 Found 2451 hits with Last Name = 'qian' and Initial = 's'
Found 2451 hits with Last Name = 'qian' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM370555
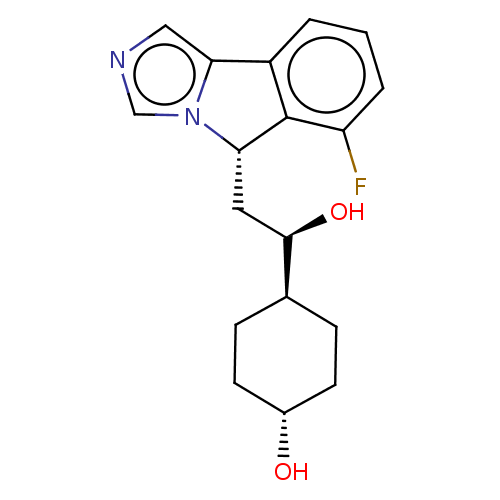
((1R,4r)-4-((R)-2-((S)-6-fluoro-5H-imidazo[5,1- a]i...)Show SMILES O[C@H](C[C@H]1c2c(cccc2F)-c2cncn12)[C@H]1CC[C@H](O)CC1 |r,wU:19.22,wD:3.2,1.0,16.19,(-.99,-3.03,;-.22,-1.7,;-.99,-.37,;-2.53,-.37,;-3.37,.92,;-4.85,.53,;-5.94,1.61,;-5.54,3.1,;-4.06,3.5,;-2.97,2.41,;-1.48,2.81,;-4.93,-1.01,;-5.9,-2.21,;-5.06,-3.5,;-3.58,-3.1,;-3.5,-1.56,;1.32,-1.7,;2.09,-3.03,;3.63,-3.03,;4.4,-1.7,;5.94,-1.7,;3.63,-.37,;2.09,-.37,)| Show InChI InChI=1S/C18H21FN2O2/c19-14-3-1-2-13-16-9-20-10-21(16)15(18(13)14)8-17(23)11-4-6-12(22)7-5-11/h1-3,9-12,15,17,22-23H,4-8H2/t11-,12-,15-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50606592

(CHEMBL5219838) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50200540

(CHEMBL3972619)Show InChI InChI=1S/C14H12BrN3O/c15-10-5-13(12-8-17-18-14(12)6-10)16-7-9-1-3-11(19)4-2-9/h1-6,8,16,19H,7H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50590775
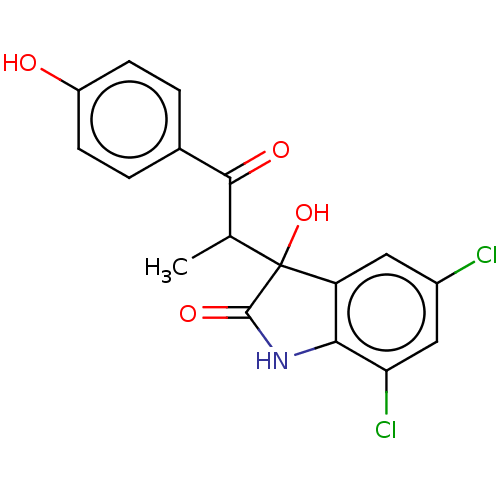
(CHEMBL1990305)Show SMILES CC(C(=O)c1ccc(O)cc1)C1(O)C(=O)Nc2c1cc(Cl)cc2Cl | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114625
BindingDB Entry DOI: 10.7270/Q2JW8JVD |
More data for this
Ligand-Target Pair | |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50590775

(CHEMBL1990305)Show SMILES CC(C(=O)c1ccc(O)cc1)C1(O)C(=O)Nc2c1cc(Cl)cc2Cl | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114625
BindingDB Entry DOI: 10.7270/Q2JW8JVD |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50200540

(CHEMBL3972619)Show InChI InChI=1S/C14H12BrN3O/c15-10-5-13(12-8-17-18-14(12)6-10)16-7-9-1-3-11(19)4-2-9/h1-6,8,16,19H,7H2,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
NAD-dependent protein deacylase sirtuin-5, mitochondrial
(Homo sapiens (Human)) | BDBM50574075

