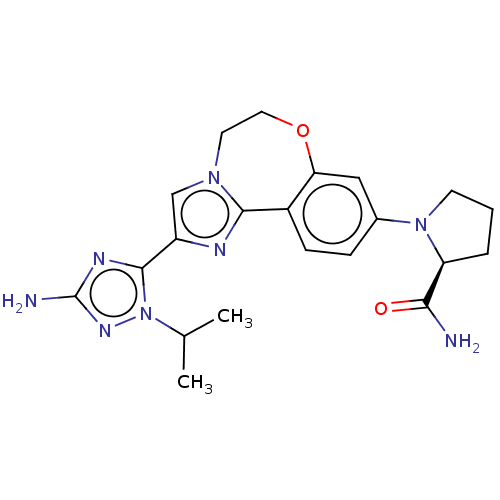
(CHEMBL5208487)Show SMILES CC(C)n1nc(N)nc1-c1cn2CCOc3cc(ccc3-c2n1)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112105

(US8623889, 103)Show SMILES Cc1cc(F)c(O)cc1-c1ccc2cc(NC(=O)C3CC3)ncc2c1 Show InChI InChI=1S/C20H17FN2O2/c1-11-6-17(21)18(24)9-16(11)14-5-4-13-8-19(22-10-15(13)7-14)23-20(25)12-2-3-12/h4-10,12,24H,2-3H2,1H3,(H,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112251

(US8623889, 249)Show SMILES Cc1ccc2[nH]ncc2c1-c1ccc2cc(NC(=O)C3CC3)ncc2c1 Show InChI InChI=1S/C21H18N4O/c1-12-2-7-18-17(11-23-25-18)20(12)15-6-5-14-9-19(22-10-16(14)8-15)24-21(26)13-3-4-13/h2,5-11,13H,3-4H2,1H3,(H,23,25)(H,22,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030757

