 Found 130 hits with Last Name = 'qian' and Initial = 'g'
Found 130 hits with Last Name = 'qian' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169371
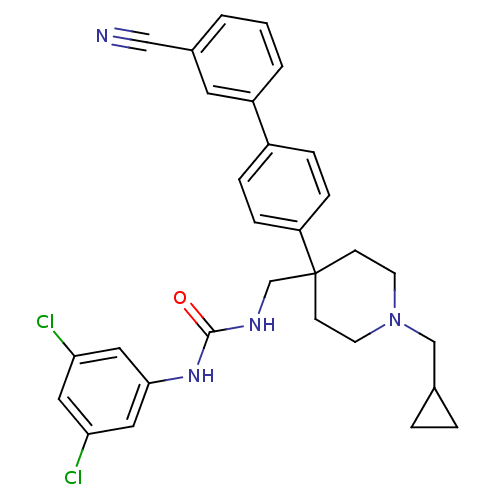
(1-[4-(3'-Cyano-biphenyl-4-yl)-1-cyclopropylmethyl-...)Show SMILES Clc1cc(Cl)cc(NC(=O)NCC2(CCN(CC3CC3)CC2)c2ccc(cc2)-c2cccc(c2)C#N)c1 Show InChI InChI=1S/C30H30Cl2N4O/c31-26-15-27(32)17-28(16-26)35-29(37)34-20-30(10-12-36(13-11-30)19-21-4-5-21)25-8-6-23(7-9-25)24-3-1-2-22(14-24)18-33/h1-3,6-9,14-17,21H,4-5,10-13,19-20H2,(H2,34,35,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169377
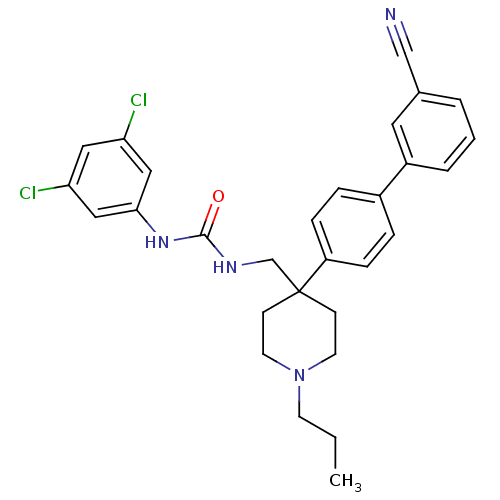
(1-[4-(3'-Cyano-biphenyl-4-yl)-1-propyl-piperidin-4...)Show SMILES CCCN1CCC(CNC(=O)Nc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C29H30Cl2N4O/c1-2-12-35-13-10-29(11-14-35,20-33-28(36)34-27-17-25(30)16-26(31)18-27)24-8-6-22(7-9-24)23-5-3-4-21(15-23)19-32/h3-9,15-18H,2,10-14,20H2,1H3,(H2,33,34,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169374
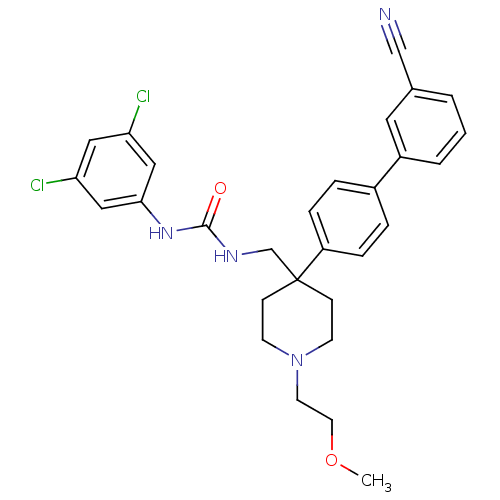
(1-[4-(3'-Cyano-biphenyl-4-yl)-1-(2-methoxy-ethyl)-...)Show SMILES COCCN1CCC(CNC(=O)Nc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C29H30Cl2N4O2/c1-37-14-13-35-11-9-29(10-12-35,20-33-28(36)34-27-17-25(30)16-26(31)18-27)24-7-5-22(6-8-24)23-4-2-3-21(15-23)19-32/h2-8,15-18H,9-14,20H2,1H3,(H2,33,34,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169390

(1-[4-(3'-Cyano-biphenyl-4-yl)-1-isopropyl-piperidi...)Show SMILES CC(C)N1CCC(CNC(=O)Nc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C29H30Cl2N4O/c1-20(2)35-12-10-29(11-13-35,19-33-28(36)34-27-16-25(30)15-26(31)17-27)24-8-6-22(7-9-24)23-5-3-4-21(14-23)18-32/h3-9,14-17,20H,10-13,19H2,1-2H3,(H2,33,34,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169389

(1-[4-(3'-Cyano-biphenyl-4-yl)-1-cyclopentyl-piperi...)Show SMILES Clc1cc(Cl)cc(NC(=O)NCC2(CCN(CC2)C2CCCC2)c2ccc(cc2)-c2cccc(c2)C#N)c1 Show InChI InChI=1S/C31H32Cl2N4O/c32-26-17-27(33)19-28(18-26)36-30(38)35-21-31(12-14-37(15-13-31)29-6-1-2-7-29)25-10-8-23(9-11-25)24-5-3-4-22(16-24)20-34/h3-5,8-11,16-19,29H,1-2,6-7,12-15,21H2,(H2,35,36,38) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169393
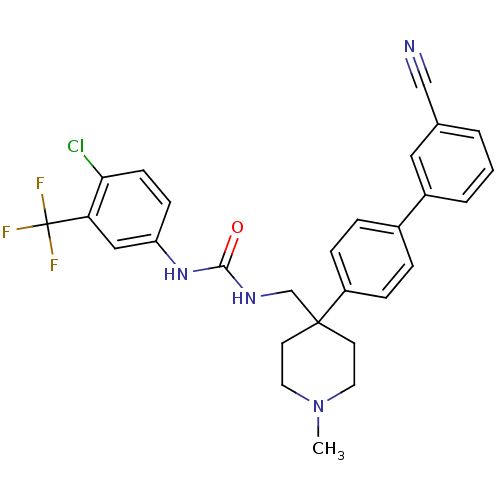
(1-(4-Chloro-3-trifluoromethyl-phenyl)-3-[4-(3'-cya...)Show SMILES CN1CCC(CNC(=O)Nc2ccc(Cl)c(c2)C(F)(F)F)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C28H26ClF3N4O/c1-36-13-11-27(12-14-36,22-7-5-20(6-8-22)21-4-2-3-19(15-21)17-33)18-34-26(37)35-23-9-10-25(29)24(16-23)28(30,31)32/h2-10,15-16H,11-14,18H2,1H3,(H2,34,35,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169382

