 Found 269 hits with Last Name = 'puttfarcken' and Initial = 'ps'
Found 269 hits with Last Name = 'puttfarcken' and Initial = 'ps' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50062639
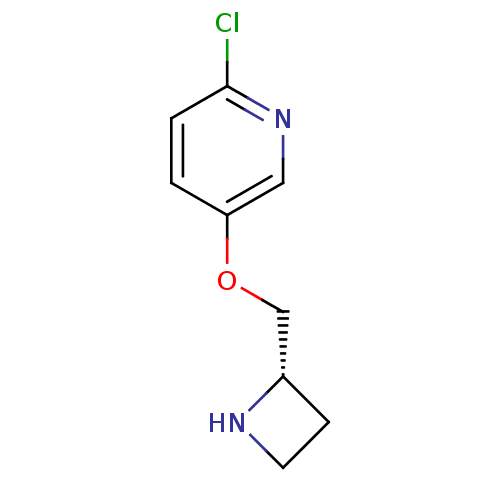
(5-((S)-1-Azetidin-2-ylmethoxy)-2-chloro-pyridine |...)Show InChI InChI=1S/C9H11ClN2O/c10-9-2-1-8(5-12-9)13-6-7-3-4-11-7/h1-2,5,7,11H,3-4,6H2/t7-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Science 279: 77-81 (1998)
Article DOI: 10.1126/science.279.5347.77
BindingDB Entry DOI: 10.7270/Q2X63KGH |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Science 279: 77-81 (1998)
Article DOI: 10.1126/science.279.5347.77
BindingDB Entry DOI: 10.7270/Q2X63KGH |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50004108