(CHEMBL3342196)Show SMILES CN1CCN(CCCNc2ncnc3n(ncc23)-c2ccc(OCCCc3sc(nc3C(O)=O)N3CCc4cccc(C(=O)Nc5nc6ccccc6s5)c4C3)cc2)CC1 Show InChI InChI=1S/C43H45N11O4S2/c1-51-20-22-52(23-21-51)18-6-17-44-38-32-25-47-54(39(32)46-27-45-38)29-12-14-30(15-13-29)58-24-5-11-36-37(41(56)57)49-43(60-36)53-19-16-28-7-4-8-31(33(28)26-53)40(55)50-42-48-34-9-2-3-10-35(34)59-42/h2-4,7-10,12-15,25,27H,5-6,11,16-24,26H2,1H3,(H,56,57)(H,44,45,46)(H,48,50,55) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr in presence of 1% human serum by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030751
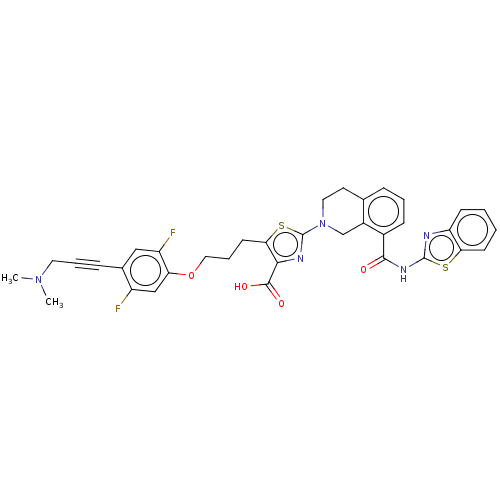
(CHEMBL3342334)Show SMILES CN(C)CC#Cc1cc(F)c(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1F Show InChI InChI=1S/C35H31F2N5O4S2/c1-41(2)15-6-9-22-18-26(37)28(19-25(22)36)46-17-7-13-30-31(33(44)45)39-35(48-30)42-16-14-21-8-5-10-23(24(21)20-42)32(43)40-34-38-27-11-3-4-12-29(27)47-34/h3-5,8,10-12,18-19H,7,13-17,20H2,1-2H3,(H,44,45)(H,38,40,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112099
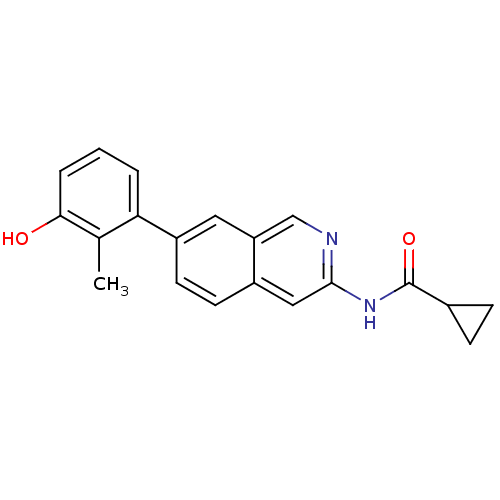
(US8623889, 97)Show InChI InChI=1S/C20H18N2O2/c1-12-17(3-2-4-18(12)23)15-8-7-14-10-19(21-11-16(14)9-15)22-20(24)13-5-6-13/h2-4,7-11,13,23H,5-6H2,1H3,(H,21,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112289
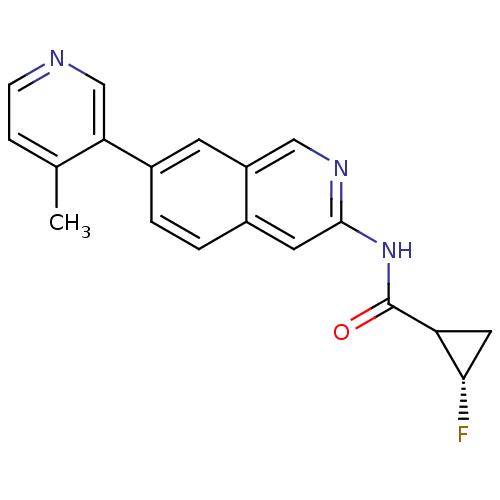
(US8623889, 287)Show SMILES Cc1ccncc1-c1ccc2cc(NC(=O)C3C[C@@H]3F)ncc2c1 |r| Show InChI InChI=1S/C19H16FN3O/c1-11-4-5-21-10-16(11)13-3-2-12-7-18(22-9-14(12)6-13)23-19(24)15-8-17(15)20/h2-7,9-10,15,17H,8H2,1H3,(H,22,23,24)/t15?,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112106
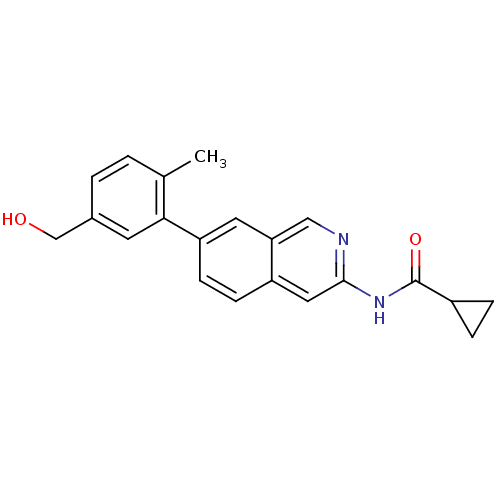
(US8623889, 104)Show InChI InChI=1S/C21H20N2O2/c1-13-2-3-14(12-24)8-19(13)17-7-6-16-10-20(22-11-18(16)9-17)23-21(25)15-4-5-15/h2-3,6-11,15,24H,4-5,12H2,1H3,(H,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112029

(US8623889, 27)Show InChI InChI=1S/C19H17N3O/c1-12-6-7-20-11-17(12)15-5-4-14-9-18(21-10-16(14)8-15)22-19(23)13-2-3-13/h4-11,13H,2-3H2,1H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112274

