 Found 8236 hits with Last Name = 'qi' and Initial = 'l'
Found 8236 hits with Last Name = 'qi' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50443101

(Cariprazine | RGH-188)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:6.5,wD:9.9,(-.77,5.69,;.57,4.92,;1.9,5.69,;.57,3.38,;-.77,2.61,;1.9,2.61,;1.9,1.07,;3.23,.3,;3.23,-1.24,;1.9,-2.01,;1.9,-3.55,;3.23,-4.32,;3.23,-5.86,;4.57,-6.63,;4.57,-8.17,;3.23,-8.94,;1.9,-8.17,;1.9,-6.63,;3.23,-10.48,;4.57,-11.25,;4.57,-12.79,;3.23,-13.56,;1.9,-12.79,;.57,-13.56,;1.9,-11.25,;.57,-10.48,;.57,-1.24,;.57,.3,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23/h3-5,16-17H,6-15H2,1-2H3,(H,24,28)/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127681
BindingDB Entry DOI: 10.7270/Q2BZ69PB |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Mus musculus) | BDBM50057514
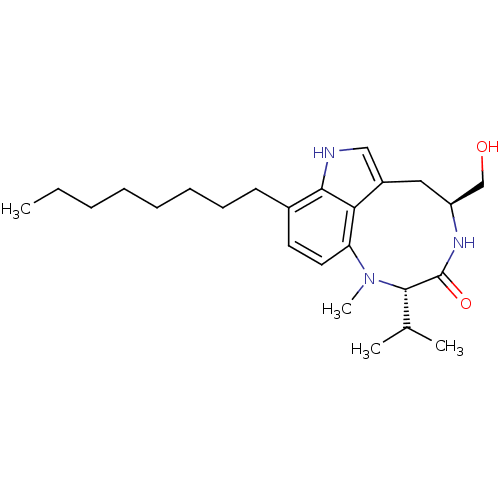
((10S,13S)-13-Hydroxymethyl-10-isopropyl-9-methyl-5...)Show SMILES CCCCCCCCc1ccc2N(C)[C@@H](C(C)C)C(=O)N[C@H](CO)Cc3c[nH]c1c23 Show InChI InChI=1S/C25H39N3O2/c1-5-6-7-8-9-10-11-18-12-13-21-22-19(15-26-23(18)22)14-20(16-29)27-25(30)24(17(2)3)28(21)4/h12-13,15,17,20,24,26,29H,5-11,14,16H2,1-4H3,(H,27,30)/t20-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]- PDBu from Protein kinase C delta C1b domain |
J Med Chem 45: 853-60 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V25 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306314

((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@H](C1)c1ccc(CN(C2CCC2)C(C)=O)cc1 |r| Show InChI InChI=1S/C24H29ClN2O2/c1-16(28)27(20-4-3-5-20)14-17-6-8-18(9-7-17)22-15-26(2)11-10-19-12-23(25)24(29)13-21(19)22/h6-9,12-13,20,22,29H,3-5,10-11,14-15H2,1-2H3/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50345941
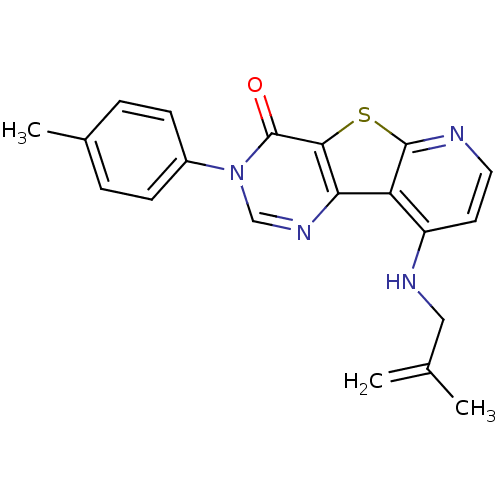
(9-(2-Methyl-allylamino)-3-p-tolyl-3H-pyrido[3',2':...)Show SMILES CC(=C)CNc1ccnc2sc3c(ncn(-c4ccc(C)cc4)c3=O)c12 Show InChI InChI=1S/C20H18N4OS/c1-12(2)10-22-15-8-9-21-19-16(15)17-18(26-19)20(25)24(11-23-17)14-6-4-13(3)5-7-14/h4-9,11H,1,10H2,2-3H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at rat mGluR1 |
Bioorg Med Chem Lett 19: 3199-203 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.104
BindingDB Entry DOI: 10.7270/Q27081SJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50537515
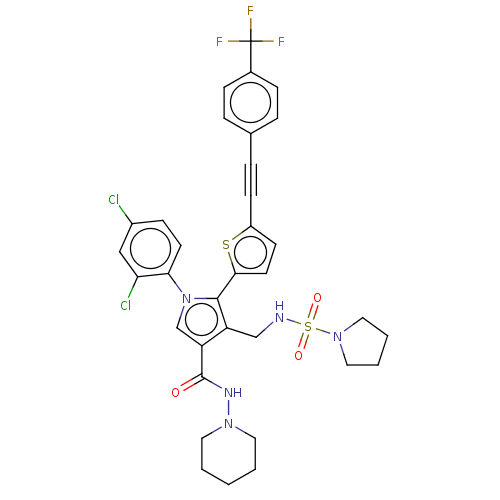
(CHEMBL4644088)Show SMILES FC(F)(F)c1ccc(cc1)C#Cc1ccc(s1)-c1c(CNS(=O)(=O)N2CCCC2)c(cn1-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C34H32Cl2F3N5O3S2/c35-25-11-14-30(29(36)20-25)44-22-28(33(45)41-42-16-2-1-3-17-42)27(21-40-49(46,47)43-18-4-5-19-43)32(44)31-15-13-26(48-31)12-8-23-6-9-24(10-7-23)34(37,38)39/h6-7,9-11,13-15,20,22,40H,1-5,16-19,21H2,(H,41,45) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C alpha expressed in Sf-9 cells |
Eur J Med Chem 162: 679-734 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.017
BindingDB Entry DOI: 10.7270/Q21839DX |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Mus musculus) | BDBM50080322

(Acetic acid (1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-9-acet...)Show SMILES C[C@@H]1[C@@H](OC(C)=O)[C@]2(OC(C)=O)[C@H]([C@@H]3C=C(CO)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]13O)C2(C)C |t:14,22| Show InChI InChI=1S/C24H32O8/c1-11-7-17-22(29,19(11)28)9-15(10-25)8-16-18-21(5,6)24(18,32-14(4)27)20(31-13(3)26)12(2)23(16,17)30/h7-8,12,16-18,20,25,29-30H,9-10H2,1-6H3/t12-,16+,17-,18?,20-,22-,23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.351 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
Binding of Indolactam-V(ILV) to wild-type Protein kinase C delta C1b domain |
J Med Chem 42: 3436-46 (1999)
Article DOI: 10.1021/jm990129n
BindingDB Entry DOI: 10.7270/Q25T3JP5 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50231010