((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Science 279: 77-81 (1998)
Article DOI: 10.1126/science.279.5347.77
BindingDB Entry DOI: 10.7270/Q2X63KGH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Science 279: 77-81 (1998)
Article DOI: 10.1126/science.279.5347.77
BindingDB Entry DOI: 10.7270/Q2X63KGH |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20458
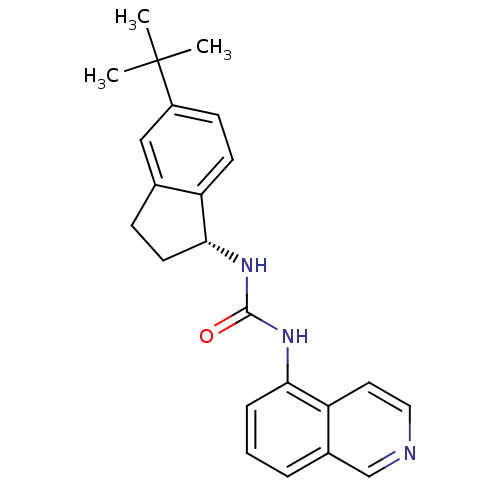
(1-[(1R)-5-tert-butyl-2,3-dihydro-1H-inden-1-yl]-3-...)Show SMILES CC(C)(C)c1ccc2[C@@H](CCc2c1)NC(=O)Nc1cccc2cnccc12 |r| Show InChI InChI=1S/C23H25N3O/c1-23(2,3)17-8-9-18-15(13-17)7-10-21(18)26-22(27)25-20-6-4-5-16-14-24-12-11-19(16)20/h4-6,8-9,11-14,21H,7,10H2,1-3H3,(H2,25,26,27)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | -46.5 | n/a | n/a | 5 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20464
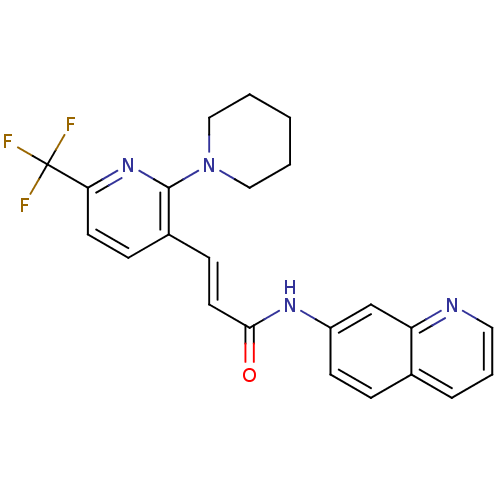
((2E)-3-[2-(piperidin-1-yl)-6-(trifluoromethyl)pyri...)Show SMILES FC(F)(F)c1ccc(\C=C\C(=O)Nc2ccc3cccnc3c2)c(n1)N1CCCCC1 Show InChI InChI=1S/C23H21F3N4O/c24-23(25,26)20-10-7-17(22(29-20)30-13-2-1-3-14-30)8-11-21(31)28-18-9-6-16-5-4-12-27-19(16)15-18/h4-12,15H,1-3,13-14H2,(H,28,31)/b11-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 27 | -43.2 | n/a | n/a | 34 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20334
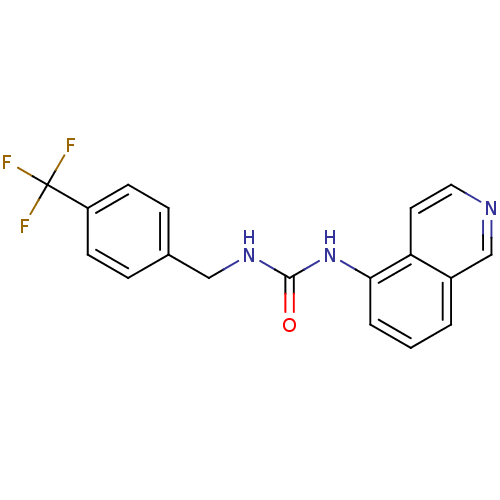
(1-Isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-ur...)Show InChI InChI=1S/C18H14F3N3O/c19-18(20,21)14-6-4-12(5-7-14)10-23-17(25)24-16-3-1-2-13-11-22-9-8-15(13)16/h1-9,11H,10H2,(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 47 | -41.8 | n/a | n/a | 11 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20465
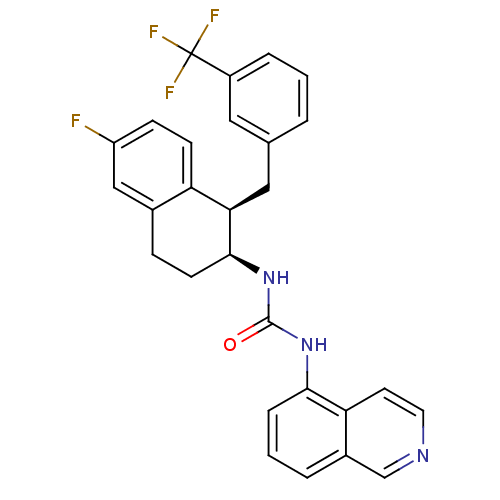
(1-[(1R,2S)-6-fluoro-1-{[3-(trifluoromethyl)phenyl]...)Show SMILES Fc1ccc2[C@@H](Cc3cccc(c3)C(F)(F)F)[C@H](CCc2c1)NC(=O)Nc1cccc2cnccc12 |r| Show InChI InChI=1S/C28H23F4N3O/c29-21-8-9-22-18(15-21)7-10-26(24(22)14-17-3-1-5-20(13-17)28(30,31)32)35-27(36)34-25-6-2-4-19-16-33-12-11-23(19)25/h1-6,8-9,11-13,15-16,24,26H,7,10,14H2,(H2,34,35,36)/t24-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 58 | -41.3 | n/a | n/a | 46 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20285
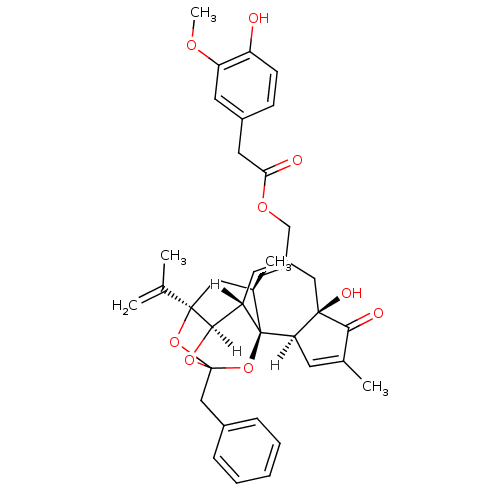
(Resiniferatoxin | [(1R,2R,6R,10S,11R,15R,17R)-13-b...)Show SMILES [H][C@]12OC3(Cc4ccccc4)O[C@]1(C[C@@H](C)[C@]1(O3)[C@]3([H])C=C(C)C(=O)[C@@]3(O)CC(COC(=O)Cc3ccc(O)c(OC)c3)=C[C@@]21[H])C(C)=C |c:47,t:23,TLB:11:3:12.14.13:44,THB:4:3:12.14.13:44| Show InChI InChI=1S/C37H40O9/c1-21(2)35-17-23(4)37-27(33(35)44-36(45-35,46-37)19-24-9-7-6-8-10-24)14-26(18-34(41)30(37)13-22(3)32(34)40)20-43-31(39)16-25-11-12-28(38)29(15-25)42-5/h6-15,23,27,30,33,38,41H,1,16-20H2,2-5H3/t23-,27+,30-,33-,34-,35-,36?,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 65 | -41.0 | n/a | n/a | 24 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20466
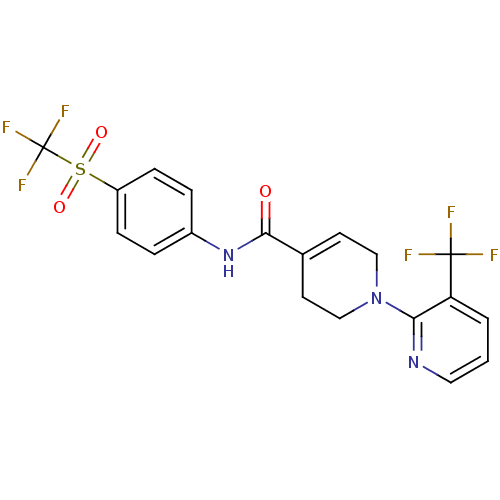
(A-784168 | CHEMBL482834 | N-[4-(trifluoromethane)s...)Show SMILES FC(F)(F)c1cccnc1N1CCC(=CC1)C(=O)Nc1ccc(cc1)S(=O)(=O)C(F)(F)F |c:14| Show InChI InChI=1S/C19H15F6N3O3S/c20-18(21,22)15-2-1-9-26-16(15)28-10-7-12(8-11-28)17(29)27-13-3-5-14(6-4-13)32(30,31)19(23,24)25/h1-7,9H,8,10-11H2,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 71 | -40.8 | n/a | n/a | 74 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20467
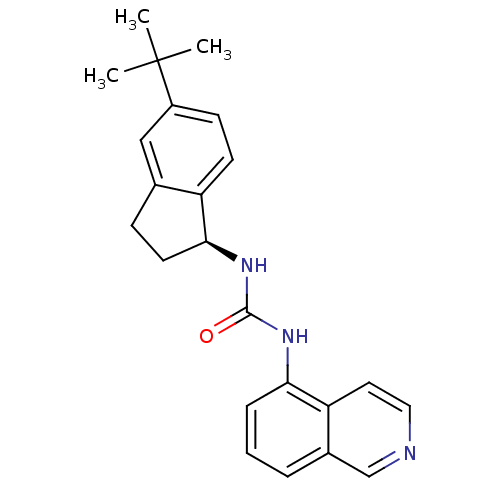
(3-[(1S)-5-tert-butyl-2,3-dihydro-1H-inden-1-yl]-1-...)Show SMILES CC(C)(C)c1ccc2[C@H](CCc2c1)NC(=O)Nc1cccc2cnccc12 |r| Show InChI InChI=1S/C23H25N3O/c1-23(2,3)17-8-9-18-15(13-17)7-10-21(18)26-22(27)25-20-6-4-5-16-14-24-12-11-19(16)20/h4-6,8-9,11-14,21H,7,10H2,1-3H3,(H2,25,26,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 112 | -39.7 | n/a | n/a | 34 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20459
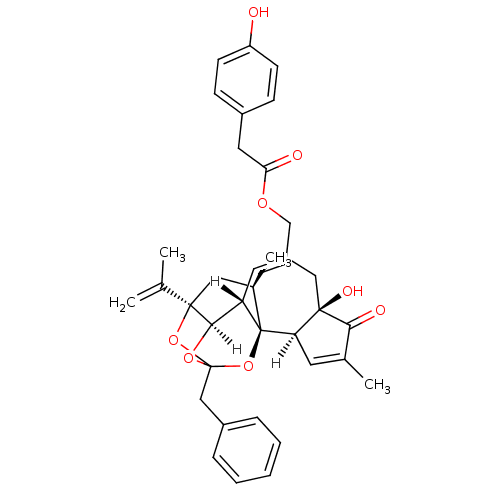
(Tinyatoxin | [(1R,2R,6R,10S,11R,15R,17R)-13-benzyl...)Show SMILES [H][C@]12OC3(Cc4ccccc4)O[C@]1(C[C@@H](C)[C@]1(O3)[C@]3([H])C=C(C)C(=O)[C@@]3(O)CC(COC(=O)Cc3ccc(O)cc3)=C[C@@]21[H])C(C)=C |c:45,t:23,TLB:11:3:12.14.13:42,THB:4:3:12.14.13:42| Show InChI InChI=1S/C36H38O8/c1-21(2)34-17-23(4)36-28(32(34)42-35(43-34,44-36)19-25-8-6-5-7-9-25)15-26(18-33(40)29(36)14-22(3)31(33)39)20-41-30(38)16-24-10-12-27(37)13-11-24/h5-15,23,28-29,32,37,40H,1,16-20H2,2-4H3/t23-,28+,29-,32-,33-,34-,35?,36-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 589 | -35.6 | n/a | n/a | 129 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20468
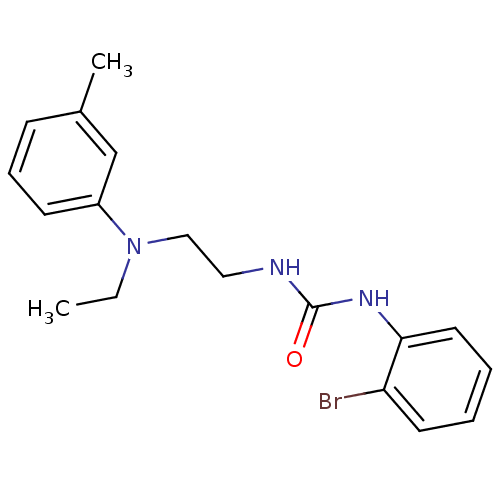
(3-(2-bromophenyl)-1-{2-[ethyl(3-methylphenyl)amino...)Show InChI InChI=1S/C18H22BrN3O/c1-3-22(15-8-6-7-14(2)13-15)12-11-20-18(23)21-17-10-5-4-9-16(17)19/h4-10,13H,3,11-12H2,1-2H3,(H2,20,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 603 | -35.5 | n/a | n/a | 95 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20284
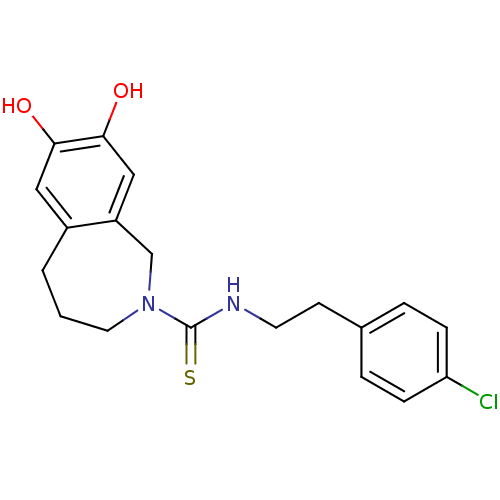
(CHEMBL391997 | CPZ | Capsazepine | N-[2-(4-chlorop...)Show InChI InChI=1S/C19H21ClN2O2S/c20-16-5-3-13(4-6-16)7-8-21-19(25)22-9-1-2-14-10-17(23)18(24)11-15(14)12-22/h3-6,10-11,23-24H,1-2,7-9,12H2,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.29E+3 | -33.6 | n/a | n/a | 282 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20460
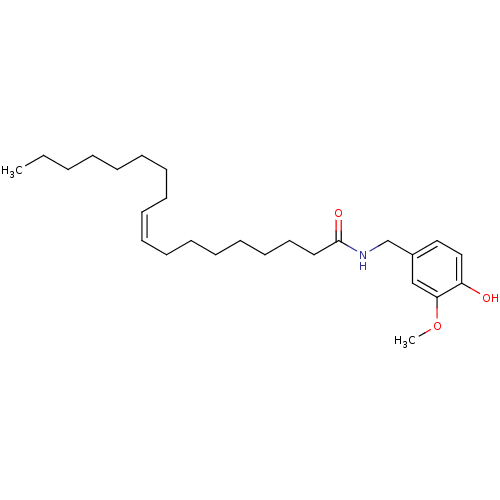
((9Z)-N-[(4-hydroxy-3-methoxyphenyl)methyl]octadec-...)Show InChI InChI=1S/C26H43NO3/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-26(29)27-22-23-19-20-24(28)25(21-23)30-2/h10-11,19-21,28H,3-9,12-18,22H2,1-2H3,(H,27,29)/b11-10- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.59E+3 | -33.1 | n/a | n/a | 132 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20462
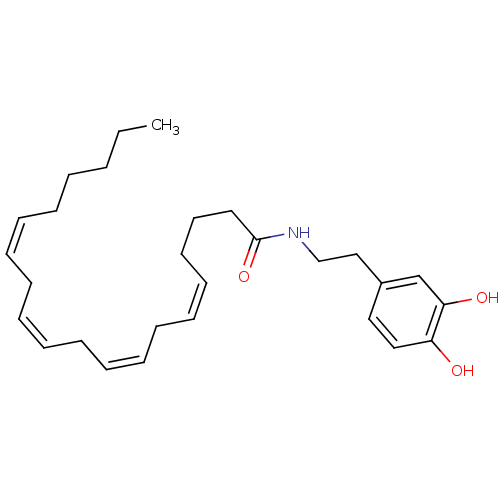
((5Z,8Z,11Z,14Z)-N-[2-(3,4-dihydroxyphenyl)ethyl]ic...)Show SMILES CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)NCCc1ccc(O)c(O)c1 Show InChI InChI=1S/C28H41NO3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-28(32)29-23-22-25-20-21-26(30)27(31)24-25/h6-7,9-10,12-13,15-16,20-21,24,30-31H,2-5,8,11,14,17-19,22-23H2,1H3,(H,29,32)/b7-6-,10-9-,13-12-,16-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >6.31E+3 | >-29.7 | n/a | n/a | 1.48E+3 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50004108