(US8623889, 272)Show SMILES Cc1cc(ncc1-c1ccc2cc(NC(=O)C3CC3)ncc2c1)[C@H](O)C(F)(F)F |r| Show InChI InChI=1S/C21H18F3N3O2/c1-11-6-17(19(28)21(22,23)24)25-10-16(11)14-5-4-13-8-18(26-9-15(13)7-14)27-20(29)12-2-3-12/h4-10,12,19,28H,2-3H2,1H3,(H,26,27,29)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112275
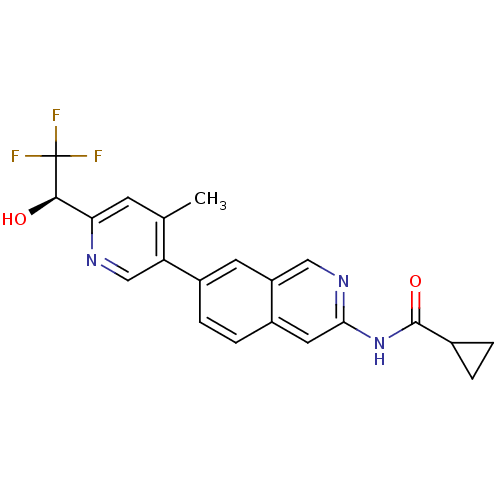
(US8623889, 273)Show SMILES Cc1cc(ncc1-c1ccc2cc(NC(=O)C3CC3)ncc2c1)[C@@H](O)C(F)(F)F |r| Show InChI InChI=1S/C21H18F3N3O2/c1-11-6-17(19(28)21(22,23)24)25-10-16(11)14-5-4-13-8-18(26-9-15(13)7-14)27-20(29)12-2-3-12/h4-10,12,19,28H,2-3H2,1H3,(H,26,27,29)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112288
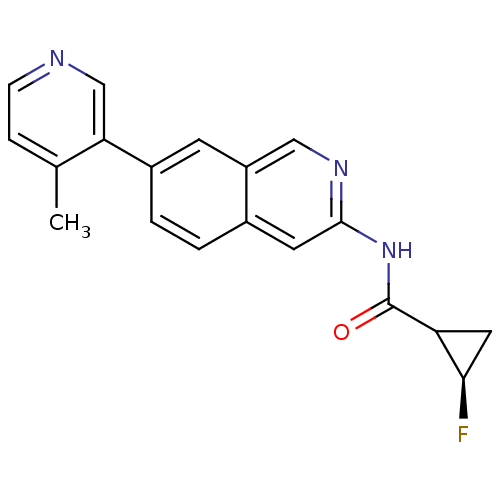
(US8623889, 286)Show SMILES Cc1ccncc1-c1ccc2cc(NC(=O)C3C[C@H]3F)ncc2c1 |r| Show InChI InChI=1S/C19H16FN3O/c1-11-4-5-21-10-16(11)13-3-2-12-7-18(22-9-14(12)6-13)23-19(24)15-8-17(15)20/h2-7,9-10,15,17H,8H2,1H3,(H,22,23,24)/t15?,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM295665
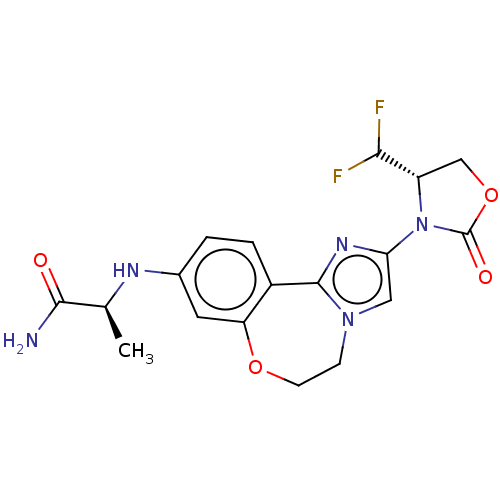