(1-[4-(3'-Cyano-biphenyl-4-yl)-1-cyclohexyl-piperid...)Show SMILES Clc1cc(Cl)cc(NC(=O)NCC2(CCN(CC2)C2CCCCC2)c2ccc(cc2)-c2cccc(c2)C#N)c1 Show InChI InChI=1S/C32H34Cl2N4O/c33-27-18-28(34)20-29(19-27)37-31(39)36-22-32(13-15-38(16-14-32)30-7-2-1-3-8-30)26-11-9-24(10-12-26)25-6-4-5-23(17-25)21-35/h4-6,9-12,17-20,30H,1-3,7-8,13-16,22H2,(H2,36,37,39) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169396

(1-[4-(3'-Cyano-biphenyl-4-yl)-1-methyl-piperidin-4...)Show SMILES CN1CCC(CNC(=O)Nc2ccc(F)c(F)c2)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C27H26F2N4O/c1-33-13-11-27(12-14-33,18-31-26(34)32-23-9-10-24(28)25(29)16-23)22-7-5-20(6-8-22)21-4-2-3-19(15-21)17-30/h2-10,15-16H,11-14,18H2,1H3,(H2,31,32,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169376

(1-[4-(3'-Cyano-biphenyl-4-yl)-1-methyl-piperidin-4...)Show SMILES CN1CCC(CNC(=O)Nc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C27H26Cl2N4O/c1-33-11-9-27(10-12-33,18-31-26(34)32-25-15-23(28)14-24(29)16-25)22-7-5-20(6-8-22)21-4-2-3-19(13-21)17-30/h2-8,13-16H,9-12,18H2,1H3,(H2,31,32,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169386
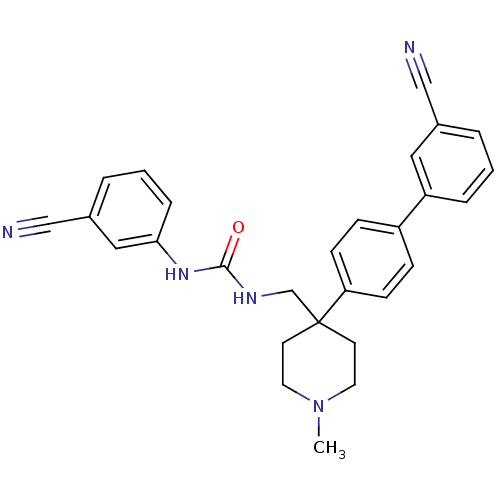
(1-[4-(3'-Cyano-biphenyl-4-yl)-1-methyl-piperidin-4...)Show SMILES CN1CCC(CNC(=O)Nc2cccc(c2)C#N)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C28H27N5O/c1-33-14-12-28(13-15-33,20-31-27(34)32-26-7-3-5-22(17-26)19-30)25-10-8-23(9-11-25)24-6-2-4-21(16-24)18-29/h2-11,16-17H,12-15,20H2,1H3,(H2,31,32,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169387

(CHEMBL366703 | N-(4'-{4-[3-(3,5-Dichloro-phenyl)-u...)Show SMILES CN1CCC(CNC(=O)Nc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccc(NC(C)=O)c1 Show InChI InChI=1S/C28H30Cl2N4O2/c1-19(35)32-25-5-3-4-21(14-25)20-6-8-22(9-7-20)28(10-12-34(2)13-11-28)18-31-27(36)33-26-16-23(29)15-24(30)17-26/h3-9,14-17H,10-13,18H2,1-2H3,(H,32,35)(H2,31,33,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169398
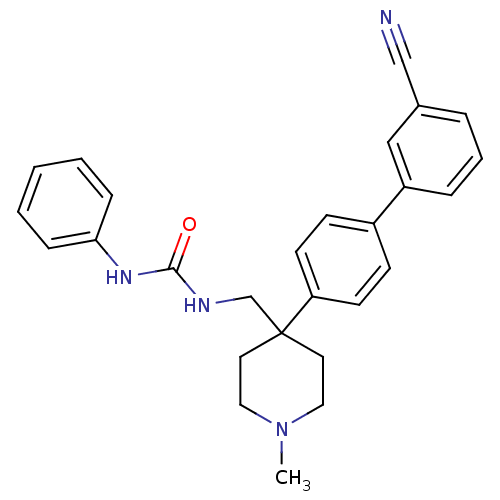
(1-[4-(3'-Cyano-biphenyl-4-yl)-1-methyl-piperidin-4...)Show SMILES CN1CCC(CNC(=O)Nc2ccccc2)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C27H28N4O/c1-31-16-14-27(15-17-31,20-29-26(32)30-25-8-3-2-4-9-25)24-12-10-22(11-13-24)23-7-5-6-21(18-23)19-28/h2-13,18H,14-17,20H2,1H3,(H2,29,30,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169388

(1-(3,5-Dichloro-phenyl)-3-[1-methanesulfonyl-4-(4-...)Show SMILES CS(=O)(=O)N1CCC(CNC(=O)Nc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccnc1 Show InChI InChI=1S/C25H26Cl2N4O3S/c1-35(33,34)31-11-8-25(9-12-31,17-29-24(32)30-23-14-21(26)13-22(27)15-23)20-6-4-18(5-7-20)19-3-2-10-28-16-19/h2-7,10,13-16H,8-9,11-12,17H2,1H3,(H2,29,30,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169370
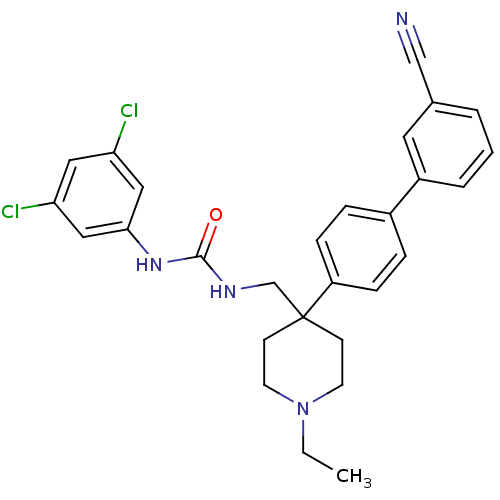
(1-[4-(3'-Cyano-biphenyl-4-yl)-1-ethyl-piperidin-4-...)Show SMILES CCN1CCC(CNC(=O)Nc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C28H28Cl2N4O/c1-2-34-12-10-28(11-13-34,19-32-27(35)33-26-16-24(29)15-25(30)17-26)23-8-6-21(7-9-23)22-5-3-4-20(14-22)18-31/h3-9,14-17H,2,10-13,19H2,1H3,(H2,32,33,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169392

(1-[4-(3'-Chloro-biphenyl-4-yl)-1-methyl-piperidin-...)Show SMILES CN1CCC(CNC(=O)Nc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccc(Cl)c1 Show InChI InChI=1S/C26H26Cl3N3O/c1-32-11-9-26(10-12-32,17-30-25(33)31-24-15-22(28)14-23(29)16-24)20-7-5-18(6-8-20)19-3-2-4-21(27)13-19/h2-8,13-16H,9-12,17H2,1H3,(H2,30,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169391