(CHEMBL4856582)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)CNCc1ccccc1)C(C)C)C(=O)N[C@@H](CCCCNC(=O)CCC(C)(C(O)=O)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](C(C)C)C(N)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SIRT5 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113803
BindingDB Entry DOI: 10.7270/Q2XG9VZ4 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50606592

(CHEMBL5219838) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tryptophan 2,3-dioxygenase
(Homo sapiens (Human)) | BDBM50350248
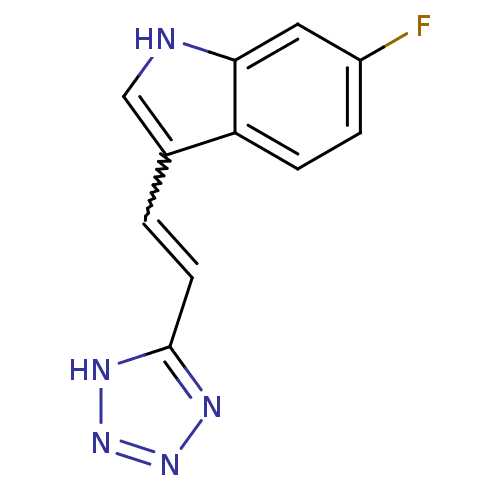
(CHEMBL1812545)Show InChI InChI=1S/C11H8FN5/c12-8-2-3-9-7(6-13-10(9)5-8)1-4-11-14-16-17-15-11/h1-6,13H,(H,14,15,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xihua University
Curated by ChEMBL
| Assay Description
Inhibition of TDO (unknown origin) |
Bioorg Med Chem 27: 1087-1098 (2019)
Article DOI: 10.1016/j.bmc.2019.02.014
BindingDB Entry DOI: 10.7270/Q2G73J3G |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50207089
(D-1-Methyltryptophan | D-1MT | Indoximod)Show InChI InChI=1S/C12H14N2O2/c1-14-7-8(6-10(13)12(15)16)9-4-2-3-5-11(9)14/h2-5,7,10H,6,13H2,1H3,(H,15,16)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00303
BindingDB Entry DOI: 10.7270/Q2474G0K |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50207089