((S)-2-((2-((S)-4-(difluoromethyl)- 2-oxooxazolidin...)Show SMILES C[C@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)F)C(N)=O |r| Show InChI InChI=1S/C18H19F2N5O4/c1-9(16(21)26)22-10-2-3-11-13(6-10)28-5-4-24-7-14(23-17(11)24)25-12(15(19)20)8-29-18(25)27/h2-3,6-7,9,12,15,22H,4-5,8H2,1H3,(H2,21,26)/t9-,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030755
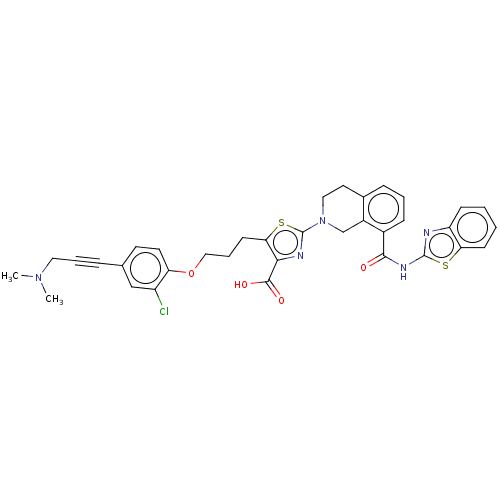
(CHEMBL3342198)Show SMILES CN(C)CC#Cc1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)c(Cl)c1 Show InChI InChI=1S/C35H32ClN5O4S2/c1-40(2)17-6-8-22-14-15-28(26(36)20-22)45-19-7-13-30-31(33(43)44)38-35(47-30)41-18-16-23-9-5-10-24(25(23)21-41)32(42)39-34-37-27-11-3-4-12-29(27)46-34/h3-5,9-12,14-15,20H,7,13,16-19,21H2,1-2H3,(H,43,44)(H,37,39,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112192
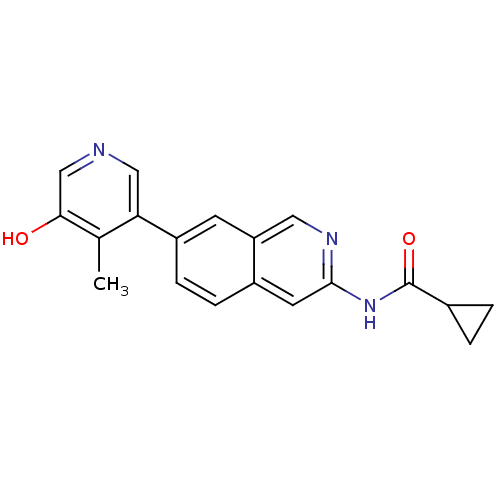
(US8623889, 190)Show InChI InChI=1S/C19H17N3O2/c1-11-16(9-20-10-17(11)23)14-5-4-13-7-18(21-8-15(13)6-14)22-19(24)12-2-3-12/h4-10,12,23H,2-3H2,1H3,(H,21,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112144
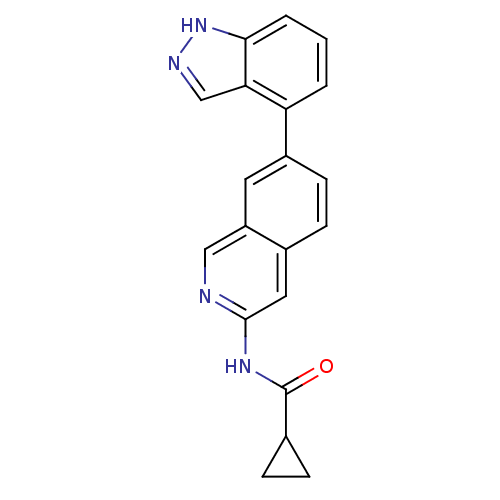
(US8623889, 142)Show SMILES O=C(Nc1cc2ccc(cc2cn1)-c1cccc2[nH]ncc12)C1CC1 Show InChI InChI=1S/C20H16N4O/c25-20(12-4-5-12)23-19-9-13-6-7-14(8-15(13)10-21-19)16-2-1-3-18-17(16)11-22-24-18/h1-3,6-12H,4-5H2,(H,22,24)(H,21,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112252