((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Science 279: 77-81 (1998)
Article DOI: 10.1126/science.279.5347.77
BindingDB Entry DOI: 10.7270/Q2X63KGH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Science 279: 77-81 (1998)
Article DOI: 10.1126/science.279.5347.77
BindingDB Entry DOI: 10.7270/Q2X63KGH |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Science 279: 77-81 (1998)
Article DOI: 10.1126/science.279.5347.77
BindingDB Entry DOI: 10.7270/Q2X63KGH |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM20461
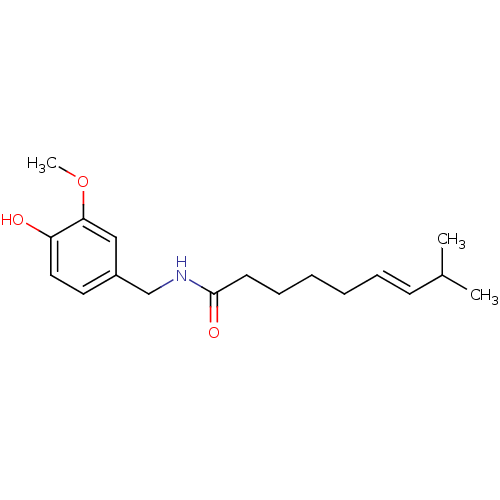
((6E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methy...)Show InChI InChI=1S/C18H27NO3/c1-14(2)8-6-4-5-7-9-18(21)19-13-15-10-11-16(20)17(12-15)22-3/h6,8,10-12,14,20H,4-5,7,9,13H2,1-3H3,(H,19,21)/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.00E+4 | -26.8 | n/a | n/a | 29 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
The hTRPV1-expressing CHO cell membranes were incubated with [3H]A-778317 and test compounds to establish equilibrium. After incubation was terminate... |
J Pharmacol Exp Ther 323: 285-93 (2007)
Article DOI: 10.1124/jpet.107.124305
BindingDB Entry DOI: 10.7270/Q28K77B0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50056351
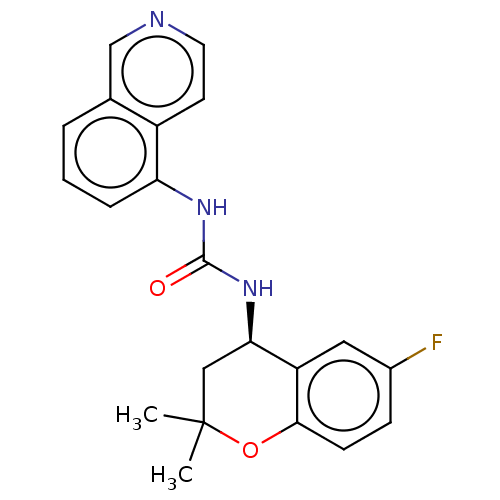
(CHEMBL3326569)Show SMILES CC1(C)C[C@@H](NC(=O)Nc2cccc3cnccc23)c2cc(F)ccc2O1 |r| Show InChI InChI=1S/C21H20FN3O2/c1-21(2)11-18(16-10-14(22)6-7-19(16)27-21)25-20(26)24-17-5-3-4-13-12-23-9-8-15(13)17/h3-10,12,18H,11H2,1-2H3,(H2,24,25,26)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in HEK293 cells assessed as capsaicin-induced calcium flux by FLIPR assay |
J Med Chem 57: 7412-24 (2014)
Article DOI: 10.1021/jm500916t
BindingDB Entry DOI: 10.7270/Q2Z3219J |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM128907