(CHEMBL312066)Show SMILES CCCCc1c(Oc2ccc(cc2)-c2ccccc2-c2nn[nH]n2)nc2c(C(O)=O)c(OCC)ccc2[n+]1[O-] Show InChI InChI=1S/C28H26N6O5/c1-3-5-10-22-27(29-25-21(34(22)37)15-16-23(38-4-2)24(25)28(35)36)39-18-13-11-17(12-14-18)19-8-6-7-9-20(19)26-30-32-33-31-26/h6-9,11-16H,3-5,10H2,1-2H3,(H,35,36)(H,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of compound in presence of bovine serum albumin (BSA) at 0% against rat adrenal Angiotensin II receptor, type 1 |
J Med Chem 36: 2335-42 (1993)
Article DOI: 10.1021/jm00068a010
BindingDB Entry DOI: 10.7270/Q21V5H6B |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50345942
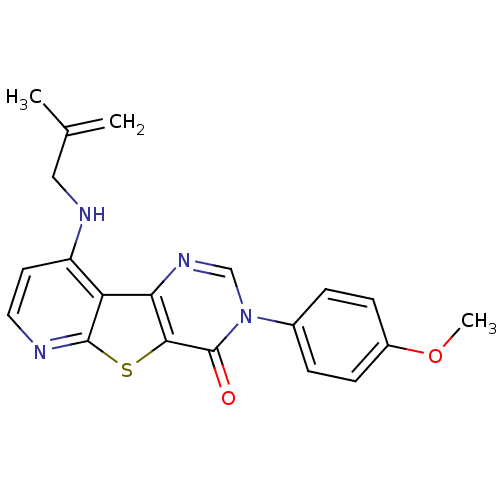
(3-(4-Methoxy-phenyl)-9-(2-methyl-allylamino)-3H-py...)Show SMILES COc1ccc(cc1)-n1cnc2c(sc3nccc(NCC(C)=C)c23)c1=O Show InChI InChI=1S/C20H18N4O2S/c1-12(2)10-22-15-8-9-21-19-16(15)17-18(27-19)20(25)24(11-23-17)13-4-6-14(26-3)7-5-13/h4-9,11H,1,10H2,2-3H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at rat mGluR1 |
Bioorg Med Chem Lett 19: 3199-203 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.104
BindingDB Entry DOI: 10.7270/Q27081SJ |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50099066
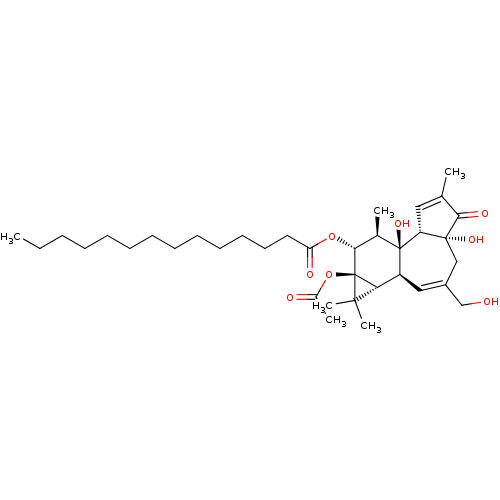
(CHEMBL279115 | phorbol 13-acetate 12-myristate)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(C)=O |r,c:33,t:22| Show InChI InChI=1S/C36H56O8/c1-7-8-9-10-11-12-13-14-15-16-17-18-29(39)43-32-24(3)35(42)27(30-33(5,6)36(30,32)44-25(4)38)20-26(22-37)21-34(41)28(35)19-23(2)31(34)40/h19-20,24,27-28,30,32,37,41-42H,7-18,21-22H2,1-6H3/t24-,27+,28-,30?,32-,34-,35-,36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C epsilon |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50443101