(1-(3,5-Dichloro-phenyl)-3-[1-methyl-4-(4-pyridin-3...)Show SMILES CN1CCC(CNC(=O)Nc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccnc1 Show InChI InChI=1S/C25H26Cl2N4O/c1-31-11-8-25(9-12-31,17-29-24(32)30-23-14-21(26)13-22(27)15-23)20-6-4-18(5-7-20)19-3-2-10-28-16-19/h2-7,10,13-16H,8-9,11-12,17H2,1H3,(H2,29,30,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50217060
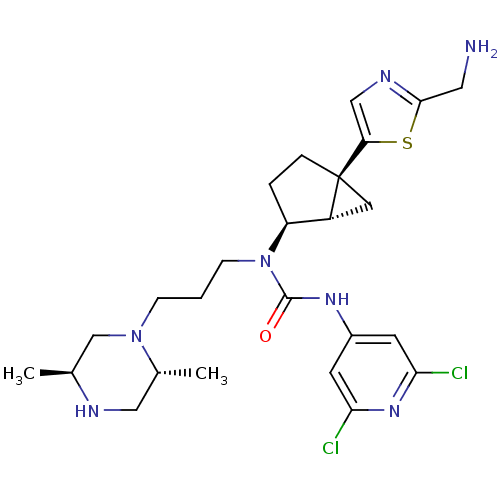
(1-((1S,2S,5S)-5-(2-(aminomethyl)thiazol-5-yl)bicyc...)Show SMILES C[C@H]1CN(CCCN([C@H]2CC[C@@]3(C[C@H]23)c2cnc(CN)s2)C(=O)Nc2cc(Cl)nc(Cl)c2)[C@H](C)CN1 Show InChI InChI=1S/C25H35Cl2N7OS/c1-15-14-33(16(2)12-29-15)6-3-7-34(24(35)31-17-8-21(26)32-22(27)9-17)19-4-5-25(10-18(19)25)20-13-30-23(11-28)36-20/h8-9,13,15-16,18-19,29H,3-7,10-12,14,28H2,1-2H3,(H,31,32,35)/t15-,16+,18+,19-,25-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Antagonist activity at MCHR1 |
Bioorg Med Chem Lett 17: 4845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.048
BindingDB Entry DOI: 10.7270/Q2V69J9H |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169372
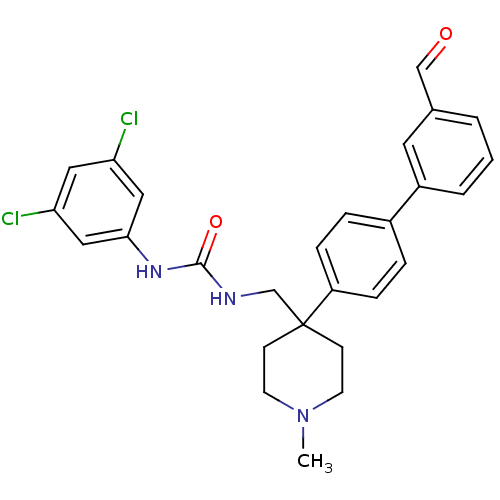
(1-(3,5-Dichloro-phenyl)-3-[4-(3'-formyl-biphenyl-4...)Show SMILES CN1CCC(CNC(=O)Nc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccc(C=O)c1 Show InChI InChI=1S/C27H27Cl2N3O2/c1-32-11-9-27(10-12-32,18-30-26(34)31-25-15-23(28)14-24(29)16-25)22-7-5-20(6-8-22)21-4-2-3-19(13-21)17-33/h2-8,13-17H,9-12,18H2,1H3,(H2,30,31,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50217070

(1-((1S,2S,5S)-5-(2-(aminomethyl)thiazol-5-yl)bicyc...)Show SMILES C[C@@H]1CN(CCCN([C@H]2CC[C@@]3(C[C@H]23)c2cnc(CN)s2)C(=O)Nc2cc(Cl)nc(Cl)c2)[C@@H](C)CN1 Show InChI InChI=1S/C25H35Cl2N7OS/c1-15-14-33(16(2)12-29-15)6-3-7-34(24(35)31-17-8-21(26)32-22(27)9-17)19-4-5-25(10-18(19)25)20-13-30-23(11-28)36-20/h8-9,13,15-16,18-19,29H,3-7,10-12,14,28H2,1-2H3,(H,31,32,35)/t15-,16+,18-,19+,25+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Antagonist activity at MCHR1 |
Bioorg Med Chem Lett 17: 4845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.048
BindingDB Entry DOI: 10.7270/Q2V69J9H |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50217066