(US8623889, 250)Show SMILES Cc1ncc2[nH]ncc2c1-c1ccc2cc(NC(=O)C3CC3)ncc2c1 Show InChI InChI=1S/C20H17N5O/c1-11-19(16-9-23-25-17(16)10-21-11)14-5-4-13-7-18(22-8-15(13)6-14)24-20(26)12-2-3-12/h4-10,12H,2-3H2,1H3,(H,23,25)(H,22,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112032
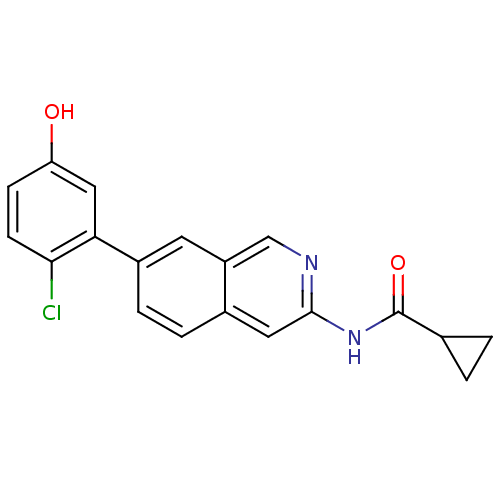
(US8623889, 30)Show SMILES Oc1ccc(Cl)c(c1)-c1ccc2cc(NC(=O)C3CC3)ncc2c1 Show InChI InChI=1S/C19H15ClN2O2/c20-17-6-5-15(23)9-16(17)13-4-3-12-8-18(21-10-14(12)7-13)22-19(24)11-1-2-11/h3-11,23H,1-2H2,(H,21,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030761
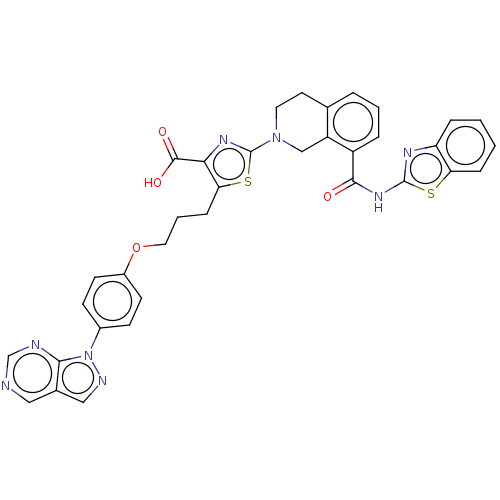
(CHEMBL3342192)Show SMILES OC(=O)c1nc(sc1CCCOc1ccc(cc1)-n1ncc2cncnc12)N1CCc2cccc(C(=O)Nc3nc4ccccc4s3)c2C1 Show InChI InChI=1S/C35H28N8O4S2/c44-32(41-34-39-27-7-1-2-8-28(27)48-34)25-6-3-5-21-14-15-42(19-26(21)25)35-40-30(33(45)46)29(49-35)9-4-16-47-24-12-10-23(11-13-24)43-31-22(18-38-43)17-36-20-37-31/h1-3,5-8,10-13,17-18,20H,4,9,14-16,19H2,(H,45,46)(H,39,41,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602320
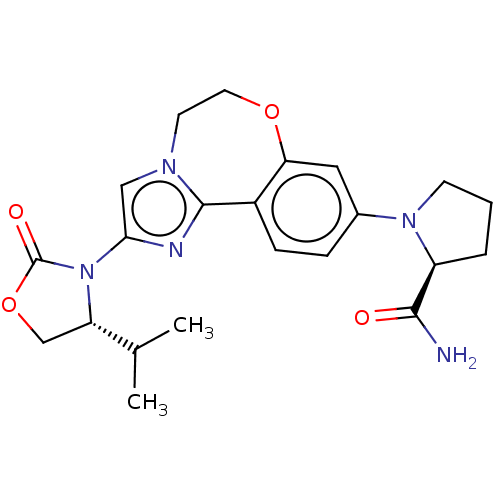
(CHEMBL5199631)Show SMILES CC(C)[C@@H]1COC(=O)N1c1cn2CCOc3cc(ccc3-c2n1)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602324
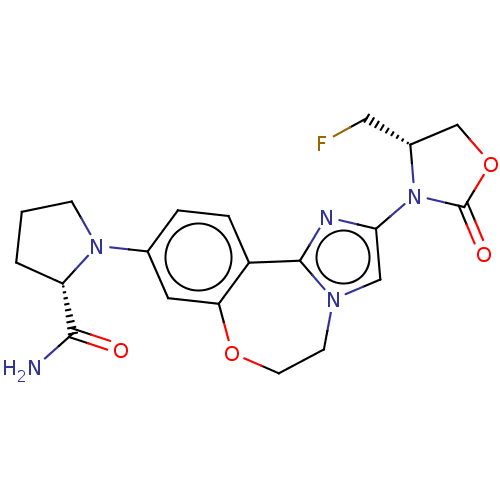
(CHEMBL5198796)Show SMILES NC(=O)[C@@H]1CCCN1c1ccc2-c3nc(cn3CCOc2c1)N1[C@H](CF)COC1=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112244