(US8802711, 76)Show SMILES Cc1cc2c(NC(=O)N[C@@H]3CC[C@@H](C3)c3cccc(F)c3)c(F)ccc2cn1 Show InChI InChI=1S/C22H21F2N3O/c1-13-9-19-16(12-25-13)6-8-20(24)21(19)27-22(28)26-18-7-5-15(11-18)14-3-2-4-17(23)10-14/h2-4,6,8-10,12,15,18H,5,7,11H2,1H3,(H2,26,27,28)/t15-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc... |
US Patent US8802711 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28NC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM128912

(US8802711, 81)Show SMILES Cn1c2cccc(NC(=O)N[C@@H]3CC[C@H](C3)c3ccccc3F)c2ccc1=O Show InChI InChI=1S/C22H22FN3O2/c1-26-20-8-4-7-19(17(20)11-12-21(26)27)25-22(28)24-15-10-9-14(13-15)16-5-2-3-6-18(16)23/h2-8,11-12,14-15H,9-10,13H2,1H3,(H2,24,25,28)/t14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc... |
US Patent US8802711 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28NC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50056358
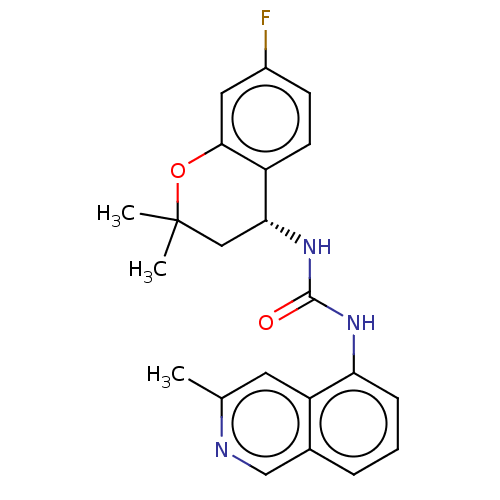
(CHEMBL3326581)Show SMILES Cc1cc2c(NC(=O)N[C@@H]3CC(C)(C)Oc4cc(F)ccc34)cccc2cn1 |r| Show InChI InChI=1S/C22H22FN3O2/c1-13-9-17-14(12-24-13)5-4-6-18(17)25-21(27)26-19-11-22(2,3)28-20-10-15(23)7-8-16(19)20/h4-10,12,19H,11H2,1-3H3,(H2,25,26,27)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in HEK293 cells assessed as capsaicin-induced calcium flux by FLIPR assay |
J Med Chem 57: 7412-24 (2014)
Article DOI: 10.1021/jm500916t
BindingDB Entry DOI: 10.7270/Q2Z3219J |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM128913

(US8802711, 82)Show SMILES Cc1cc2c(NC(=O)N[C@@H]3CC[C@H](C3)c3ccccc3F)c(F)ccc2cn1 Show InChI InChI=1S/C22H21F2N3O/c1-13-10-18-15(12-25-13)7-9-20(24)21(18)27-22(28)26-16-8-6-14(11-16)17-4-2-3-5-19(17)23/h2-5,7,9-10,12,14,16H,6,8,11H2,1H3,(H2,26,27,28)/t14-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc... |
US Patent US8802711 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28NC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM128905

(US8802711, 74)Show SMILES Cc1cc2c(NC(=O)N[C@H]3CC[C@@H](C3)c3cccc(F)c3)c(F)ccc2cn1 Show InChI InChI=1S/C22H21F2N3O/c1-13-9-19-16(12-25-13)6-8-20(24)21(19)27-22(28)26-18-7-5-15(11-18)14-3-2-4-17(23)10-14/h2-4,6,8-10,12,15,18H,5,7,11H2,1H3,(H2,26,27,28)/t15-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc... |
US Patent US8802711 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28NC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50319456
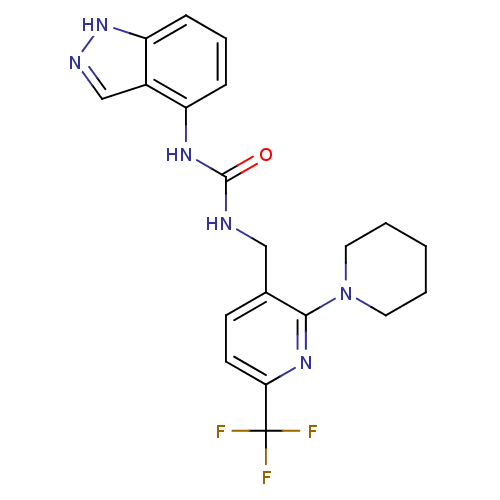
(1-(1H-indazol-4-yl)-3-((2-(piperidin-1-yl)-6-(trif...)Show SMILES FC(F)(F)c1ccc(CNC(=O)Nc2cccc3[nH]ncc23)c(n1)N1CCCCC1 Show InChI InChI=1S/C20H21F3N6O/c21-20(22,23)17-8-7-13(18(27-17)29-9-2-1-3-10-29)11-24-19(30)26-15-5-4-6-16-14(15)12-25-28-16/h4-8,12H,1-3,9-11H2,(H,25,28)(H2,24,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 |
Bioorg Med Chem Lett 20: 3291-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.047
BindingDB Entry DOI: 10.7270/Q2B27VF7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM128982