(Cariprazine | RGH-188)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:6.5,wD:9.9,(-.77,5.69,;.57,4.92,;1.9,5.69,;.57,3.38,;-.77,2.61,;1.9,2.61,;1.9,1.07,;3.23,.3,;3.23,-1.24,;1.9,-2.01,;1.9,-3.55,;3.23,-4.32,;3.23,-5.86,;4.57,-6.63,;4.57,-8.17,;3.23,-8.94,;1.9,-8.17,;1.9,-6.63,;3.23,-10.48,;4.57,-11.25,;4.57,-12.79,;3.23,-13.56,;1.9,-12.79,;.57,-13.56,;1.9,-11.25,;.57,-10.48,;.57,-1.24,;.57,.3,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23/h3-5,16-17H,6-15H2,1-2H3,(H,24,28)/t16-,17- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-spiperone from recombinant human D2L receptor expressed in CHO cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127681
BindingDB Entry DOI: 10.7270/Q2BZ69PB |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306322
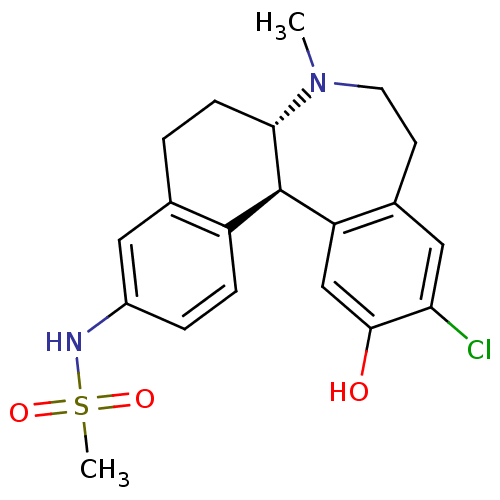
(CHEMBL597909 | N-((6aS,13bR)-11-chloro-12-hydroxy-...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@@H]2[C@@H]1CCc1cc(NS(C)(=O)=O)ccc21 |r| Show InChI InChI=1S/C20H23ClN2O3S/c1-23-8-7-13-10-17(21)19(24)11-16(13)20-15-5-4-14(22-27(2,25)26)9-12(15)3-6-18(20)23/h4-5,9-11,18,20,22,24H,3,6-8H2,1-2H3/t18-,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50549018
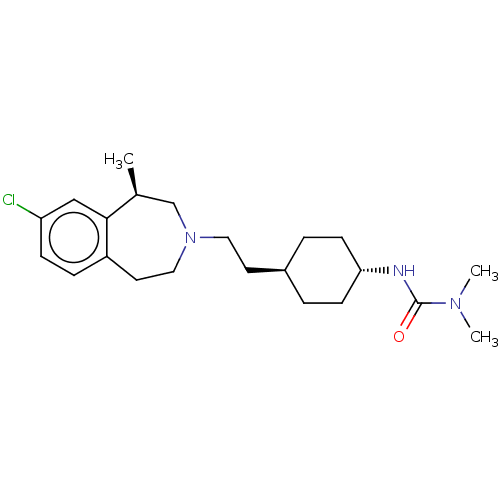
(CHEMBL4799863)Show SMILES C[C@H]1CN(CC[C@H]2CC[C@@H](CC2)NC(=O)N(C)C)CCc2ccc(Cl)cc12 |r,wU:9.12,wD:6.5,1.0,(18.24,-31.84,;17.89,-33.34,;16.38,-33.68,;15.71,-35.08,;14.17,-35.08,;13.4,-36.42,;11.86,-36.42,;11.09,-35.08,;9.55,-35.08,;8.78,-36.42,;9.55,-37.75,;11.09,-37.75,;7.24,-36.42,;6.47,-37.75,;7.24,-39.08,;4.93,-37.75,;4.16,-36.42,;4.16,-39.08,;16.38,-36.48,;17.89,-36.82,;19.1,-35.85,;20.44,-36.62,;21.78,-35.85,;21.77,-34.3,;23.1,-33.52,;20.44,-33.54,;19.11,-34.31,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127681
BindingDB Entry DOI: 10.7270/Q2BZ69PB |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306316
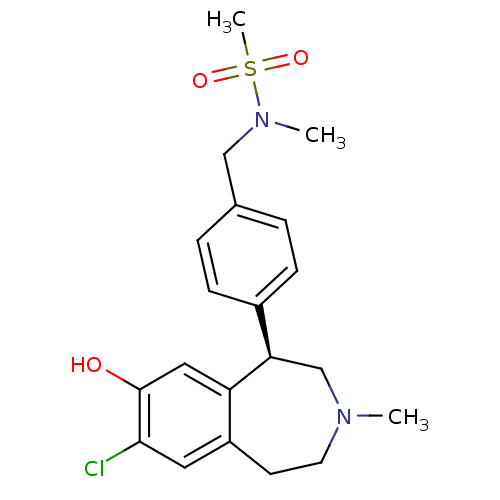
((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...)Show SMILES CN(Cc1ccc(cc1)[C@H]1CN(C)CCc2cc(Cl)c(O)cc12)S(C)(=O)=O |r| Show InChI InChI=1S/C20H25ClN2O3S/c1-22-9-8-16-10-19(21)20(24)11-17(16)18(13-22)15-6-4-14(5-7-15)12-23(2)27(3,25)26/h4-7,10-11,18,24H,8-9,12-13H2,1-3H3/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306315
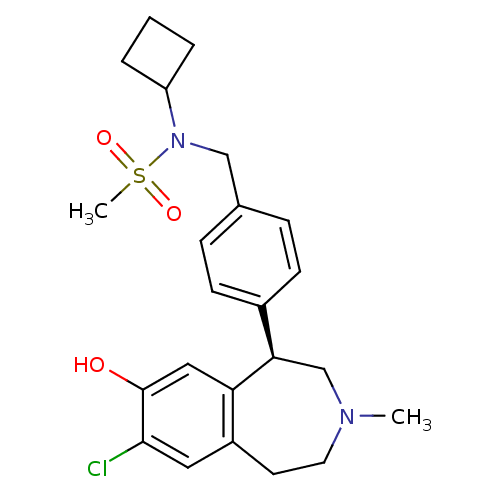
((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@H](C1)c1ccc(CN(C2CCC2)S(C)(=O)=O)cc1 |r| Show InChI InChI=1S/C23H29ClN2O3S/c1-25-11-10-18-12-22(24)23(27)13-20(18)21(15-25)17-8-6-16(7-9-17)14-26(30(2,28)29)19-4-3-5-19/h6-9,12-13,19,21,27H,3-5,10-11,14-15H2,1-2H3/t21-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306317
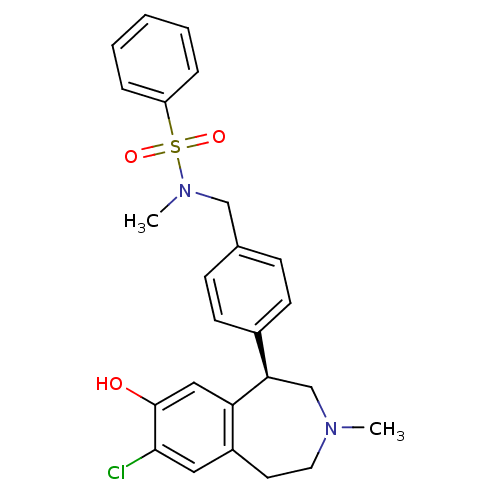
((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...)Show SMILES CN(Cc1ccc(cc1)[C@H]1CN(C)CCc2cc(Cl)c(O)cc12)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C25H27ClN2O3S/c1-27-13-12-20-14-24(26)25(29)15-22(20)23(17-27)19-10-8-18(9-11-19)16-28(2)32(30,31)21-6-4-3-5-7-21/h3-11,14-15,23,29H,12-13,16-17H2,1-2H3/t23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50345947
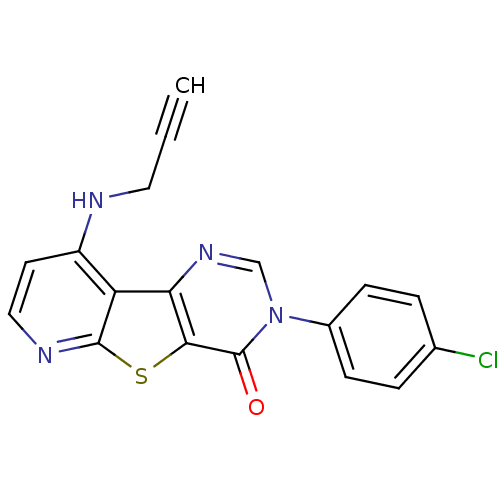
(3-(4-Chloro-phenyl)-9-prop-2-ynylamino-3H-pyrido[3...)Show SMILES Clc1ccc(cc1)-n1cnc2c(sc3nccc(NCC#C)c23)c1=O Show InChI InChI=1S/C18H11ClN4OS/c1-2-8-20-13-7-9-21-17-14(13)15-16(25-17)18(24)23(10-22-15)12-5-3-11(19)4-6-12/h1,3-7,9-10H,8H2,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at rat mGluR1 |
Bioorg Med Chem Lett 19: 3199-203 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.104
BindingDB Entry DOI: 10.7270/Q27081SJ |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306325