(D-1-Methyltryptophan | D-1MT | Indoximod)Show InChI InChI=1S/C12H14N2O2/c1-14-7-8(6-10(13)12(15)16)9-4-2-3-5-11(9)14/h2-5,7,10H,6,13H2,1H3,(H,15,16)/t10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114625
BindingDB Entry DOI: 10.7270/Q2JW8JVD |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245192
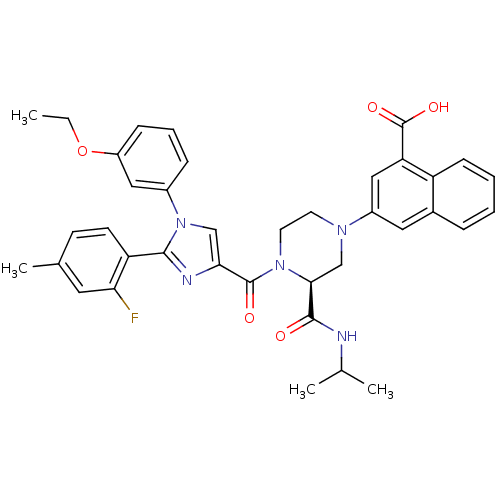
(3-((S)-4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylp...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1C(=O)NC(C)C)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C38H38FN5O5/c1-5-49-28-11-8-10-26(19-28)44-21-33(41-35(44)30-14-13-24(4)17-32(30)39)37(46)43-16-15-42(22-34(43)36(45)40-23(2)3)27-18-25-9-6-7-12-29(25)31(20-27)38(47)48/h6-14,17-21,23,34H,5,15-16,22H2,1-4H3,(H,40,45)(H,47,48)/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245186
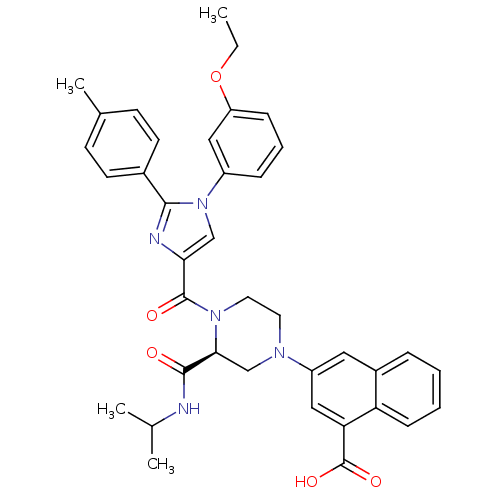
(3-((S)-4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1C(=O)NC(C)C)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C38H39N5O5/c1-5-48-30-11-8-10-28(20-30)43-22-33(40-35(43)26-15-13-25(4)14-16-26)37(45)42-18-17-41(23-34(42)36(44)39-24(2)3)29-19-27-9-6-7-12-31(27)32(21-29)38(46)47/h6-16,19-22,24,34H,5,17-18,23H2,1-4H3,(H,39,44)(H,46,47)/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245205
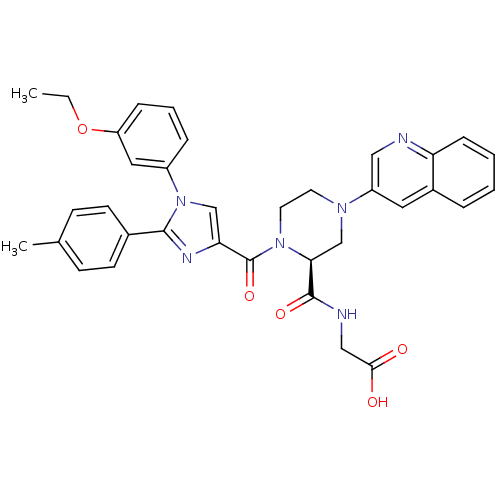
(2-((S)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1C(=O)NCC(O)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H34N6O5/c1-3-46-28-9-6-8-26(18-28)41-21-30(38-33(41)24-13-11-23(2)12-14-24)35(45)40-16-15-39(22-31(40)34(44)37-20-32(42)43)27-17-25-7-4-5-10-29(25)36-19-27/h4-14,17-19,21,31H,3,15-16,20,22H2,1-2H3,(H,37,44)(H,42,43)/t31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263230
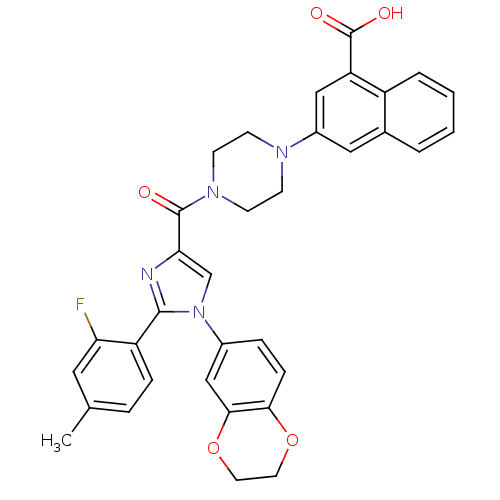
(3-(4-(1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-(2...)Show SMILES Cc1ccc(-c2nc(cn2-c2ccc3OCCOc3c2)C(=O)N2CCN(CC2)c2cc(C(O)=O)c3ccccc3c2)c(F)c1 Show InChI InChI=1S/C34H29FN4O5/c1-21-6-8-26(28(35)16-21)32-36-29(20-39(32)23-7-9-30-31(19-23)44-15-14-43-30)33(40)38-12-10-37(11-13-38)24-17-22-4-2-3-5-25(22)27(18-24)34(41)42/h2-9,16-20H,10-15H2,1H3,(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263229
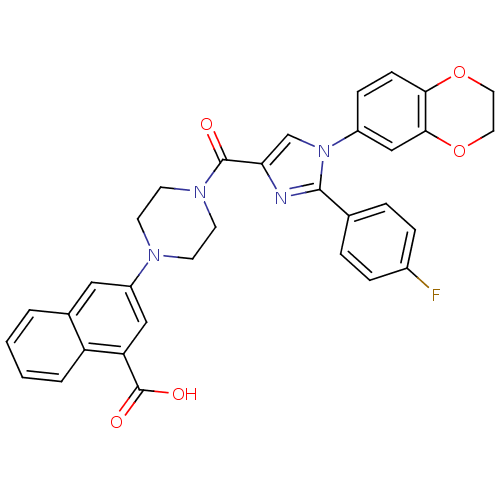
(3-(4-(1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-(4...)Show SMILES OC(=O)c1cc(cc2ccccc12)N1CCN(CC1)C(=O)c1cn(c(n1)-c1ccc(F)cc1)-c1ccc2OCCOc2c1 Show InChI InChI=1S/C33H27FN4O5/c34-23-7-5-21(6-8-23)31-35-28(20-38(31)24-9-10-29-30(19-24)43-16-15-42-29)32(39)37-13-11-36(12-14-37)25-17-22-3-1-2-4-26(22)27(18-25)33(40)41/h1-10,17-20H,11-16H2,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50343722
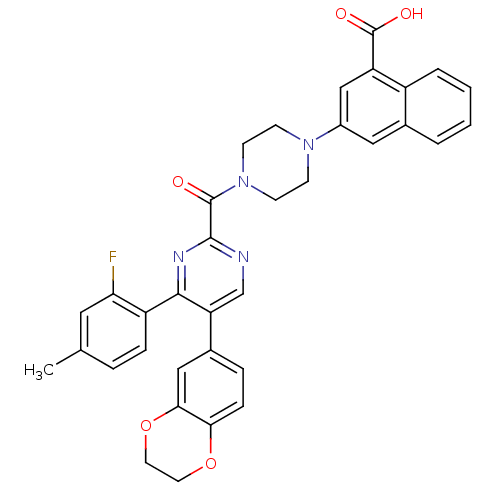
(3-(4-(5-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-4-(2...)Show SMILES Cc1ccc(c(F)c1)-c1nc(ncc1-c1ccc2OCCOc2c1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C35H29FN4O5/c1-21-6-8-26(29(36)16-21)32-28(23-7-9-30-31(18-23)45-15-14-44-30)20-37-33(38-32)34(41)40-12-10-39(11-13-40)24-17-22-4-2-3-5-25(22)27(19-24)35(42)43/h2-9,16-20H,10-15H2,1H3,(H,42,43) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 21: 2911-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.069
BindingDB Entry DOI: 10.7270/Q2TB177X |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245195
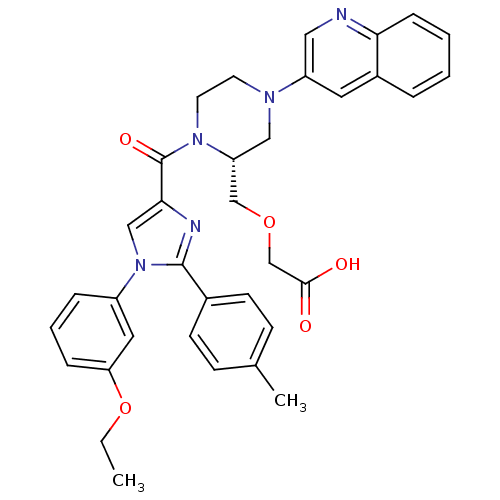
(2-(((R)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazo...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1COCC(O)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H35N5O5/c1-3-45-30-9-6-8-27(18-30)40-21-32(37-34(40)25-13-11-24(2)12-14-25)35(43)39-16-15-38(20-29(39)22-44-23-33(41)42)28-17-26-7-4-5-10-31(26)36-19-28/h4-14,17-19,21,29H,3,15-16,20,22-23H2,1-2H3,(H,41,42)/t29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245187