(US8802711, G)Show SMILES O[C@@H]1CCc2cccc(NC(=O)N[C@@H]3CC[C@@H](C3)c3ccccc3)c2C1 Show InChI InChI=1S/C22H26N2O2/c25-19-12-10-16-7-4-8-21(20(16)14-19)24-22(26)23-18-11-9-17(13-18)15-5-2-1-3-6-15/h1-8,17-19,25H,9-14H2,(H2,23,24,26)/t17-,18+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc... |
US Patent US8802711 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28NC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM128976

(US8802711, A)Show SMILES O[C@@H]1CCc2cccc(NC(=O)N[C@H]3CC[C@@H](C3)c3ccccc3)c2C1 Show InChI InChI=1S/C22H26N2O2/c25-19-12-10-16-7-4-8-21(20(16)14-19)24-22(26)23-18-11-9-17(13-18)15-5-2-1-3-6-15/h1-8,17-19,25H,9-14H2,(H2,23,24,26)/t17-,18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc... |
US Patent US8802711 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28NC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
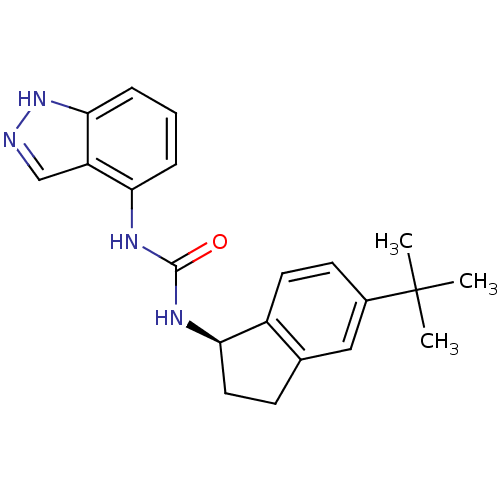
(Homo sapiens (Human)) | BDBM50232114
((R)-1-(5-tert-butyl-2,3-dihydro-1H-inden-1-yl)-3-(...)Show SMILES CC(C)(C)c1ccc2[C@@H](CCc2c1)NC(=O)Nc1cccc2[nH]ncc12 Show InChI InChI=1S/C21H24N4O/c1-21(2,3)14-8-9-15-13(11-14)7-10-18(15)24-20(26)23-17-5-4-6-19-16(17)12-22-25-19/h4-6,8-9,11-12,18H,7,10H2,1-3H3,(H,22,25)(H2,23,24,26)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in HEK293 cells assessed as capsaicin-induced calcium flux by FLIPR assay |
J Med Chem 57: 7412-24 (2014)
Article DOI: 10.1021/jm500916t
BindingDB Entry DOI: 10.7270/Q2Z3219J |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
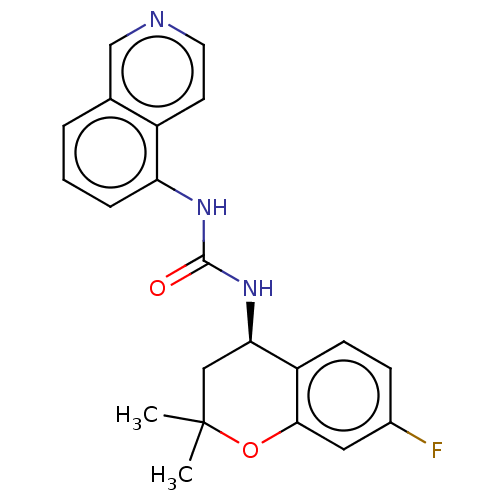
(Homo sapiens (Human)) | BDBM50056352
(CHEMBL3326570)Show SMILES CC1(C)C[C@@H](NC(=O)Nc2cccc3cnccc23)c2ccc(F)cc2O1 |r| Show InChI InChI=1S/C21H20FN3O2/c1-21(2)11-18(16-7-6-14(22)10-19(16)27-21)25-20(26)24-17-5-3-4-13-12-23-9-8-15(13)17/h3-10,12,18H,11H2,1-2H3,(H2,24,25,26)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in HEK293 cells assessed as capsaicin-induced calcium flux by FLIPR assay |
J Med Chem 57: 7412-24 (2014)
Article DOI: 10.1021/jm500916t
BindingDB Entry DOI: 10.7270/Q2Z3219J |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM128837

(US8802711, 6)Show SMILES Cc1cc2c(NC(=O)N[C@@H]3CC[C@@H](C3)c3ccccc3)c(F)ccc2cn1 Show InChI InChI=1S/C22H22FN3O/c1-14-11-19-17(13-24-14)8-10-20(23)21(19)26-22(27)25-18-9-7-16(12-18)15-5-3-2-4-6-15/h2-6,8,10-11,13,16,18H,7,9,12H2,1H3,(H2,25,26,27)/t16-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc... |
US Patent US8802711 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28NC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
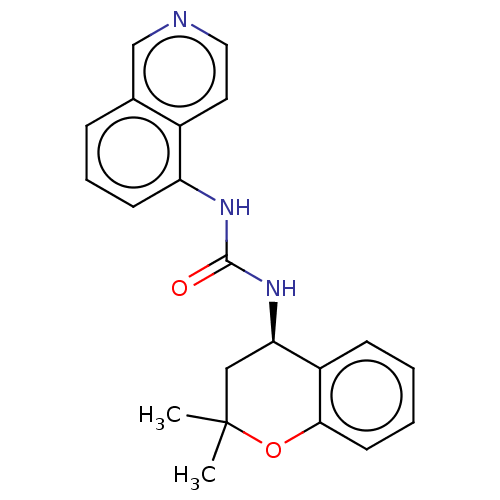
(Homo sapiens (Human)) | BDBM50056434
(CHEMBL3326575)Show SMILES CC1(C)C[C@@H](NC(=O)Nc2cccc3cnccc23)c2ccccc2O1 |r| Show InChI InChI=1S/C21H21N3O2/c1-21(2)12-18(16-7-3-4-9-19(16)26-21)24-20(25)23-17-8-5-6-14-13-22-11-10-15(14)17/h3-11,13,18H,12H2,1-2H3,(H2,23,24,25)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in HEK293 cells assessed as capsaicin-induced calcium flux by FLIPR assay |
J Med Chem 57: 7412-24 (2014)
Article DOI: 10.1021/jm500916t
BindingDB Entry DOI: 10.7270/Q2Z3219J |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50319471