(1-((6aS,13bR)-11-chloro-12-hydroxy-7-methyl-6,6a,7...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@@H]2[C@@H]1CCc1cc(NC(=O)Nc3c(Cl)cccc3Cl)ccc21 |r| Show InChI InChI=1S/C26H24Cl3N3O2/c1-32-10-9-15-12-21(29)23(33)13-18(15)24-17-7-6-16(11-14(17)5-8-22(24)32)30-26(34)31-25-19(27)3-2-4-20(25)28/h2-4,6-7,11-13,22,24,33H,5,8-10H2,1H3,(H2,30,31,34)/t22-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306323
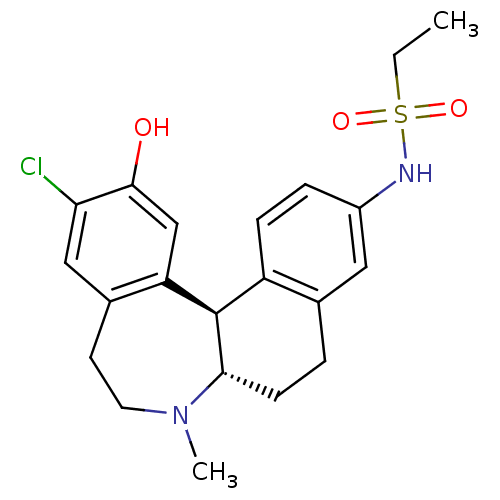
(CHEMBL600986 | N-((6aS,13bR)-11-chloro-12-hydroxy-...)Show SMILES CCS(=O)(=O)Nc1ccc2[C@H]3[C@H](CCc2c1)N(C)CCc1cc(Cl)c(O)cc31 |r| Show InChI InChI=1S/C21H25ClN2O3S/c1-3-28(26,27)23-15-5-6-16-13(10-15)4-7-19-21(16)17-12-20(25)18(22)11-14(17)8-9-24(19)2/h5-6,10-12,19,21,23,25H,3-4,7-9H2,1-2H3/t19-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306319
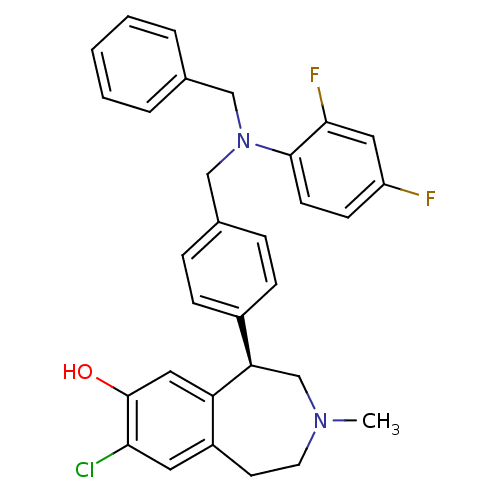
((R)-5-(4-((benzyl(2,4-difluorophenyl)amino)methyl)...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@H](C1)c1ccc(CN(Cc2ccccc2)c2ccc(F)cc2F)cc1 |r| Show InChI InChI=1S/C31H29ClF2N2O/c1-35-14-13-24-15-28(32)31(37)17-26(24)27(20-35)23-9-7-22(8-10-23)19-36(18-21-5-3-2-4-6-21)30-12-11-25(33)16-29(30)34/h2-12,15-17,27,37H,13-14,18-20H2,1H3/t27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50345934
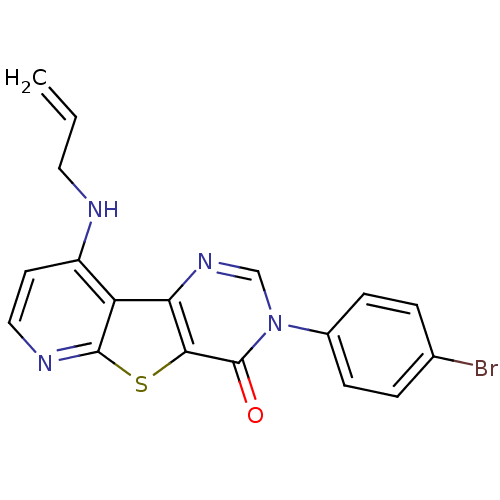
(9-Allylamino-3-(4-bromo-phenyl)-3H-pyrido[3',2':4,...)Show SMILES Brc1ccc(cc1)-n1cnc2c(sc3nccc(NCC=C)c23)c1=O Show InChI InChI=1S/C18H13BrN4OS/c1-2-8-20-13-7-9-21-17-14(13)15-16(25-17)18(24)23(10-22-15)12-5-3-11(19)4-6-12/h2-7,9-10H,1,8H2,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at rat mGluR1 |
Bioorg Med Chem Lett 19: 3199-203 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.104
BindingDB Entry DOI: 10.7270/Q27081SJ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50345950
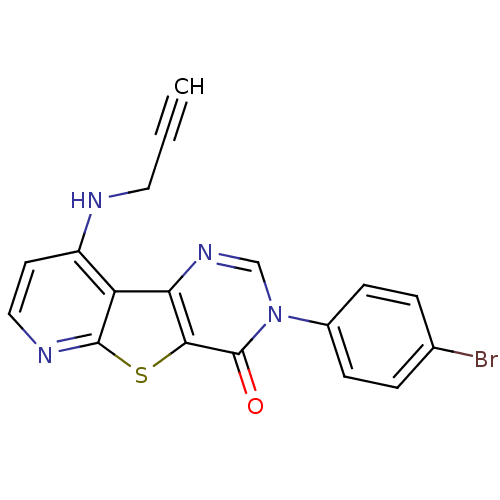
(3-(4-Bromo-phenyl)-9-prop-2-ynylamino-3H-pyrido[3'...)Show SMILES Brc1ccc(cc1)-n1cnc2c(sc3nccc(NCC#C)c23)c1=O Show InChI InChI=1S/C18H11BrN4OS/c1-2-8-20-13-7-9-21-17-14(13)15-16(25-17)18(24)23(10-22-15)12-5-3-11(19)4-6-12/h1,3-7,9-10H,8H2,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at rat mGluR1 |
Bioorg Med Chem Lett 19: 3199-203 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.104
BindingDB Entry DOI: 10.7270/Q27081SJ |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to adrenergic alpha1 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127681
BindingDB Entry DOI: 10.7270/Q2BZ69PB |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50099066

(CHEMBL279115 | phorbol 13-acetate 12-myristate)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(C)=O |r,c:33,t:22| Show InChI InChI=1S/C36H56O8/c1-7-8-9-10-11-12-13-14-15-16-17-18-29(39)43-32-24(3)35(42)27(30-33(5,6)36(30,32)44-25(4)38)20-26(22-37)21-34(41)28(35)19-23(2)31(34)40/h19-20,24,27-28,30,32,37,41-42H,7-18,21-22H2,1-6H3/t24-,27+,28-,30?,32-,34-,35-,36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C delta |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Mus musculus) | BDBM50080322

(Acetic acid (1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-9-acet...)Show SMILES C[C@@H]1[C@@H](OC(C)=O)[C@]2(OC(C)=O)[C@H]([C@@H]3C=C(CO)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]13O)C2(C)C |t:14,22| Show InChI InChI=1S/C24H32O8/c1-11-7-17-22(29,19(11)28)9-15(10-25)8-16-18-21(5,6)24(18,32-14(4)27)20(31-13(3)26)12(2)23(16,17)30/h7-8,12,16-18,20,25,29-30H,9-10H2,1-6H3/t12-,16+,17-,18?,20-,22-,23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
Binding of Indolactam-V(ILV) to mutant Protein kinase C delta C1b domain (Phe13 to Gly) |
J Med Chem 42: 3436-46 (1999)
Article DOI: 10.1021/jm990129n
BindingDB Entry DOI: 10.7270/Q25T3JP5 |
More data for this
Ligand-Target Pair | |
RAS guanyl-releasing protein 1
(Rattus norvegicus) | BDBM50057514