(1-((1S,2S,5S)-5-(2-(aminomethyl)thiazol-5-yl)bicyc...)Show SMILES NCc1ncc(s1)[C@@]12C[C@@H]1[C@H](CC2)N(CCCN1CC[C@@H](O)C1)C(=O)Nc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C25H31F4N5O2S/c26-19-3-2-15(10-17(19)25(27,28)29)32-23(36)34(8-1-7-33-9-5-16(35)14-33)20-4-6-24(11-18(20)24)21-13-31-22(12-30)37-21/h2-3,10,13,16,18,20,35H,1,4-9,11-12,14,30H2,(H,32,36)/t16-,18-,20+,24+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Antagonist activity at MCHR1 |
Bioorg Med Chem Lett 17: 4845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.048
BindingDB Entry DOI: 10.7270/Q2V69J9H |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50164248
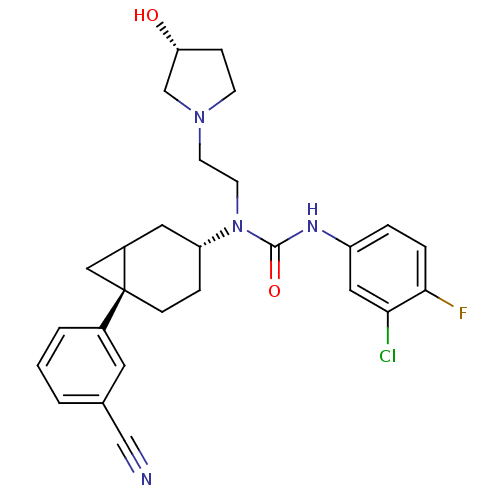
(3-(3-Chloro-4-fluoro-phenyl)-1-[(3R,6S)-6-(3-cyano...)Show SMILES O[C@@H]1CCN(CCN([C@@H]2CC[C@@]3(CC3C2)c2cccc(c2)C#N)C(=O)Nc2ccc(F)c(Cl)c2)C1 Show InChI InChI=1S/C27H30ClFN4O2/c28-24-14-21(4-5-25(24)29)31-26(35)33(11-10-32-9-7-23(34)17-32)22-6-8-27(15-20(27)13-22)19-3-1-2-18(12-19)16-30/h1-5,12,14,20,22-23,34H,6-11,13,15,17H2,(H,31,35)/t20?,22-,23-,27-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Antagonist activity at MCHR1 |
Bioorg Med Chem Lett 17: 4845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.048
BindingDB Entry DOI: 10.7270/Q2V69J9H |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169381
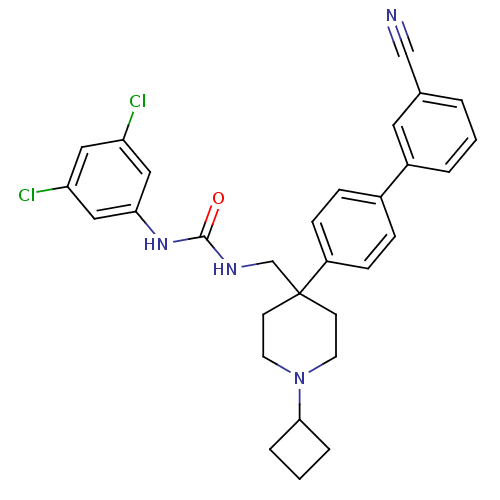
(1-[4-(3'-Cyano-biphenyl-4-yl)-1-cyclobutyl-piperid...)Show SMILES Clc1cc(Cl)cc(NC(=O)NCC2(CCN(CC2)C2CCC2)c2ccc(cc2)-c2cccc(c2)C#N)c1 Show InChI InChI=1S/C30H30Cl2N4O/c31-25-16-26(32)18-27(17-25)35-29(37)34-20-30(11-13-36(14-12-30)28-5-2-6-28)24-9-7-22(8-10-24)23-4-1-3-21(15-23)19-33/h1,3-4,7-10,15-18,28H,2,5-6,11-14,20H2,(H2,34,35,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169395
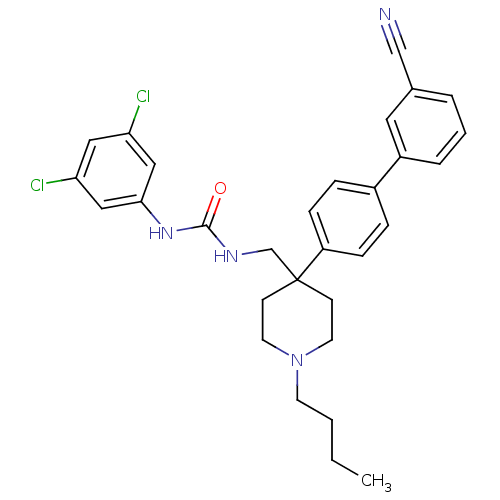
(1-[1-Butyl-4-(3'-cyano-biphenyl-4-yl)-piperidin-4-...)Show SMILES CCCCN1CCC(CNC(=O)Nc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C30H32Cl2N4O/c1-2-3-13-36-14-11-30(12-15-36,21-34-29(37)35-28-18-26(31)17-27(32)19-28)25-9-7-23(8-10-25)24-6-4-5-22(16-24)20-33/h4-10,16-19H,2-3,11-15,21H2,1H3,(H2,34,35,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169394
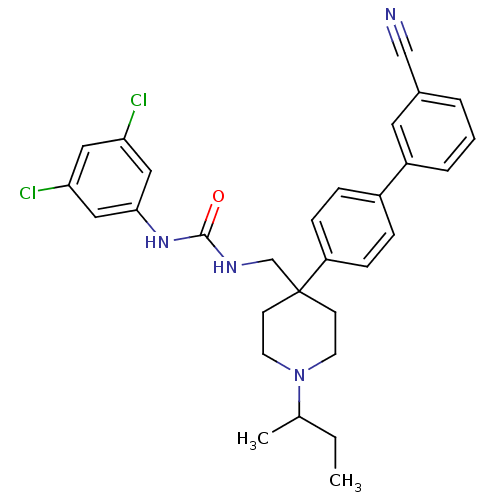
(1-[1-sec-Butyl-4-(3'-cyano-biphenyl-4-yl)-piperidi...)Show SMILES CCC(C)N1CCC(CNC(=O)Nc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C30H32Cl2N4O/c1-3-21(2)36-13-11-30(12-14-36,20-34-29(37)35-28-17-26(31)16-27(32)18-28)25-9-7-23(8-10-25)24-6-4-5-22(15-24)19-33/h4-10,15-18,21H,3,11-14,20H2,1-2H3,(H2,34,35,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50217057
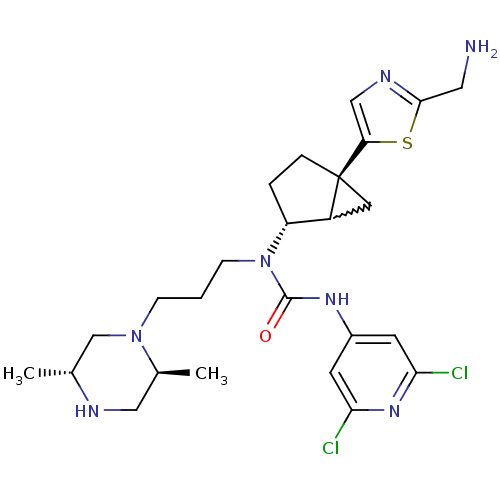
(1-((2R,5S)-5-(2-(aminomethyl)thiazol-5-yl)bicyclo[...)Show SMILES C[C@@H]1CN(CCCN([C@@H]2CC[C@@]3(CC23)c2cnc(CN)s2)C(=O)Nc2cc(Cl)nc(Cl)c2)[C@@H](C)CN1 |w:13.12| Show InChI InChI=1S/C25H35Cl2N7OS/c1-15-14-33(16(2)12-29-15)6-3-7-34(24(35)31-17-8-21(26)32-22(27)9-17)19-4-5-25(10-18(19)25)20-13-30-23(11-28)36-20/h8-9,13,15-16,18-19,29H,3-7,10-12,14,28H2,1-2H3,(H,31,32,35)/t15-,16+,18?,19-,25+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Antagonist activity at MCHR1 |
Bioorg Med Chem Lett 17: 4845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.048
BindingDB Entry DOI: 10.7270/Q2V69J9H |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50215659