(1-(2-(3,3-dimethylbutyl)-4-(trifluoromethyl)benzyl...)Show SMILES CC(C)(C)CCc1cc(ccc1CNC(=O)Nc1cccc2[nH]ncc12)C(F)(F)F Show InChI InChI=1S/C22H25F3N4O/c1-21(2,3)10-9-14-11-16(22(23,24)25)8-7-15(14)12-26-20(30)28-18-5-4-6-19-17(18)13-27-29-19/h4-8,11,13H,9-10,12H2,1-3H3,(H,27,29)(H2,26,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 |
Bioorg Med Chem Lett 20: 3291-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.047
BindingDB Entry DOI: 10.7270/Q2B27VF7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50056387

(CHEMBL3326582)Show SMILES Cc1cc2c(NC(=O)N[C@@H]3CC(C)(C)Oc4c(F)c(F)ccc34)cccc2cn1 |r| Show InChI InChI=1S/C22H21F2N3O2/c1-12-9-15-13(11-25-12)5-4-6-17(15)26-21(28)27-18-10-22(2,3)29-20-14(18)7-8-16(23)19(20)24/h4-9,11,18H,10H2,1-3H3,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in HEK293 cells assessed as capsaicin-induced calcium flux by FLIPR assay |
J Med Chem 57: 7412-24 (2014)
Article DOI: 10.1021/jm500916t
BindingDB Entry DOI: 10.7270/Q2Z3219J |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50319465
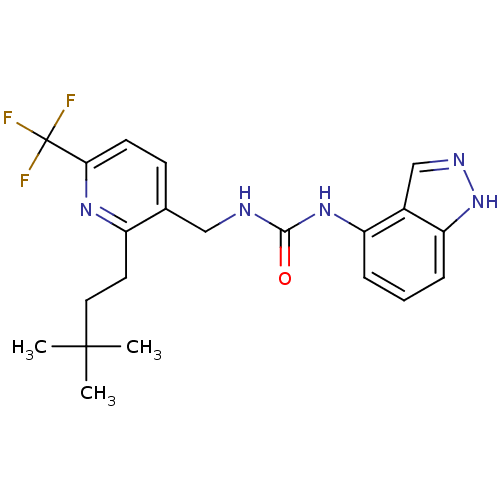
(1-((2-(3,3-dimethylbutyl)-6-(trifluoromethyl)pyrid...)Show SMILES CC(C)(C)CCc1nc(ccc1CNC(=O)Nc1cccc2[nH]ncc12)C(F)(F)F Show InChI InChI=1S/C21H24F3N5O/c1-20(2,3)10-9-15-13(7-8-18(27-15)21(22,23)24)11-25-19(30)28-16-5-4-6-17-14(16)12-26-29-17/h4-8,12H,9-11H2,1-3H3,(H,26,29)(H2,25,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 |
Bioorg Med Chem Lett 20: 3291-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.047
BindingDB Entry DOI: 10.7270/Q2B27VF7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM128851