((10S,13S)-13-Hydroxymethyl-10-isopropyl-9-methyl-5...)Show SMILES CCCCCCCCc1ccc2N(C)[C@@H](C(C)C)C(=O)N[C@H](CO)Cc3c[nH]c1c23 Show InChI InChI=1S/C25H39N3O2/c1-5-6-7-8-9-10-11-18-12-13-21-22-19(15-26-23(18)22)14-20(16-29)27-25(30)24(17(2)3)28(21)4/h12-13,15,17,20,24,26,29H,5-11,14,16H2,1-4H3,(H,27,30)/t20-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
Binding affinity against RAS guanyl releasing protein using [3H]- PDBu as the labeled ligand |
J Med Chem 45: 853-60 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V25 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306313
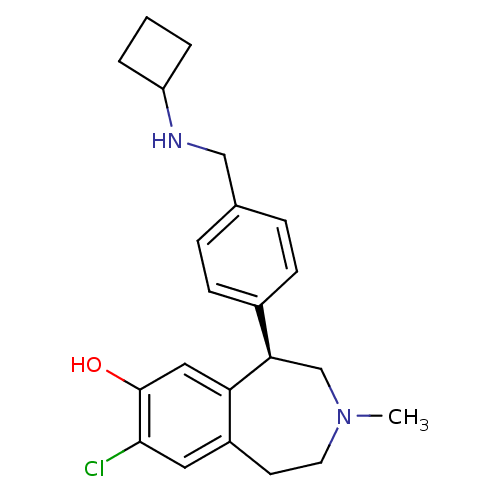
((R)-8-chloro-5-(4-((cyclobutylamino)methyl)phenyl)...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@H](C1)c1ccc(CNC2CCC2)cc1 |r| Show InChI InChI=1S/C22H27ClN2O/c1-25-10-9-17-11-21(23)22(26)12-19(17)20(14-25)16-7-5-15(6-8-16)13-24-18-3-2-4-18/h5-8,11-12,18,20,24,26H,2-4,9-10,13-14H2,1H3/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50549038

(CHEBI:71257 | CHEMBL504548)Show SMILES [H][C@]12CN(C)C[C@]1([H])c1cc(Cl)ccc1Oc1ccccc21 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to H1 histamine receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127681
BindingDB Entry DOI: 10.7270/Q2BZ69PB |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50186790
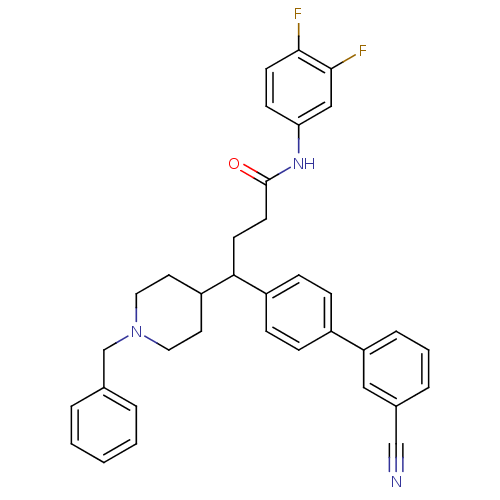
(4-(1-benzyl-piperidin-4-yl)-4-(3'-cyano-biphenyl-4...)Show SMILES Fc1ccc(NC(=O)CCC(C2CCN(Cc3ccccc3)CC2)c2ccc(cc2)-c2cccc(c2)C#N)cc1F Show InChI InChI=1S/C35H33F2N3O/c36-33-15-13-31(22-34(33)37)39-35(41)16-14-32(29-17-19-40(20-18-29)24-25-5-2-1-3-6-25)28-11-9-27(10-12-28)30-8-4-7-26(21-30)23-38/h1-13,15,21-22,29,32H,14,16-20,24H2,(H,39,41) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human MCHR1 assessed as inhibition of MCH-mediated calcium ion influx by FLIPR assay |
Bioorg Med Chem Lett 16: 3668-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.061
BindingDB Entry DOI: 10.7270/Q2251HT9 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50345931
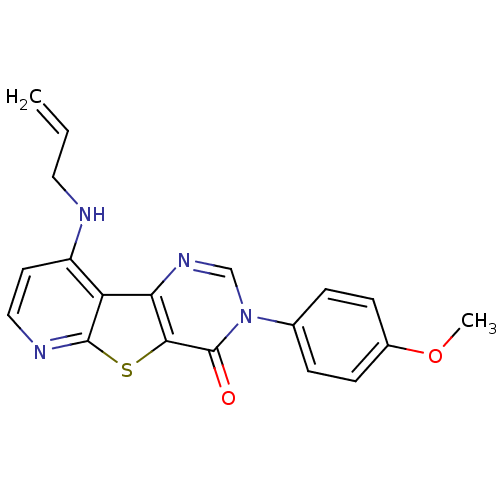
(9-Allylamino-3-(4-methoxy-phenyl)-3H-pyrido[3',2':...)Show SMILES COc1ccc(cc1)-n1cnc2c(sc3nccc(NCC=C)c23)c1=O Show InChI InChI=1S/C19H16N4O2S/c1-3-9-20-14-8-10-21-18-15(14)16-17(26-18)19(24)23(11-22-16)12-4-6-13(25-2)7-5-12/h3-8,10-11H,1,9H2,2H3,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at rat mGluR1 |
Bioorg Med Chem Lett 19: 3199-203 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.104
BindingDB Entry DOI: 10.7270/Q27081SJ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50345933
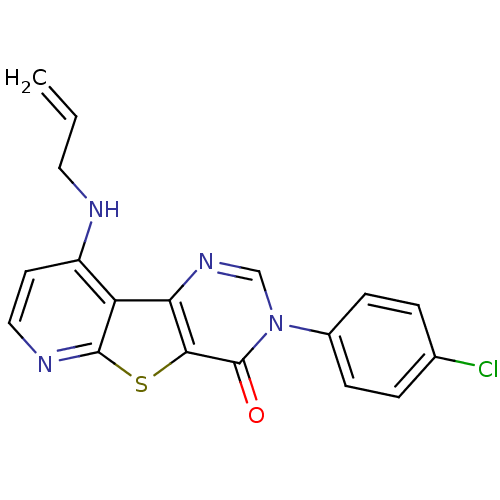
(9-Allylamino-3-(4-chloro-phenyl)-3H-pyrido[3',2':4...)Show SMILES Clc1ccc(cc1)-n1cnc2c(sc3nccc(NCC=C)c23)c1=O Show InChI InChI=1S/C18H13ClN4OS/c1-2-8-20-13-7-9-21-17-14(13)15-16(25-17)18(24)23(10-22-15)12-5-3-11(19)4-6-12/h2-7,9-10H,1,8H2,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at rat mGluR1 |
Bioorg Med Chem Lett 19: 3199-203 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.104
BindingDB Entry DOI: 10.7270/Q27081SJ |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50549021