(US8623889, 242)Show SMILES C[C@@H](O)c1cc(C)c(cn1)-c1ccc2cc(NC(=O)C3CC3)ncc2c1 |r| Show InChI InChI=1S/C21H21N3O2/c1-12-7-19(13(2)25)22-11-18(12)16-6-5-15-9-20(23-10-17(15)8-16)24-21(26)14-3-4-14/h5-11,13-14,25H,3-4H2,1-2H3,(H,23,24,26)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112255

(US8623889, 253)Show SMILES Cc1ccncc1-c1ccc2cc(NC(=O)[C@@H]3C[C@@H]3F)ncc2c1 |r| Show InChI InChI=1S/C19H16FN3O/c1-11-4-5-21-10-16(11)13-3-2-12-7-18(22-9-14(12)6-13)23-19(24)15-8-17(15)20/h2-7,9-10,15,17H,8H2,1H3,(H,22,23,24)/t15-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50030758

(CHEMBL3342195)Show SMILES CN(C)CCCNc1ncnc2n(ncc12)-c1ccc(OCCCc2sc(nc2C(O)=O)N2CCc3cccc(C(=O)Nc4nc5ccccc5s4)c3C2)cc1 Show InChI InChI=1S/C40H40N10O4S2/c1-48(2)19-7-18-41-35-29-22-44-50(36(29)43-24-42-35)26-13-15-27(16-14-26)54-21-6-12-33-34(38(52)53)46-40(56-33)49-20-17-25-8-5-9-28(30(25)23-49)37(51)47-39-45-31-10-3-4-11-32(31)55-39/h3-5,8-11,13-16,22,24H,6-7,12,17-21,23H2,1-2H3,(H,52,53)(H,41,42,43)(H,45,47,51) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of BCL-XL (unknown origin) incubated for 1 hr in presence of 1% human serum by TR-FRET assay |
ACS Med Chem Lett 5: 1088-93 (2014)
Article DOI: 10.1021/ml5001867
BindingDB Entry DOI: 10.7270/Q2VX0J43 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112245