(3-(4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylpheny...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H31FN4O4/c1-3-43-26-9-6-8-24(19-26)39-21-31(36-32(39)28-12-11-22(2)17-30(28)35)33(40)38-15-13-37(14-16-38)25-18-23-7-4-5-10-27(23)29(20-25)34(41)42/h4-12,17-21H,3,13-16H2,1-2H3,(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245188
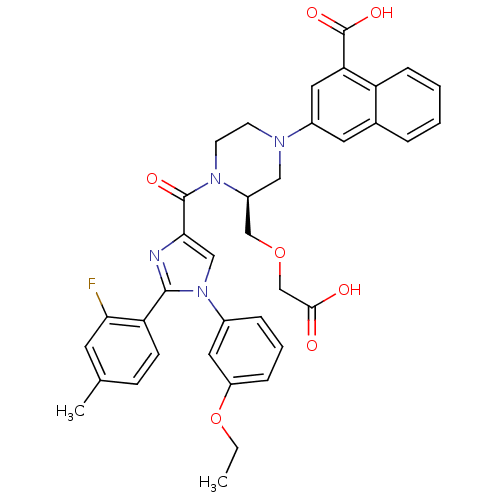
(3-((S)-3-((carboxymethoxy)methyl)-4-(1-(3-ethoxyph...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1COCC(O)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H35FN4O7/c1-3-49-28-9-6-8-25(17-28)42-20-33(39-35(42)30-12-11-23(2)15-32(30)38)36(45)41-14-13-40(19-27(41)21-48-22-34(43)44)26-16-24-7-4-5-10-29(24)31(18-26)37(46)47/h4-12,15-18,20,27H,3,13-14,19,21-22H2,1-2H3,(H,43,44)(H,46,47)/t27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245201
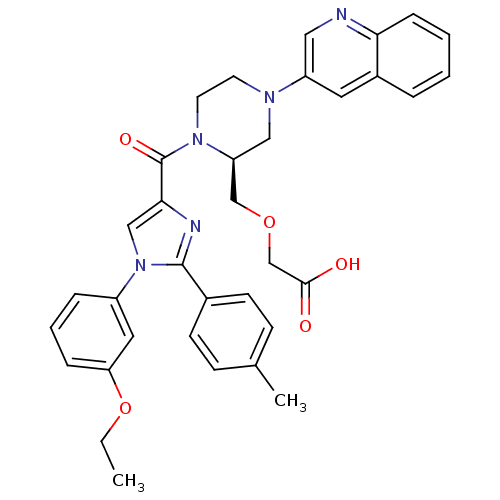
(3-(((S)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazo...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1COCC(O)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H35N5O5/c1-3-45-30-9-6-8-27(18-30)40-21-32(37-34(40)25-13-11-24(2)12-14-25)35(43)39-16-15-38(20-29(39)22-44-23-33(41)42)28-17-26-7-4-5-10-31(26)36-19-28/h4-14,17-19,21,29H,3,15-16,20,22-23H2,1-2H3,(H,41,42)/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245189