(1-(4-(2-(aminomethyl)thiazol-5-yl)cyclohex-3-enyl)...)Show SMILES NCc1ncc(s1)C1=CCC(CC1)N(CCN1CCCC1)C(=O)Nc1ccc(F)c(Cl)c1 |w:10.14,t:8| Show InChI InChI=1S/C23H29ClFN5OS/c24-19-13-17(5-8-20(19)25)28-23(31)30(12-11-29-9-1-2-10-29)18-6-3-16(4-7-18)21-15-27-22(14-26)32-21/h3,5,8,13,15,18H,1-2,4,6-7,9-12,14,26H2,(H,28,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Antagonist activity at MCHR1 |
Bioorg Med Chem Lett 17: 4845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.048
BindingDB Entry DOI: 10.7270/Q2V69J9H |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50217056
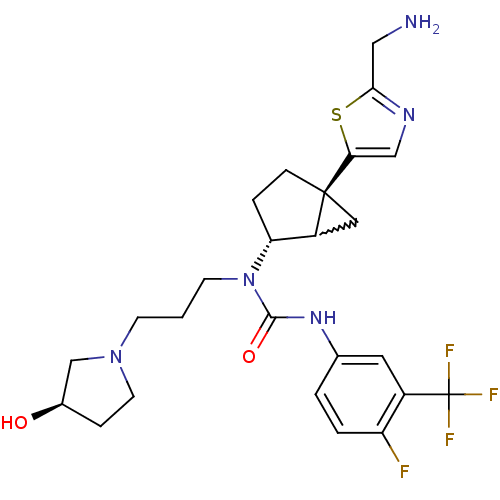
(1-((2R,5S)-5-(2-(aminomethyl)thiazol-5-yl)bicyclo[...)Show SMILES NCc1ncc(s1)[C@@]12CC1[C@@H](CC2)N(CCCN1CC[C@@H](O)C1)C(=O)Nc1ccc(F)c(c1)C(F)(F)F |w:9.9| Show InChI InChI=1S/C25H31F4N5O2S/c26-19-3-2-15(10-17(19)25(27,28)29)32-23(36)34(8-1-7-33-9-5-16(35)14-33)20-4-6-24(11-18(20)24)21-13-31-22(12-30)37-21/h2-3,10,13,16,18,20,35H,1,4-9,11-12,14,30H2,(H,32,36)/t16-,18?,20-,24+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Antagonist activity at MCHR1 |
Bioorg Med Chem Lett 17: 4845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.048
BindingDB Entry DOI: 10.7270/Q2V69J9H |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50217063

(1-((2R,5S)-5-(2-(aminomethyl)thiazol-5-yl)bicyclo[...)Show SMILES C[C@@H]1CN(CCCN([C@@H]2CC[C@@]3(CC23)c2cnc(CN)s2)C(=O)Nc2ccc(F)c(c2)C(F)(F)F)[C@@H](C)CN1 |w:13.12| Show InChI InChI=1S/C27H36F4N6OS/c1-16-15-36(17(2)13-33-16)8-3-9-37(25(38)35-18-4-5-21(28)19(10-18)27(29,30)31)22-6-7-26(11-20(22)26)23-14-34-24(12-32)39-23/h4-5,10,14,16-17,20,22,33H,3,6-9,11-13,15,32H2,1-2H3,(H,35,38)/t16-,17+,20?,22-,26+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Antagonist activity at MCHR1 |
Bioorg Med Chem Lett 17: 4845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.048
BindingDB Entry DOI: 10.7270/Q2V69J9H |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50217068
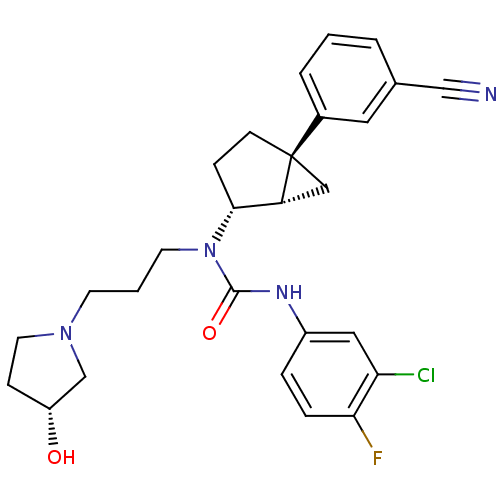
(3-(3-chloro-4-fluorophenyl)-1-((1S,2R,5S)-5-(3-cya...)Show SMILES O[C@@H]1CCN(CCCN([C@@H]2CC[C@@]3(C[C@H]23)c2cccc(c2)C#N)C(=O)Nc2ccc(F)c(Cl)c2)C1 Show InChI InChI=1S/C27H30ClFN4O2/c28-23-14-20(5-6-24(23)29)31-26(35)33(11-2-10-32-12-8-21(34)17-32)25-7-9-27(15-22(25)27)19-4-1-3-18(13-19)16-30/h1,3-6,13-14,21-22,25,34H,2,7-12,15,17H2,(H,31,35)/t21-,22-,25-,27-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Antagonist activity at MCHR1 |
Bioorg Med Chem Lett 17: 4845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.048
BindingDB Entry DOI: 10.7270/Q2V69J9H |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169373
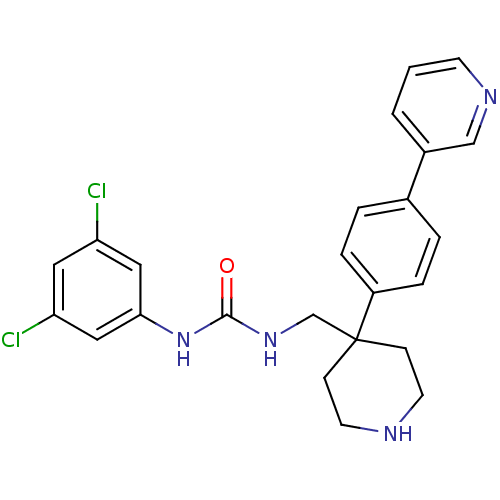
(1-(3,5-Dichloro-phenyl)-3-[4-(4-pyridin-3-yl-pheny...)Show SMILES Clc1cc(Cl)cc(NC(=O)NCC2(CCNCC2)c2ccc(cc2)-c2cccnc2)c1 Show InChI InChI=1S/C24H24Cl2N4O/c25-20-12-21(26)14-22(13-20)30-23(31)29-16-24(7-10-27-11-8-24)19-5-3-17(4-6-19)18-2-1-9-28-15-18/h1-6,9,12-15,27H,7-8,10-11,16H2,(H2,29,30,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50217058

(1-((2R,5S)-5-(2-(aminomethyl)thiazol-5-yl)bicyclo[...)Show SMILES NCc1ncc(s1)[C@@]12CC1[C@@H](CC2)N(CCCN1CC[C@H](O)C1)C(=O)Nc1ccc(F)c(c1)C(F)(F)F |w:9.9| Show InChI InChI=1S/C25H31F4N5O2S/c26-19-3-2-15(10-17(19)25(27,28)29)32-23(36)34(8-1-7-33-9-5-16(35)14-33)20-4-6-24(11-18(20)24)21-13-31-22(12-30)37-21/h2-3,10,13,16,18,20,35H,1,4-9,11-12,14,30H2,(H,32,36)/t16-,18?,20+,24-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Antagonist activity at MCHR1 |
Bioorg Med Chem Lett 17: 4845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.048
BindingDB Entry DOI: 10.7270/Q2V69J9H |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50217062