(CHEMBL4797996)Show SMILES C[C@H]1CN(CC[C@H]2CC[C@@H](CC2)NC(=O)N2CCCC2)CCc2ccc(Cl)cc12 |r,wU:9.12,wD:6.5,1.0,(48.42,-47.98,;48.08,-49.48,;46.56,-49.83,;45.89,-51.23,;44.36,-51.23,;43.58,-52.56,;42.04,-52.56,;41.28,-51.23,;39.74,-51.23,;38.97,-52.56,;39.74,-53.9,;41.27,-53.9,;37.43,-52.56,;36.66,-53.9,;37.42,-55.23,;35.12,-53.9,;34.2,-52.65,;32.74,-53.13,;32.74,-54.67,;34.2,-55.15,;46.56,-52.63,;48.07,-52.97,;49.29,-52,;50.62,-52.77,;51.96,-52,;51.95,-50.44,;53.28,-49.67,;50.62,-49.68,;49.29,-50.45,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127681
BindingDB Entry DOI: 10.7270/Q2BZ69PB |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50004823
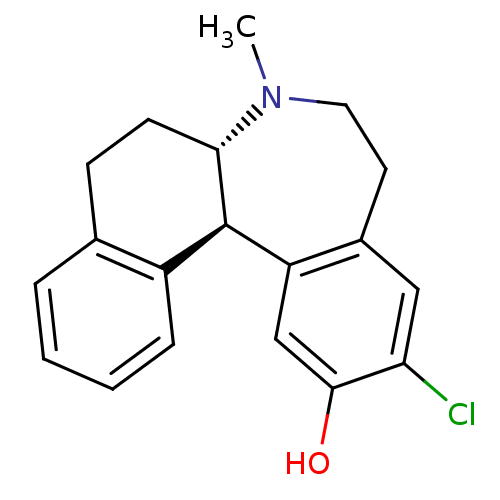
((6aS,13bR)-11-Chloro-7-methyl-5,6a,7,8,9,13b-hexah...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@@H]2[C@@H]1CCc1ccccc21 Show InChI InChI=1S/C19H20ClNO/c1-21-9-8-13-10-16(20)18(22)11-15(13)19-14-5-3-2-4-12(14)6-7-17(19)21/h2-5,10-11,17,19,22H,6-9H2,1H3/t17-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50549019
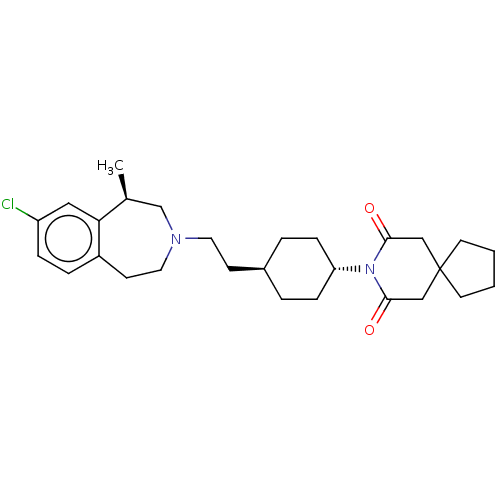
(CHEMBL4753808)Show SMILES C[C@H]1CN(CC[C@H]2CC[C@@H](CC2)N2C(=O)CC3(CCCC3)CC2=O)CCc2ccc(Cl)cc12 |r,wU:9.12,wD:6.5,1.0,(78.22,-16.9,;77.88,-18.4,;76.36,-18.74,;75.69,-20.14,;74.15,-20.14,;73.38,-21.48,;71.84,-21.48,;71.07,-20.14,;69.53,-20.14,;68.76,-21.48,;69.53,-22.81,;71.07,-22.81,;67.22,-21.48,;66.46,-22.81,;67.23,-24.15,;64.91,-22.81,;64.15,-21.47,;63.25,-20.22,;61.79,-20.69,;61.78,-22.23,;63.24,-22.71,;64.92,-20.14,;66.46,-20.14,;67.23,-18.81,;76.36,-21.54,;77.87,-21.88,;79.09,-20.91,;80.42,-21.68,;81.76,-20.91,;81.75,-19.36,;83.08,-18.58,;80.42,-18.6,;79.09,-19.37,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127681
BindingDB Entry DOI: 10.7270/Q2BZ69PB |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM50306317

((R)-N-(4-(7-chloro-8-hydroxy-3-methyl-2,3,4,5-tetr...)Show SMILES CN(Cc1ccc(cc1)[C@H]1CN(C)CCc2cc(Cl)c(O)cc12)S(=O)(=O)c1ccccc1 |r| Show InChI InChI=1S/C25H27ClN2O3S/c1-27-13-12-20-14-24(26)25(29)15-22(20)23(17-27)19-10-8-18(9-11-19)16-28(2)32(30,31)21-6-4-3-5-7-21/h3-11,14-15,23,29H,12-13,16-17H2,1-2H3/t23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D5 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50345940
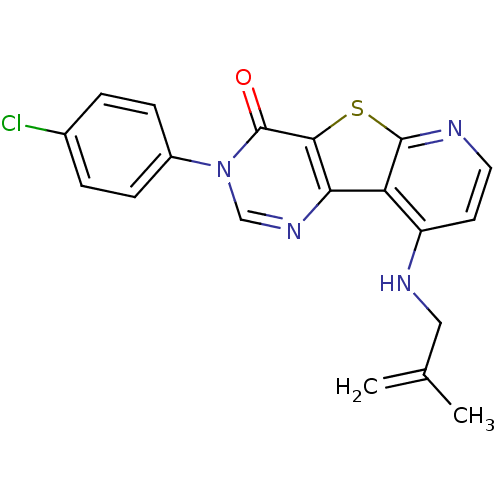
(3-(4-Chloro-phenyl)-9-(2-methyl-allylamino)-3H-pyr...)Show SMILES CC(=C)CNc1ccnc2sc3c(ncn(-c4ccc(Cl)cc4)c3=O)c12 Show InChI InChI=1S/C19H15ClN4OS/c1-11(2)9-22-14-7-8-21-18-15(14)16-17(26-18)19(25)24(10-23-16)13-5-3-12(20)4-6-13/h3-8,10H,1,9H2,2H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at rat mGluR1 |
Bioorg Med Chem Lett 19: 3199-203 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.104
BindingDB Entry DOI: 10.7270/Q27081SJ |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50549038

(CHEBI:71257 | CHEMBL504548)Show SMILES [H][C@]12CN(C)C[C@]1([H])c1cc(Cl)ccc1Oc1ccccc21 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to adrenergic alpha1 receptor (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127681
BindingDB Entry DOI: 10.7270/Q2BZ69PB |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50186811
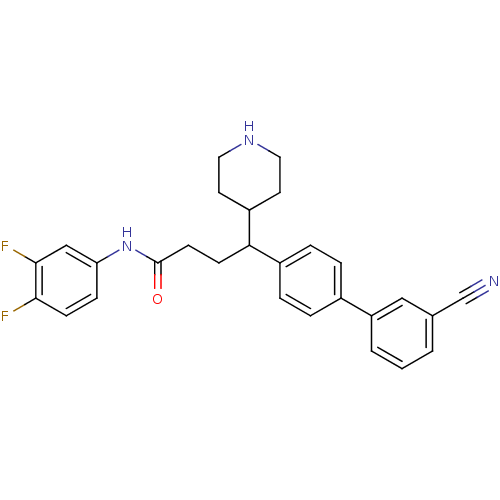
(4a-(3'-cyano-biphenyl-4-yl)-N-(3,4-difluoro-phenyl...)Show SMILES Fc1ccc(NC(=O)CCC(C2CCNCC2)c2ccc(cc2)-c2cccc(c2)C#N)cc1F Show InChI InChI=1S/C28H27F2N3O/c29-26-10-8-24(17-27(26)30)33-28(34)11-9-25(22-12-14-32-15-13-22)21-6-4-20(5-7-21)23-3-1-2-19(16-23)18-31/h1-8,10,16-17,22,25,32H,9,11-15H2,(H,33,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human MCHR1 assessed as inhibition of MCH-mediated calcium ion influx by FLIPR assay |
Bioorg Med Chem Lett 16: 3668-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.061
BindingDB Entry DOI: 10.7270/Q2251HT9 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Mus musculus) | BDBM50080322