(US8623889, 243)Show SMILES C[C@H](O)c1cc(C)c(cn1)-c1ccc2cc(NC(=O)C3CC3)ncc2c1 |r| Show InChI InChI=1S/C21H21N3O2/c1-12-7-19(13(2)25)22-11-18(12)16-6-5-15-9-20(23-10-17(15)8-16)24-21(26)14-3-4-14/h5-11,13-14,25H,3-4H2,1-2H3,(H,23,24,26)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM295669
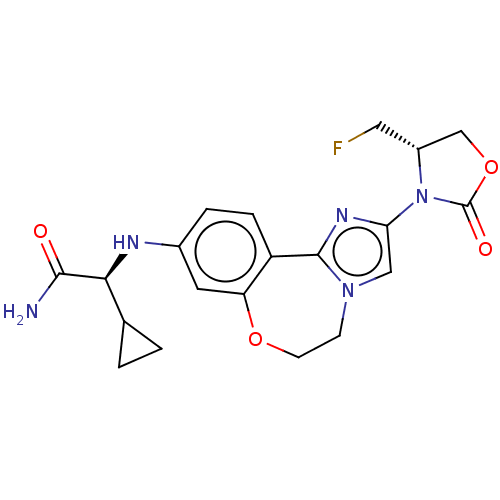
((S)-2-cyclopropyl-2-((2-((S)-4- (fluoromethyl)-2-o...)Show SMILES NC(=O)[C@@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@H](CF)COC1=O)C1CC1 |r| Show InChI InChI=1S/C20H22FN5O4/c21-8-13-10-30-20(28)26(13)16-9-25-5-6-29-15-7-12(3-4-14(15)19(25)24-16)23-17(18(22)27)11-1-2-11/h3-4,7,9,11,13,17,23H,1-2,5-6,8,10H2,(H2,22,27)/t13-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602323
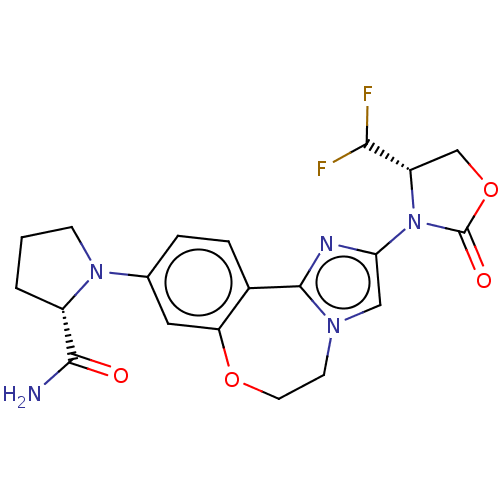
(CHEMBL5209168)Show SMILES NC(=O)[C@@H]1CCCN1c1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)F |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM475607
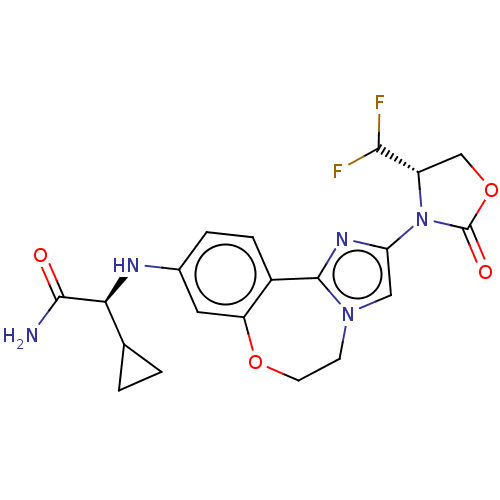
(US10851091, Compound 103)Show SMILES NC(=O)[C@@H](Nc1ccc2-c3nc(cn3CCOc2c1)N1[C@@H](COC1=O)C(F)F)C1CC1 |r| Show InChI InChI=1S/C20H21F2N5O4/c21-17(22)13-9-31-20(29)27(13)15-8-26-5-6-30-14-7-11(3-4-12(14)19(26)25-15)24-16(18(23)28)10-1-2-10/h3-4,7-8,10,13,16-17,24H,1-2,5-6,9H2,(H2,23,28)/t13-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112078