(1-(3-(2,5-diaza-bicyclo[2.2.1]heptan-2-yl)propyl)-...)Show SMILES NCc1ncc(s1)[C@@]12CC1[C@@H](CC2)N(CCCN1CC2CC1CN2)C(=O)Nc1ccc(F)c(c1)C(F)(F)F |w:19.22,21.23,9.9,TLB:16:17:20:22.23| Show InChI InChI=1S/C26H32F4N6OS/c27-20-3-2-15(9-18(20)26(28,29)30)34-24(37)36(7-1-6-35-14-16-8-17(35)12-32-16)21-4-5-25(10-19(21)25)22-13-33-23(11-31)38-22/h2-3,9,13,16-17,19,21,32H,1,4-8,10-12,14,31H2,(H,34,37)/t16?,17?,19?,21-,25+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Antagonist activity at MCHR1 |
Bioorg Med Chem Lett 17: 4845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.048
BindingDB Entry DOI: 10.7270/Q2V69J9H |
More data for this
Ligand-Target Pair | |
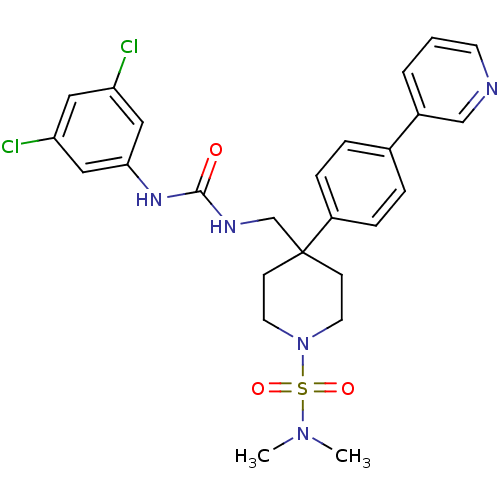
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169380
(4-[3-(3,5-Dichloro-phenyl)-ureidomethyl]-4-(4-pyri...)Show SMILES CN(C)S(=O)(=O)N1CCC(CNC(=O)Nc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccnc1 Show InChI InChI=1S/C26H29Cl2N5O3S/c1-32(2)37(35,36)33-12-9-26(10-13-33,18-30-25(34)31-24-15-22(27)14-23(28)16-24)21-7-5-19(6-8-21)20-4-3-11-29-17-20/h3-8,11,14-17H,9-10,12-13,18H2,1-2H3,(H2,30,31,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
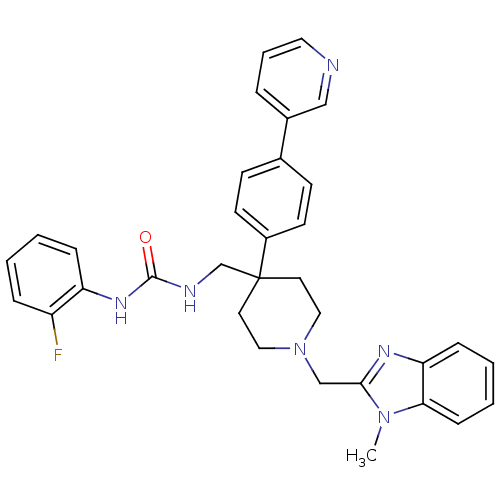
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169384
(1-(2-Fluoro-phenyl)-3-[1-(1-methyl-1H-benzoimidazo...)Show SMILES Cn1c(CN2CCC(CNC(=O)Nc3ccccc3F)(CC2)c2ccc(cc2)-c2cccnc2)nc2ccccc12 Show InChI InChI=1S/C33H33FN6O/c1-39-30-11-5-4-10-29(30)37-31(39)22-40-19-16-33(17-20-40,23-36-32(41)38-28-9-3-2-8-27(28)34)26-14-12-24(13-15-26)25-7-6-18-35-21-25/h2-15,18,21H,16-17,19-20,22-23H2,1H3,(H2,36,38,41) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
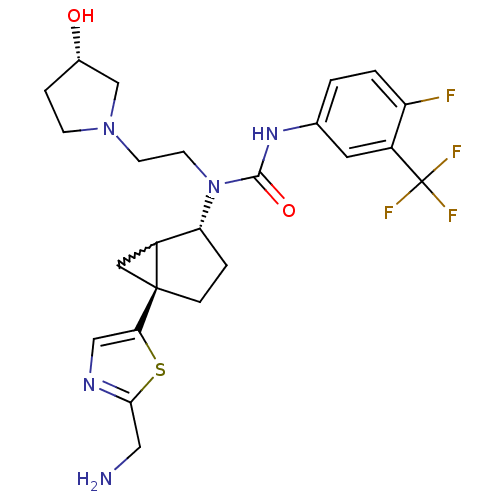
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50217069
(1-((2R,5S)-5-(2-(aminomethyl)thiazol-5-yl)bicyclo[...)Show SMILES NCc1ncc(s1)[C@@]12CC1[C@@H](CC2)N(CCN1CC[C@H](O)C1)C(=O)Nc1ccc(F)c(c1)C(F)(F)F |w:9.9| Show InChI InChI=1S/C24H29F4N5O2S/c25-18-2-1-14(9-16(18)24(26,27)28)31-22(35)33(8-7-32-6-4-15(34)13-32)19-3-5-23(10-17(19)23)20-12-30-21(11-29)36-20/h1-2,9,12,15,17,19,34H,3-8,10-11,13,29H2,(H,31,35)/t15-,17?,19+,23-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Antagonist activity at MCHR1 |
Bioorg Med Chem Lett 17: 4845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.048
BindingDB Entry DOI: 10.7270/Q2V69J9H |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50217064

(1-((2R,5S)-5-(2-(aminomethyl)thiazol-5-yl)bicyclo[...)Show SMILES NCc1ncc(s1)[C@@]12CC1[C@@H](CC2)N(CCN1CCCC1)C(=O)Nc1ccc(F)c(c1)C(F)(F)F |w:9.9| Show InChI InChI=1S/C24H29F4N5OS/c25-18-4-3-15(11-16(18)24(26,27)28)31-22(34)33(10-9-32-7-1-2-8-32)19-5-6-23(12-17(19)23)20-14-30-21(13-29)35-20/h3-4,11,14,17,19H,1-2,5-10,12-13,29H2,(H,31,34)/t17?,19-,23+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Antagonist activity at MCHR1 |
Bioorg Med Chem Lett 17: 4845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.048
BindingDB Entry DOI: 10.7270/Q2V69J9H |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50217065
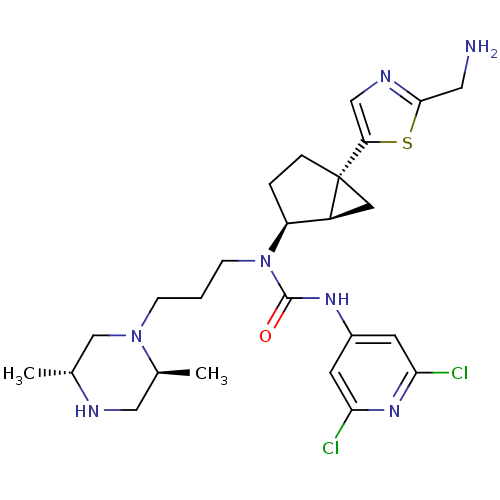
(1-((1R,2S,5R)-5-(2-(aminomethyl)thiazol-5-yl)bicyc...)Show SMILES C[C@@H]1CN(CCCN([C@H]2CC[C@]3(C[C@@H]23)c2cnc(CN)s2)C(=O)Nc2cc(Cl)nc(Cl)c2)[C@@H](C)CN1 Show InChI InChI=1S/C25H35Cl2N7OS/c1-15-14-33(16(2)12-29-15)6-3-7-34(24(35)31-17-8-21(26)32-22(27)9-17)19-4-5-25(10-18(19)25)20-13-30-23(11-28)36-20/h8-9,13,15-16,18-19,29H,3-7,10-12,14,28H2,1-2H3,(H,31,32,35)/t15-,16+,18+,19+,25-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Antagonist activity at MCHR1 |
Bioorg Med Chem Lett 17: 4845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.048
BindingDB Entry DOI: 10.7270/Q2V69J9H |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169397