(3-((R)-3-(acetamidomethyl)-4-(1-(3-ethoxyphenyl)-2...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1CNC(C)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H36FN5O5/c1-4-48-29-10-7-9-26(18-29)43-22-34(40-35(43)31-13-12-23(2)16-33(31)38)36(45)42-15-14-41(21-28(42)20-39-24(3)44)27-17-25-8-5-6-11-30(25)32(19-27)37(46)47/h5-13,16-19,22,28H,4,14-15,20-21H2,1-3H3,(H,39,44)(H,46,47)/t28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245187

(3-(4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylpheny...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H31FN4O4/c1-3-43-26-9-6-8-24(19-26)39-21-31(36-32(39)28-12-11-22(2)17-30(28)35)33(40)38-15-13-37(14-16-38)25-18-23-7-4-5-10-27(23)29(20-25)34(41)42/h4-12,17-21H,3,13-16H2,1-2H3,(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245190
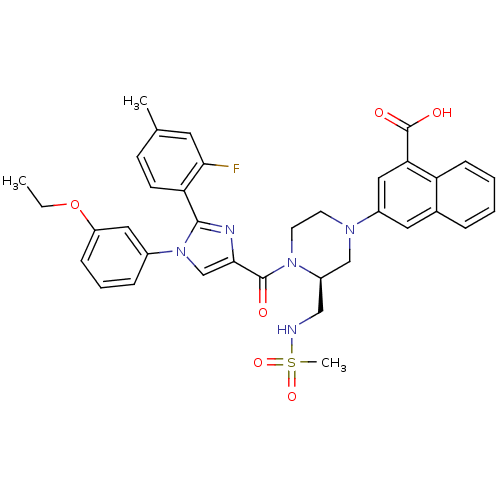
(3-((S)-4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylp...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1CNS(C)(=O)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C36H36FN5O6S/c1-4-48-28-10-7-9-25(18-28)42-22-33(39-34(42)30-13-12-23(2)16-32(30)37)35(43)41-15-14-40(21-27(41)20-38-49(3,46)47)26-17-24-8-5-6-11-29(24)31(19-26)36(44)45/h5-13,16-19,22,27,38H,4,14-15,20-21H2,1-3H3,(H,44,45)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263227