(US8802711, 20)Show SMILES Cc1cc2c(NC(=O)N[C@@H]3CC[C@H](C3)c3ccccc3)c(F)ccc2cn1 Show InChI InChI=1S/C22H22FN3O/c1-14-11-19-17(13-24-14)8-10-20(23)21(19)26-22(27)25-18-9-7-16(12-18)15-5-3-2-4-6-15/h2-6,8,10-11,13,16,18H,7,9,12H2,1H3,(H2,25,26,27)/t16-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc... |
US Patent US8802711 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28NC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50056389
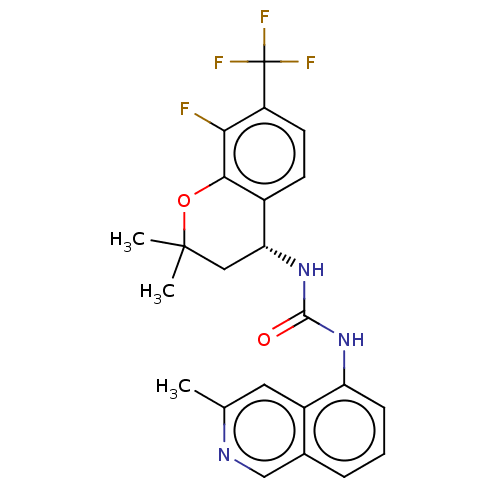
(CHEMBL3326584)Show SMILES Cc1cc2c(NC(=O)N[C@@H]3CC(C)(C)Oc4c(F)c(ccc34)C(F)(F)F)cccc2cn1 |r| Show InChI InChI=1S/C23H21F4N3O2/c1-12-9-15-13(11-28-12)5-4-6-17(15)29-21(31)30-18-10-22(2,3)32-20-14(18)7-8-16(19(20)24)23(25,26)27/h4-9,11,18H,10H2,1-3H3,(H2,29,30,31)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in HEK293 cells assessed as capsaicin-induced calcium flux by FLIPR assay |
J Med Chem 57: 7412-24 (2014)
Article DOI: 10.1021/jm500916t
BindingDB Entry DOI: 10.7270/Q2Z3219J |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM128981
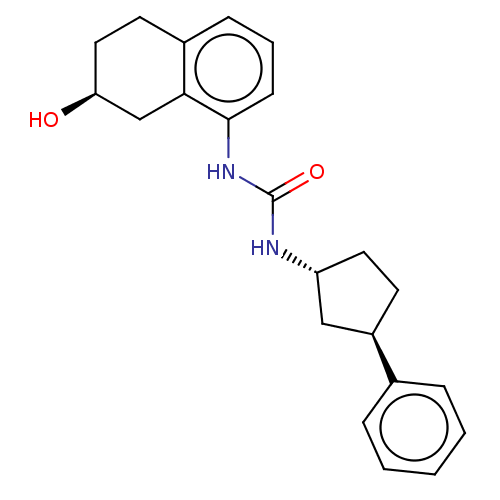
(US8802711, F)Show SMILES O[C@H]1CCc2cccc(NC(=O)N[C@@H]3CC[C@H](C3)c3ccccc3)c2C1 Show InChI InChI=1S/C22H26N2O2/c25-19-12-10-16-7-4-8-21(20(16)14-19)24-22(26)23-18-11-9-17(13-18)15-5-2-1-3-6-15/h1-8,17-19,25H,9-14H2,(H2,23,24,26)/t17-,18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc... |
US Patent US8802711 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28NC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50056408
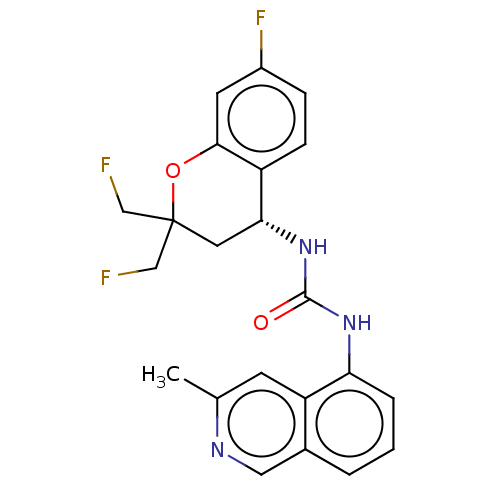
(CHEMBL3326587)Show SMILES Cc1cc2c(NC(=O)N[C@@H]3CC(CF)(CF)Oc4cc(F)ccc34)cccc2cn1 |r| Show InChI InChI=1S/C22H20F3N3O2/c1-13-7-17-14(10-26-13)3-2-4-18(17)27-21(29)28-19-9-22(11-23,12-24)30-20-8-15(25)5-6-16(19)20/h2-8,10,19H,9,11-12H2,1H3,(H2,27,28,29)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in HEK293 cells assessed as capsaicin-induced calcium flux by FLIPR assay |
J Med Chem 57: 7412-24 (2014)
Article DOI: 10.1021/jm500916t
BindingDB Entry DOI: 10.7270/Q2Z3219J |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM128978
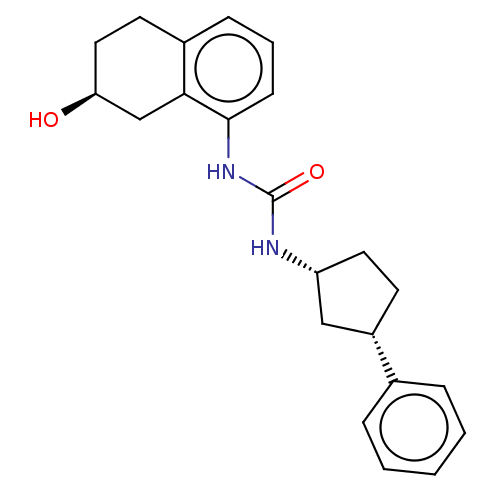
(US8802711, C)Show SMILES O[C@H]1CCc2cccc(NC(=O)N[C@@H]3CC[C@@H](C3)c3ccccc3)c2C1 Show InChI InChI=1S/C22H26N2O2/c25-19-12-10-16-7-4-8-21(20(16)14-19)24-22(26)23-18-11-9-17(13-18)15-5-2-1-3-6-15/h1-8,17-19,25H,9-14H2,(H2,23,24,26)/t17-,18+,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc... |
US Patent US8802711 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28NC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
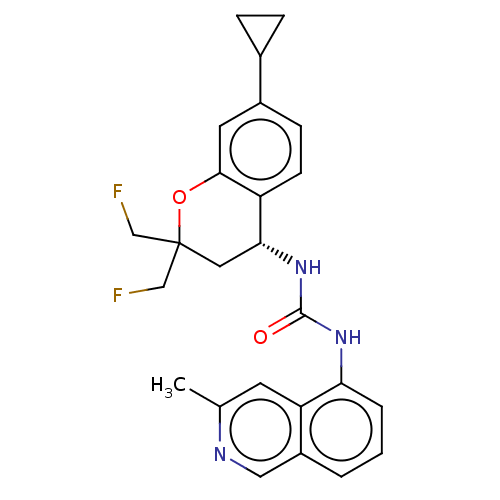
(Homo sapiens (Human)) | BDBM50056426
(CHEMBL3326589)Show SMILES Cc1cc2c(NC(=O)N[C@@H]3CC(CF)(CF)Oc4cc(ccc34)C3CC3)cccc2cn1 |r| Show InChI InChI=1S/C25H25F2N3O2/c1-15-9-20-18(12-28-15)3-2-4-21(20)29-24(31)30-22-11-25(13-26,14-27)32-23-10-17(16-5-6-16)7-8-19(22)23/h2-4,7-10,12,16,22H,5-6,11,13-14H2,1H3,(H2,29,30,31)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in HEK293 cells assessed as capsaicin-induced calcium flux by FLIPR assay |
J Med Chem 57: 7412-24 (2014)
Article DOI: 10.1021/jm500916t
BindingDB Entry DOI: 10.7270/Q2Z3219J |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
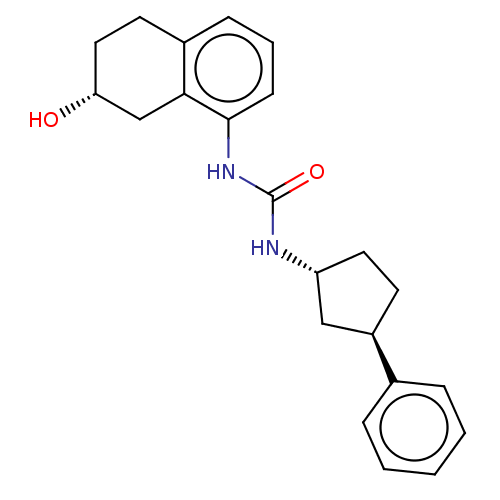
(Homo sapiens (Human)) | BDBM128980
(US8802711, E)Show SMILES O[C@@H]1CCc2cccc(NC(=O)N[C@@H]3CC[C@H](C3)c3ccccc3)c2C1 Show InChI InChI=1S/C22H26N2O2/c25-19-12-10-16-7-4-8-21(20(16)14-19)24-22(26)23-18-11-9-17(13-18)15-5-2-1-3-6-15/h1-8,17-19,25H,9-14H2,(H2,23,24,26)/t17-,18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc... |
US Patent US8802711 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28NC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM128914