(1-[4-(3'-Cyano-biphenyl-4-yl)-1-methyl-piperidin-4...)Show SMILES CN1CCC(CNC(=O)Nc2cc(Cl)ccc2Cl)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C27H26Cl2N4O/c1-33-13-11-27(12-14-33,18-31-26(34)32-25-16-23(28)9-10-24(25)29)22-7-5-20(6-8-22)21-4-2-3-19(15-21)17-30/h2-10,15-16H,11-14,18H2,1H3,(H2,31,32,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169378
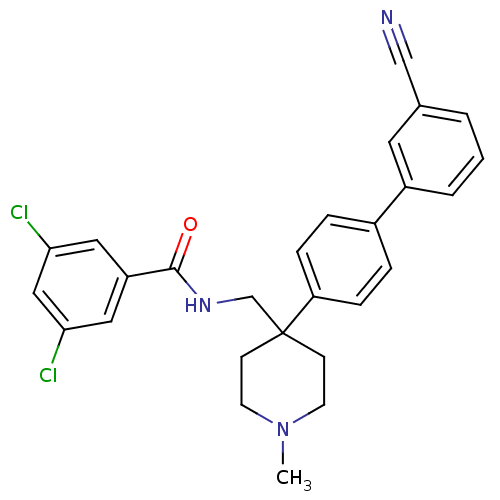
(3,5-Dichloro-N-[4-(3'-cyano-biphenyl-4-yl)-1-methy...)Show SMILES CN1CCC(CNC(=O)c2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C27H25Cl2N3O/c1-32-11-9-27(10-12-32,18-31-26(33)22-14-24(28)16-25(29)15-22)23-7-5-20(6-8-23)21-4-2-3-19(13-21)17-30/h2-8,13-16H,9-12,18H2,1H3,(H,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169383

(CHEMBL178549 | N-[4-(3'-Cyano-biphenyl-4-yl)-1-met...)Show SMILES CN1CCC(CNC(=O)Cc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C28H27Cl2N3O/c1-33-11-9-28(10-12-33,19-32-27(34)16-21-14-25(29)17-26(30)15-21)24-7-5-22(6-8-24)23-4-2-3-20(13-23)18-31/h2-8,13-15,17H,9-12,16,19H2,1H3,(H,32,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50217061
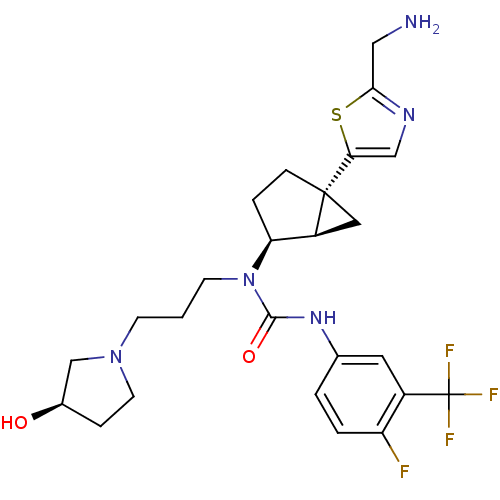
(1-((1R,2S,5R)-5-(2-(aminomethyl)thiazol-5-yl)bicyc...)Show SMILES NCc1ncc(s1)[C@]12C[C@H]1[C@H](CC2)N(CCCN1CC[C@@H](O)C1)C(=O)Nc1ccc(F)c(c1)C(F)(F)F Show InChI InChI=1S/C25H31F4N5O2S/c26-19-3-2-15(10-17(19)25(27,28)29)32-23(36)34(8-1-7-33-9-5-16(35)14-33)20-4-6-24(11-18(20)24)21-13-31-22(12-30)37-21/h2-3,10,13,16,18,20,35H,1,4-9,11-12,14,30H2,(H,32,36)/t16-,18+,20+,24-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Antagonist activity at MCHR1 |
Bioorg Med Chem Lett 17: 4845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.048
BindingDB Entry DOI: 10.7270/Q2V69J9H |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50217067

(1-((1R,2S,5R)-5-(2-(aminomethyl)thiazol-5-yl)bicyc...)Show SMILES C[C@H]1CN(CCCN([C@H]2CC[C@]3(C[C@@H]23)c2cnc(CN)s2)C(=O)Nc2cc(Cl)nc(Cl)c2)[C@H](C)CN1 Show InChI InChI=1S/C25H35Cl2N7OS/c1-15-14-33(16(2)12-29-15)6-3-7-34(24(35)31-17-8-21(26)32-22(27)9-17)19-4-5-25(10-18(19)25)20-13-30-23(11-28)36-20/h8-9,13,15-16,18-19,29H,3-7,10-12,14,28H2,1-2H3,(H,31,32,35)/t15-,16+,18-,19-,25+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 171 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Antagonist activity at MCHR1 |
Bioorg Med Chem Lett 17: 4845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.048
BindingDB Entry DOI: 10.7270/Q2V69J9H |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169369