(3-(4-(2-(2,4-difluorophenyl)-1-(3-ethoxyphenyl)-1H...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(F)cc1F)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C33H28F2N4O4/c1-2-43-25-8-5-7-23(18-25)39-20-30(36-31(39)27-11-10-22(34)17-29(27)35)32(40)38-14-12-37(13-15-38)24-16-21-6-3-4-9-26(21)28(19-24)33(41)42/h3-11,16-20H,2,12-15H2,1H3,(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245184
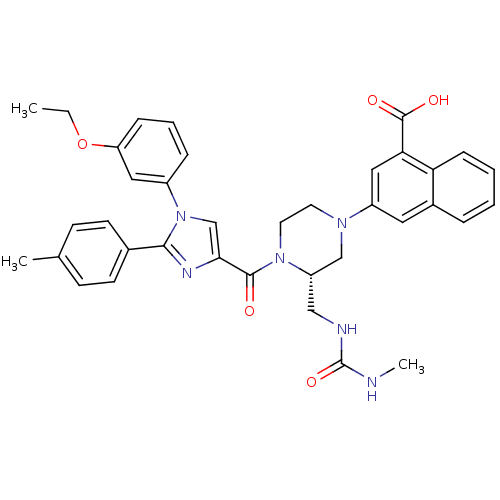
(3-((S)-4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNC(=O)NC)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H38N6O5/c1-4-48-30-10-7-9-27(19-30)43-23-33(40-34(43)25-14-12-24(2)13-15-25)35(44)42-17-16-41(22-29(42)21-39-37(47)38-3)28-18-26-8-5-6-11-31(26)32(20-28)36(45)46/h5-15,18-20,23,29H,4,16-17,21-22H2,1-3H3,(H,45,46)(H2,38,39,47)/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245182

(3-((S)-3-(acetamidomethyl)-4-(1-(3-ethoxyphenyl)-2...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNC(C)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H37N5O5/c1-4-47-31-10-7-9-28(19-31)42-23-34(39-35(42)26-14-12-24(2)13-15-26)36(44)41-17-16-40(22-30(41)21-38-25(3)43)29-18-27-8-5-6-11-32(27)33(20-29)37(45)46/h5-15,18-20,23,30H,4,16-17,21-22H2,1-3H3,(H,38,43)(H,45,46)/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263226
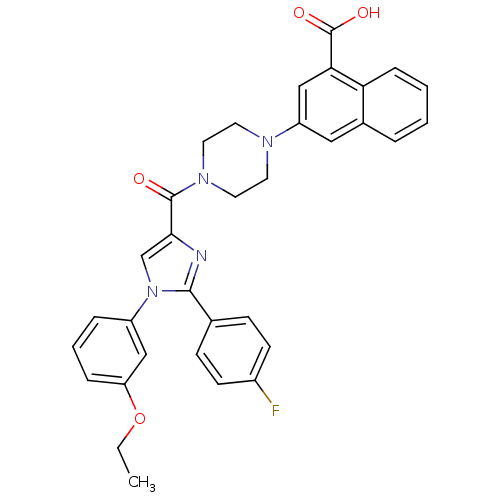
(3-(4-(1-(3-ethoxyphenyl)-2-(4-fluorophenyl)-1H-imi...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(F)cc1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C33H29FN4O4/c1-2-42-27-8-5-7-25(19-27)38-21-30(35-31(38)22-10-12-24(34)13-11-22)32(39)37-16-14-36(15-17-37)26-18-23-6-3-4-9-28(23)29(20-26)33(40)41/h3-13,18-21H,2,14-17H2,1H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245191
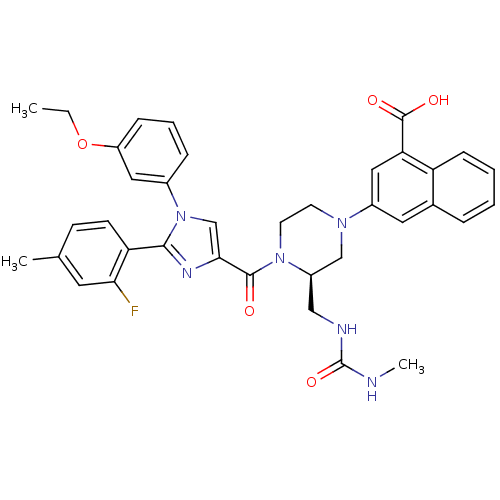
(3-((R)-4-(1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylp...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(C[C@H]1CNC(=O)NC)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H37FN6O5/c1-4-49-28-10-7-9-25(18-28)44-22-33(41-34(44)30-13-12-23(2)16-32(30)38)35(45)43-15-14-42(21-27(43)20-40-37(48)39-3)26-17-24-8-5-6-11-29(24)31(19-26)36(46)47/h5-13,16-19,22,27H,4,14-15,20-21H2,1-3H3,(H,46,47)(H2,39,40,48)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245185