(Acetic acid (1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-9-acet...)Show SMILES C[C@@H]1[C@@H](OC(C)=O)[C@]2(OC(C)=O)[C@H]([C@@H]3C=C(CO)C[C@@]4(O)[C@@H](C=C(C)C4=O)[C@@]13O)C2(C)C |t:14,22| Show InChI InChI=1S/C24H32O8/c1-11-7-17-22(29,19(11)28)9-15(10-25)8-16-18-21(5,6)24(18,32-14(4)27)20(31-13(3)26)12(2)23(16,17)30/h7-8,12,16-18,20,25,29-30H,9-10H2,1-6H3/t12-,16+,17-,18?,20-,22-,23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
Binding of Indolactam-V(ILV) to mutant Protein kinase C delta C1b domain (Thr12 to Gly) |
J Med Chem 42: 3436-46 (1999)
Article DOI: 10.1021/jm990129n
BindingDB Entry DOI: 10.7270/Q25T3JP5 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50231010

(CHEMBL312066)Show SMILES CCCCc1c(Oc2ccc(cc2)-c2ccccc2-c2nn[nH]n2)nc2c(C(O)=O)c(OCC)ccc2[n+]1[O-] Show InChI InChI=1S/C28H26N6O5/c1-3-5-10-22-27(29-25-21(34(22)37)15-16-23(38-4-2)24(25)28(35)36)39-18-13-11-17(12-14-18)19-8-6-7-9-20(19)26-30-32-33-31-26/h6-9,11-16H,3-5,10H2,1-2H3,(H,35,36)(H,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of compound in presence of bovine serum albumin (BSA) at 0.01% against rat adrenal Angiotensin II receptor, type 1 |
J Med Chem 36: 2335-42 (1993)
Article DOI: 10.1021/jm00068a010
BindingDB Entry DOI: 10.7270/Q21V5H6B |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50306334
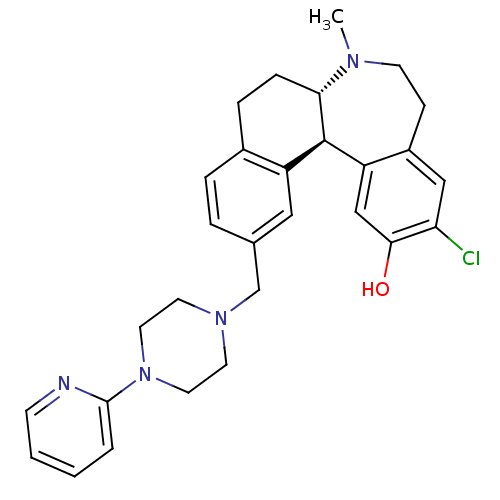
((6aS,13bR)-11-chloro-7-methyl-2-((4-(pyridin-2-yl)...)Show SMILES CN1CCc2cc(Cl)c(O)cc2[C@@H]2[C@@H]1CCc1ccc(CN3CCN(CC3)c3ccccn3)cc21 |r| Show InChI InChI=1S/C29H33ClN4O/c1-32-11-9-22-17-25(30)27(35)18-24(22)29-23-16-20(5-6-21(23)7-8-26(29)32)19-33-12-14-34(15-13-33)28-4-2-3-10-31-28/h2-6,10,16-18,26,29,35H,7-9,11-15,19H2,1H3/t26-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D2 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/B
(RAT) | BDBM50231010

(CHEMBL312066)Show SMILES CCCCc1c(Oc2ccc(cc2)-c2ccccc2-c2nn[nH]n2)nc2c(C(O)=O)c(OCC)ccc2[n+]1[O-] Show InChI InChI=1S/C28H26N6O5/c1-3-5-10-22-27(29-25-21(34(22)37)15-16-23(38-4-2)24(25)28(35)36)39-18-13-11-17(12-14-18)19-8-6-7-9-20(19)26-30-32-33-31-26/h6-9,11-16H,3-5,10H2,1-2H3,(H,35,36)(H,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 in rat adrenal cortical membranes using [125I]Sar1, Ile8-angiotensin II |
J Med Chem 36: 2335-42 (1993)
Article DOI: 10.1021/jm00068a010
BindingDB Entry DOI: 10.7270/Q21V5H6B |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306326

(CHEMBL603485 | ethyl(6aS,13bR)-11-chloro-12-hydrox...)Show SMILES CCOC(=O)Nc1ccc2[C@H]3[C@H](CCc2c1)N(C)CCc1cc(Cl)c(O)cc31 |r| Show InChI InChI=1S/C22H25ClN2O3/c1-3-28-22(27)24-15-5-6-16-13(10-15)4-7-19-21(16)17-12-20(26)18(23)11-14(17)8-9-25(19)2/h5-6,10-12,19,21,26H,3-4,7-9H2,1-2H3,(H,24,27)/t19-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50549032