(3,5-Dichloro-N-[4-(3'-cyano-biphenyl-4-yl)-1-methy...)Show SMILES CN1CCC(CNS(=O)(=O)c2cc(Cl)cc(Cl)c2)(CC1)c1ccc(cc1)-c1cccc(c1)C#N Show InChI InChI=1S/C26H25Cl2N3O2S/c1-31-11-9-26(10-12-31,18-30-34(32,33)25-15-23(27)14-24(28)16-25)22-7-5-20(6-8-22)21-4-2-3-19(13-21)17-29/h2-8,13-16,30H,9-12,18H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 281 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169385
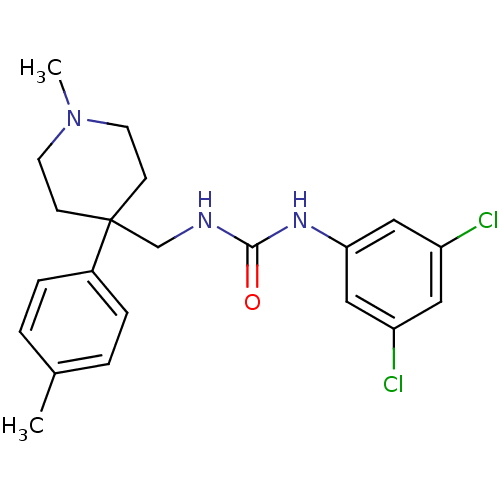
(1-(3,5-Dichloro-phenyl)-3-(1-methyl-4-p-tolyl-pipe...)Show SMILES CN1CCC(CNC(=O)Nc2cc(Cl)cc(Cl)c2)(CC1)c1ccc(C)cc1 Show InChI InChI=1S/C21H25Cl2N3O/c1-15-3-5-16(6-4-15)21(7-9-26(2)10-8-21)14-24-20(27)25-19-12-17(22)11-18(23)13-19/h3-6,11-13H,7-10,14H2,1-2H3,(H2,24,25,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50217059

(1-((3R,6S)-6-(5-(aminomethyl)thiazol-2-yl)bicyclo[...)Show SMILES NCc1cnc(s1)[C@@]12CC1C[C@@H](CC2)N(CCN1CCCC1)C(=O)Nc1ccc(F)c(c1)C(F)(F)F |w:9.9| Show InChI InChI=1S/C25H31F4N5OS/c26-21-4-3-17(12-20(21)25(27,28)29)32-23(35)34(10-9-33-7-1-2-8-33)18-5-6-24(13-16(24)11-18)22-31-15-19(14-30)36-22/h3-4,12,15-16,18H,1-2,5-11,13-14,30H2,(H,32,35)/t16?,18-,24+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 431 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute K15 2545
Curated by ChEMBL
| Assay Description
Antagonist activity at MCHR1 |
Bioorg Med Chem Lett 17: 4845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.048
BindingDB Entry DOI: 10.7270/Q2V69J9H |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50169375
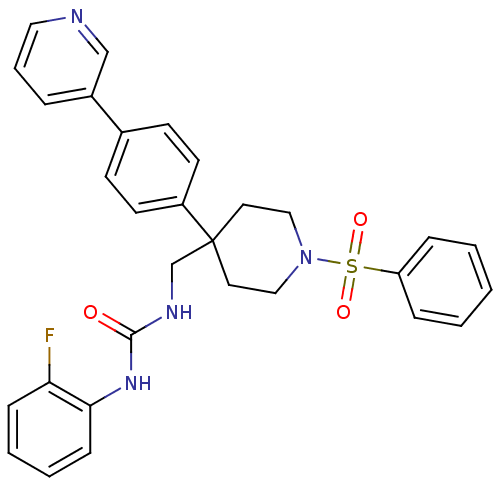
(1-[1-Benzenesulfonyl-4-(4-pyridin-3-yl-phenyl)-pip...)Show SMILES Fc1ccccc1NC(=O)NCC1(CCN(CC1)S(=O)(=O)c1ccccc1)c1ccc(cc1)-c1cccnc1 Show InChI InChI=1S/C30H29FN4O3S/c31-27-10-4-5-11-28(27)34-29(36)33-22-30(25-14-12-23(13-15-25)24-7-6-18-32-21-24)16-19-35(20-17-30)39(37,38)26-8-2-1-3-9-26/h1-15,18,21H,16-17,19-20,22H2,(H2,33,34,36) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Ability to displace [125-I]-MCH()0.5 nM from human MCH1R(2.5 uM) expressed in CHO cells |
Bioorg Med Chem Lett 15: 3696-700 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.085
BindingDB Entry DOI: 10.7270/Q2ZS2W16 |
More data for this
Ligand-Target Pair | |
Wee1-like protein kinase
(Homo sapiens (Human)) | CHEMBL5278196
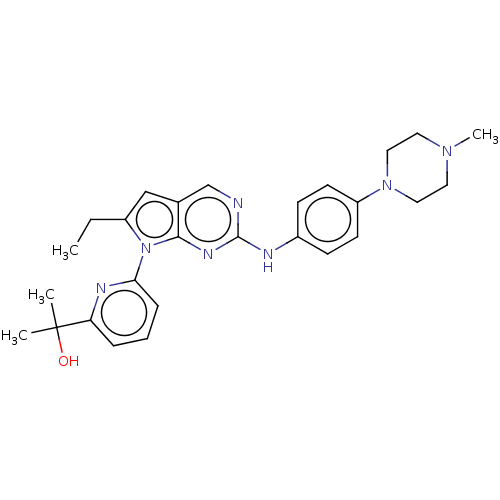
Show InChI InChI=1S/C13H14ClN5O3/c14-8-2-4-9(5-3-8)22-7-1-6-16-11-10(19-21)12(20)18-13(15)17-11/h2-5H,1,6-7H2,(H4,15,16,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of angiotensin converting enzyme isolated from rat lung. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Wee1-like protein kinase
(Homo sapiens (Human)) | CHEMBL5284925
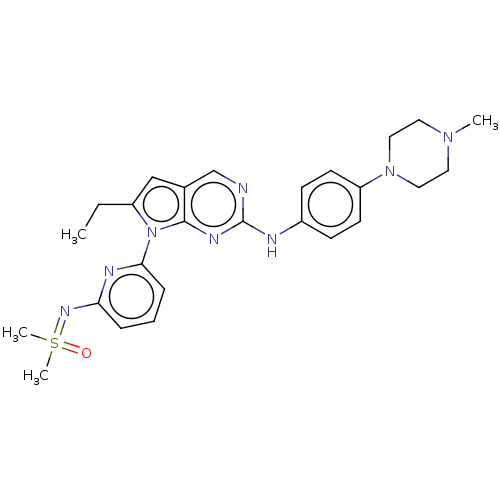
Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(cc1)-[#6](=O)-[#8]-c1ccccc1 Show InChI InChI=1S/C14H13N3O2/c15-14(16)17-11-8-6-10(7-9-11)13(18)19-12-4-2-1-3-5-12/h1-9H,(H4,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of angiotensin converting enzyme isolated from rat lung. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Wee1-like protein kinase
(Homo sapiens (Human)) | CHEMBL5279054

Show InChI InChI=1S/C13H15N5O4/c14-13-16-11(10(18-21)12(20)17-13)15-6-1-7-22-9-4-2-8(19)3-5-9/h2-5,19H,1,6-7H2,(H4,14,15,16,17,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of Neutral Endopeptidase isolated from rat kidney. |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Wee1-like protein kinase
(Homo sapiens (Human)) | CHEMBL5282382

Show InChI InChI=1S/C14H17N5O4/c1-22-9-3-5-10(6-4-9)23-8-2-7-16-12-11(19-21)13(20)18-14(15)17-12/h3-6H,2,7-8H2,1H3,(H4,15,16,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibition of Neutral Endopeptidase isolated from rat kidney. |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data