(US8802711, 83)Show SMILES Cc1cc2c(NC(=O)N[C@H]3CC[C@H](C3)c3ccccc3F)c(F)ccc2cn1 Show InChI InChI=1S/C22H21F2N3O/c1-13-10-18-15(12-25-13)7-9-20(24)21(18)27-22(28)26-16-8-6-14(11-16)17-4-2-3-5-19(17)23/h2-5,7,9-10,12,14,16H,6,8,11H2,1H3,(H2,26,27,28)/t14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc... |
US Patent US8802711 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28NC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
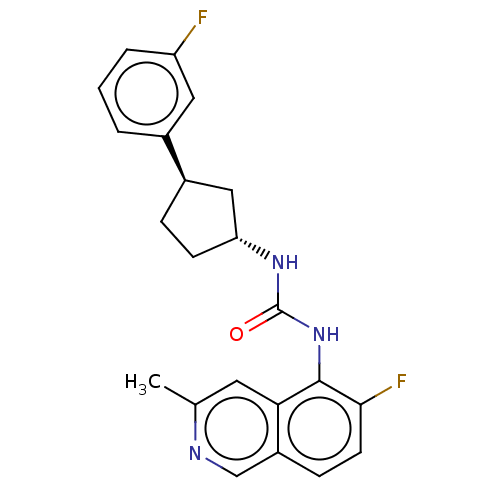
(Homo sapiens (Human)) | BDBM128915
(US8802711, 84)Show SMILES Cc1cc2c(NC(=O)N[C@@H]3CC[C@H](C3)c3cccc(F)c3)c(F)ccc2cn1 Show InChI InChI=1S/C22H21F2N3O/c1-13-9-19-16(12-25-13)6-8-20(24)21(19)27-22(28)26-18-7-5-15(11-18)14-3-2-4-17(23)10-14/h2-4,6,8-10,12,15,18H,5,7,11H2,1H3,(H2,26,27,28)/t15-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc... |
US Patent US8802711 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28NC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM128901
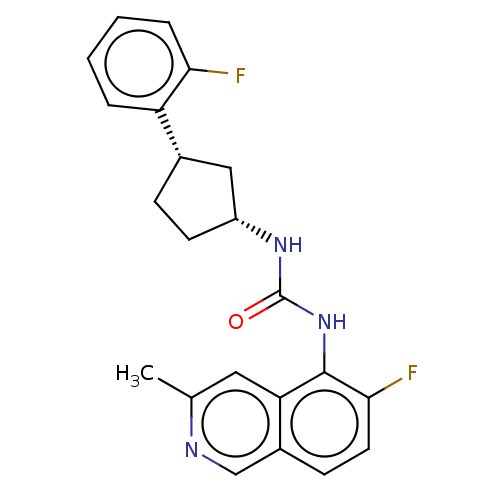
(US8802711, 70)Show SMILES Cc1cc2c(NC(=O)N[C@@H]3CC[C@@H](C3)c3ccccc3F)c(F)ccc2cn1 Show InChI InChI=1S/C22H21F2N3O/c1-13-10-18-15(12-25-13)7-9-20(24)21(18)27-22(28)26-16-8-6-14(11-16)17-4-2-3-5-19(17)23/h2-5,7,9-10,12,14,16H,6,8,11H2,1H3,(H2,26,27,28)/t14-,16+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc... |
US Patent US8802711 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28NC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM128977

(US8802711, B)Show SMILES O[C@H]1CCc2cccc(NC(=O)N[C@H]3CC[C@H](C3)c3ccccc3)c2C1 Show InChI InChI=1S/C22H26N2O2/c25-19-12-10-16-7-4-8-21(20(16)14-19)24-22(26)23-18-11-9-17(13-18)15-5-2-1-3-6-15/h1-8,17-19,25H,9-14H2,(H2,23,24,26)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
US Patent
| Assay Description
The functional activity of compounds at the TRPV1 receptor was determined by measurement of intracellular Ca2+ levels ([Ca2+]i) using the Fluorescenc... |
US Patent US8802711 (2014)
BindingDB Entry DOI: 10.7270/Q2QZ28NC |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50319470
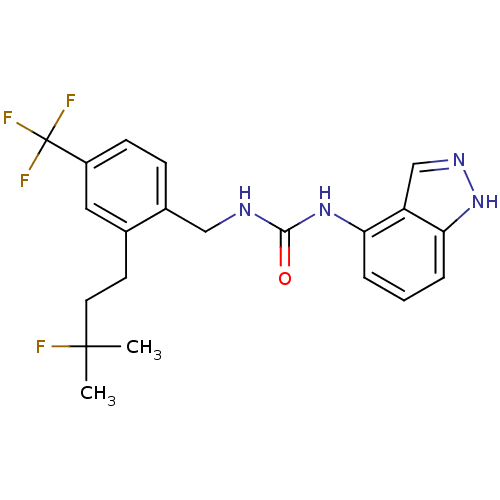
(1-(2-(3-fluoro-3-methylbutyl)-4-(trifluoromethyl)b...)Show SMILES CC(C)(F)CCc1cc(ccc1CNC(=O)Nc1cccc2[nH]ncc12)C(F)(F)F Show InChI InChI=1S/C21H22F4N4O/c1-20(2,22)9-8-13-10-15(21(23,24)25)7-6-14(13)11-26-19(30)28-17-4-3-5-18-16(17)12-27-29-18/h3-7,10,12H,8-9,11H2,1-2H3,(H,27,29)(H2,26,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 |
Bioorg Med Chem Lett 20: 3291-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.047
BindingDB Entry DOI: 10.7270/Q2B27VF7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50319464

(1-(1H-indazol-4-yl)-3-((2-(neopentyloxy)-6-(triflu...)Show SMILES CC(C)(C)COc1nc(ccc1CNC(=O)Nc1cccc2[nH]ncc12)C(F)(F)F Show InChI InChI=1S/C20H22F3N5O2/c1-19(2,3)11-30-17-12(7-8-16(27-17)20(21,22)23)9-24-18(29)26-14-5-4-6-15-13(14)10-25-28-15/h4-8,10H,9,11H2,1-3H3,(H,25,28)(H2,24,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 |
Bioorg Med Chem Lett 20: 3291-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.047
BindingDB Entry DOI: 10.7270/Q2B27VF7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50056354

(CHEMBL3326577)Show SMILES CCC1(CC)C[C@@H](NC(=O)Nc2cccc3cnccc23)c2cc(F)ccc2O1 |r| Show InChI InChI=1S/C23H24FN3O2/c1-3-23(4-2)13-20(18-12-16(24)8-9-21(18)29-23)27-22(28)26-19-7-5-6-15-14-25-11-10-17(15)19/h5-12,14,20H,3-4,13H2,1-2H3,(H2,26,27,28)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity against human TRPV1 expressed in HEK293 cells assessed as capsaicin-induced calcium flux by FLIPR assay |
J Med Chem 57: 7412-24 (2014)
Article DOI: 10.1021/jm500916t
BindingDB Entry DOI: 10.7270/Q2Z3219J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data