(CHEMBL4759975)Show SMILES C[C@H]1CN(CC[C@H]2CC[C@@H](CC2)NC(=O)Nc2ccccc2)CCc2ccc(Cl)cc12 |r,wU:9.12,wD:6.5,1.0,(80.06,-46.54,;79.71,-48.04,;78.2,-48.38,;77.53,-49.79,;75.99,-49.79,;75.22,-51.12,;73.68,-51.12,;72.91,-49.78,;71.37,-49.78,;70.6,-51.12,;71.37,-52.45,;72.91,-52.45,;69.06,-51.12,;68.29,-52.45,;69.06,-53.79,;66.75,-52.45,;65.98,-53.79,;64.44,-53.78,;63.67,-55.11,;64.44,-56.45,;65.99,-56.44,;66.75,-55.11,;78.2,-51.18,;79.71,-51.52,;80.92,-50.55,;82.26,-51.32,;83.59,-50.55,;83.59,-49,;84.92,-48.23,;82.25,-48.24,;80.93,-49.01,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127681
BindingDB Entry DOI: 10.7270/Q2BZ69PB |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50306324
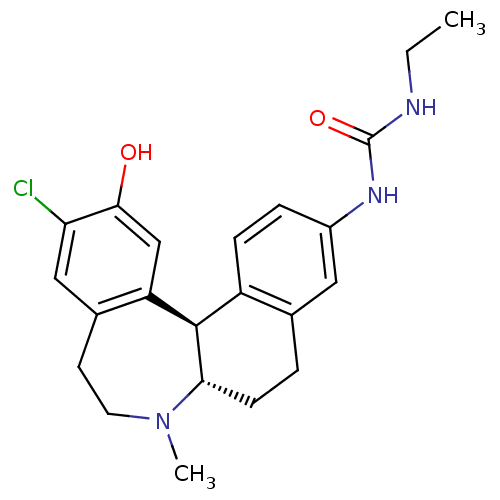
(1-((6aS,13bR)-11-chloro-12-hydroxy-7-methyl-6,6a,7...)Show SMILES CCNC(=O)Nc1ccc2[C@H]3[C@H](CCc2c1)N(C)CCc1cc(Cl)c(O)cc31 |r| Show InChI InChI=1S/C22H26ClN3O2/c1-3-24-22(28)25-15-5-6-16-13(10-15)4-7-19-21(16)17-12-20(27)18(23)11-14(17)8-9-26(19)2/h5-6,10-12,19,21,27H,3-4,7-9H2,1-2H3,(H2,24,25,28)/t19-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 20: 836-40 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.100
BindingDB Entry DOI: 10.7270/Q23N23HT |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50192623
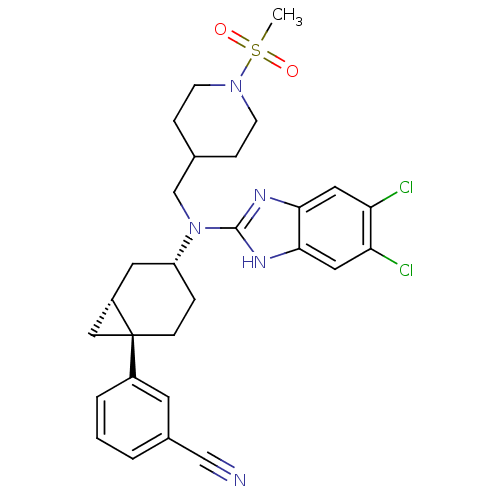
(3-((1R,4R,6R)-4-((5,6-dichloro-1H-benzo[d]imidazol...)Show SMILES CS(=O)(=O)N1CCC(CN([C@@H]2CC[C@@]3(C[C@@H]3C2)c2cccc(c2)C#N)c2nc3cc(Cl)c(Cl)cc3[nH]2)CC1 Show InChI InChI=1S/C28H31Cl2N5O2S/c1-38(36,37)34-9-6-18(7-10-34)17-35(27-32-25-13-23(29)24(30)14-26(25)33-27)22-5-8-28(15-21(28)12-22)20-4-2-3-19(11-20)16-31/h2-4,11,13-14,18,21-22H,5-10,12,15,17H2,1H3,(H,32,33)/t21-,22+,28+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding activity against human MCH-R1 |
Bioorg Med Chem Lett 16: 5427-31 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.058
BindingDB Entry DOI: 10.7270/Q228076K |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50345943
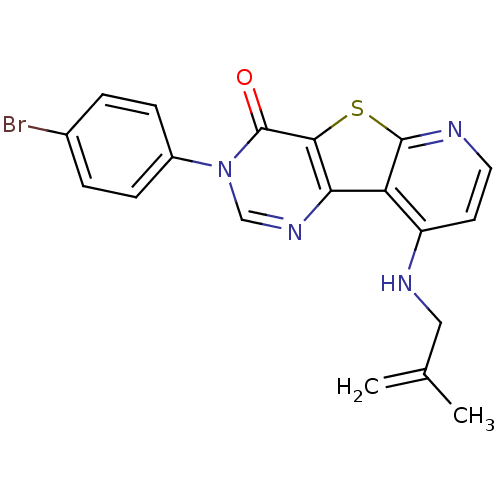
(3-(4-Bromo-phenyl)-9-(2-methyl-allylamino)-3H-pyri...)Show SMILES CC(=C)CNc1ccnc2sc3c(ncn(-c4ccc(Br)cc4)c3=O)c12 Show InChI InChI=1S/C19H15BrN4OS/c1-11(2)9-22-14-7-8-21-18-15(14)16-17(26-18)19(25)24(10-23-16)13-5-3-12(20)4-6-13/h3-8,10H,1,9H2,2H3,(H,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at rat mGluR1 |
Bioorg Med Chem Lett 19: 3199-203 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.104
BindingDB Entry DOI: 10.7270/Q27081SJ |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 1
(RAT) | BDBM50345948
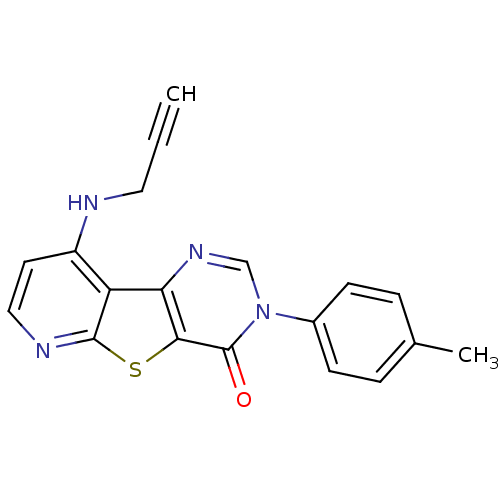
(9-Prop-2-ynylamino-3-p-tolyl-3H-pyrido[3',2':4,5]t...)Show InChI InChI=1S/C19H14N4OS/c1-3-9-20-14-8-10-21-18-15(14)16-17(25-18)19(24)23(11-22-16)13-6-4-12(2)5-7-13/h1,4-8,10-11H,9H2,2H3,(H,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at rat mGluR1 |
Bioorg Med Chem Lett 19: 3199-203 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.104
BindingDB Entry DOI: 10.7270/Q27081SJ |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50099066

(CHEMBL279115 | phorbol 13-acetate 12-myristate)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(C)=O |r,c:33,t:22| Show InChI InChI=1S/C36H56O8/c1-7-8-9-10-11-12-13-14-15-16-17-18-29(39)43-32-24(3)35(42)27(30-33(5,6)36(30,32)44-25(4)38)20-26(22-37)21-34(41)28(35)19-23(2)31(34)40/h19-20,24,27-28,30,32,37,41-42H,7-18,21-22H2,1-6H3/t24-,27+,28-,30?,32-,34-,35-,36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetotwn University
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-phorbol 12,13-dibutyrate (PDBu) binding to human recombinant protein kinase C beta |
Bioorg Med Chem Lett 11: 955-9 (2001)
BindingDB Entry DOI: 10.7270/Q2D799PV |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50549033

(CHEMBL4741702)Show SMILES C[C@H]1CN(CC[C@H]2CC[C@@H](CC2)NC(=O)N2CCOCC2)CCc2ccc(Cl)cc12 |r,wU:9.12,wD:6.5,1.0,(18.81,-48,;18.47,-49.5,;16.96,-49.85,;16.29,-51.25,;14.75,-51.25,;13.98,-52.58,;12.44,-52.58,;11.67,-51.25,;10.13,-51.25,;9.36,-52.58,;10.13,-53.91,;11.67,-53.91,;7.82,-52.58,;7.05,-53.91,;7.82,-55.25,;5.51,-53.91,;4.74,-55.25,;3.2,-55.25,;2.43,-53.91,;3.2,-52.58,;4.74,-52.58,;16.95,-52.64,;18.47,-52.98,;19.68,-52.01,;21.01,-52.78,;22.35,-52.01,;22.35,-50.46,;23.68,-49.69,;21.01,-49.7,;19.69,-50.47,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]methyl-spiperone from recombinant human D3 receptor expressed in CHO cells |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127681
BindingDB Entry DOI: 10.7270/Q2BZ69PB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data