(US8623889, 76)Show SMILES Fc1ccc(-c2ccc3cc(NC(=O)C4CC4)ncc3c2)c(c1)C#N Show InChI InChI=1S/C20H14FN3O/c21-17-5-6-18(15(8-17)10-22)14-4-3-13-9-19(23-11-16(13)7-14)24-20(25)12-1-2-12/h3-9,11-12H,1-2H2,(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112254
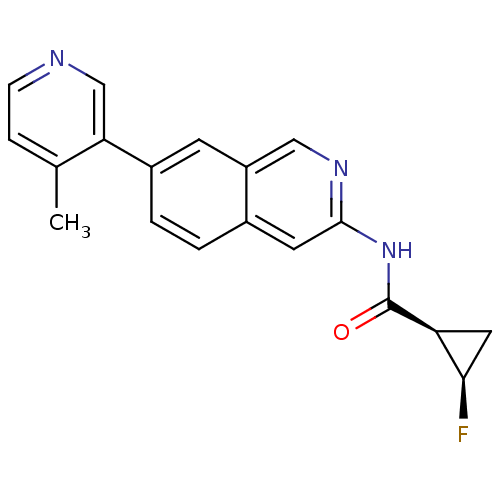
(US8623889, 252)Show SMILES Cc1ccncc1-c1ccc2cc(NC(=O)[C@H]3C[C@H]3F)ncc2c1 |r| Show InChI InChI=1S/C19H16FN3O/c1-11-4-5-21-10-16(11)13-3-2-12-7-18(22-9-14(12)6-13)23-19(24)15-8-17(15)20/h2-7,9-10,15,17H,8H2,1H3,(H,22,23,24)/t15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50602305
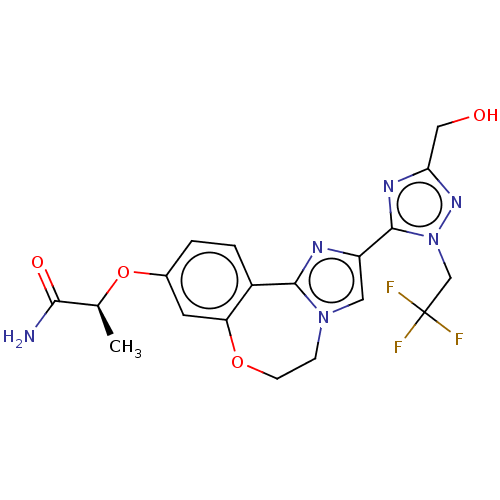
(CHEMBL5209048)Show SMILES C[C@H](Oc1ccc2-c3nc(cn3CCOc2c1)-c1nc(CO)nn1CC(F)(F)F)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01422
BindingDB Entry DOI: 10.7270/Q2S46X11 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112289

(US8623889, 287)Show SMILES Cc1ccncc1-c1ccc2cc(NC(=O)C3C[C@@H]3F)ncc2c1 |r| Show InChI InChI=1S/C19H16FN3O/c1-11-4-5-21-10-16(11)13-3-2-12-7-18(22-9-14(12)6-13)23-19(24)15-8-17(15)20/h2-7,9-10,15,17H,8H2,1H3,(H,22,23,24)/t15?,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112194

(US8623889, 192)Show InChI InChI=1S/C20H19N3O2/c1-12-6-7-21-17(11-24)19(12)15-5-4-14-9-18(22-10-16(14)8-15)23-20(25)13-2-3-13/h4-10,13,24H,2-3,11H2,1H3,(H,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112157

(US8623889, 155)Show InChI InChI=1S/C18H14ClN3O/c19-16-5-6-20-10-15(16)13-4-3-12-8-17(21-9-14(12)7-13)22-18(23)11-1-2-11/h3-11H,1-2H2,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112065

(US8623889, 63)Show SMILES Fc1cc(C#N)c(cc1F)-c1ccc2cc(NC(=O)C3CC3)ncc2c1 Show InChI InChI=1S/C20H13F2N3O/c21-17-6-14(9-23)16(8-18(17)22)13-4-3-12-7-19(24-10-15(12)5-13)25-20(26)11-1-2-11/h3-8,10-11H,1-2H2,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM112288

(US8623889, 286)Show SMILES Cc1ccncc1-c1ccc2cc(NC(=O)C3C[C@H]3F)ncc2c1 |r| Show InChI InChI=1S/C19H16FN3O/c1-11-4-5-21-10-16(11)13-3-2-12-7-18(22-9-14(12)6-13)23-19(24)15-8-17(15)20/h2-7,9-10,15,17H,8H2,1H3,(H,22,23,24)/t15?,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
Using the following procedure, varying concentration of compounds of the invention were assessed for their ability to inhibit c-Abl enzyme's phos... |
US Patent US8623889 (2014)
BindingDB Entry DOI: 10.7270/Q2JQ0ZNX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data