(3-((R)-4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1C(=O)NC(C)C)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C38H39N5O5/c1-5-48-30-11-8-10-28(20-30)43-22-33(40-35(43)26-15-13-25(4)14-16-26)37(45)42-18-17-41(23-34(42)36(44)39-24(2)3)29-19-27-9-6-7-12-31(27)32(21-29)38(46)47/h6-16,19-22,24,34H,5,17-18,23H2,1-4H3,(H,39,44)(H,46,47)/t34-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245180
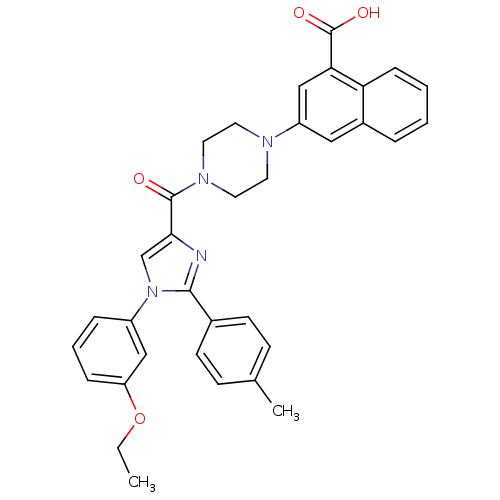
(3-(4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazole-4-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H32N4O4/c1-3-42-28-9-6-8-26(20-28)38-22-31(35-32(38)24-13-11-23(2)12-14-24)33(39)37-17-15-36(16-18-37)27-19-25-7-4-5-10-29(25)30(21-27)34(40)41/h4-14,19-22H,3,15-18H2,1-2H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263225
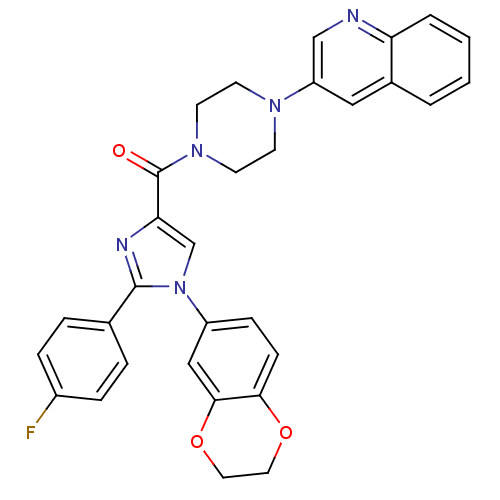
((1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-(4-fluo...)Show SMILES Fc1ccc(cc1)-c1nc(cn1-c1ccc2OCCOc2c1)C(=O)N1CCN(CC1)c1cnc2ccccc2c1 Show InChI InChI=1S/C31H26FN5O3/c32-23-7-5-21(6-8-23)30-34-27(20-37(30)24-9-10-28-29(18-24)40-16-15-39-28)31(38)36-13-11-35(12-14-36)25-17-22-3-1-2-4-26(22)33-19-25/h1-10,17-20H,11-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245180

(3-(4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazole-4-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H32N4O4/c1-3-42-28-9-6-8-26(20-28)38-22-31(35-32(38)24-13-11-23(2)12-14-24)33(39)37-17-15-36(16-18-37)27-19-25-7-4-5-10-29(25)30(21-27)34(40)41/h4-14,19-22H,3,15-18H2,1-2H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245183
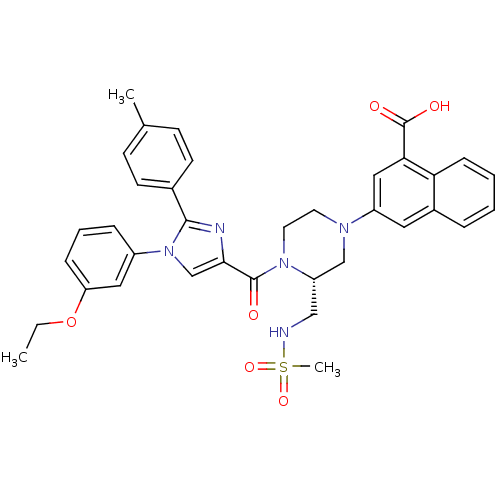
(3-((R)-4-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNS(C)(=O)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C36H37N5O6S/c1-4-47-30-10-7-9-27(19-30)41-23-33(38-34(41)25-14-12-24(2)13-15-25)35(42)40-17-16-39(22-29(40)21-37-48(3,45)46)28-18-26-8-5-6-11-31(26)32(20-28)36(43)44/h5-15,18-20,23,29,37H,4,16-17,21-22H2,1-3H3,(H,43,44)/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262862

(3-(4-(1-(3-methoxyphenyl)-2-p-tolyl-1H-imidazole-4...)Show SMILES COc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C33H30N4O4/c1-22-10-12-23(13-11-22)31-34-30(21-37(31)25-7-5-8-27(19-25)41-2)32(38)36-16-14-35(15-17-36)26-18-24-6-3-4-9-28(24)29(20-26)33(39)40/h3-13,18-21H,14-17H2,1-2H3,(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263228
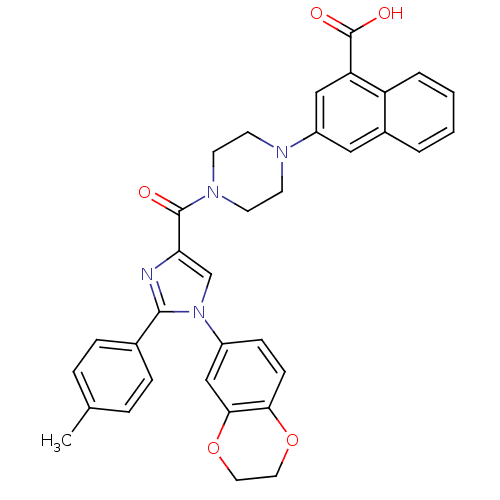
(3-(4-(1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-p-...)Show SMILES Cc1ccc(cc1)-c1nc(cn1-c1ccc2OCCOc2c1)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H30N4O5/c1-22-6-8-23(9-7-22)32-35-29(21-38(32)25-10-11-30-31(20-25)43-17-16-42-30)33(39)37-14-12-36(13-15-37)26-18-24-4-2-3-5-27(24)28(19-26)34(40)41/h2-11,18-21H,12-17H2,1H3,(H,40,41) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245181
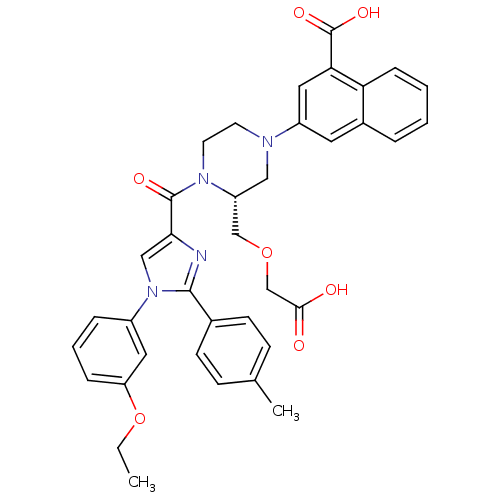
(3-((R)-3-((carboxymethoxy)methyl)-4-(1-(3-ethoxyph...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1COCC(O)=O)c1cc(C(O)=O)c2ccccc2c1 |r| Show InChI InChI=1S/C37H36N4O7/c1-3-48-30-9-6-8-27(18-30)41-21-33(38-35(41)25-13-11-24(2)12-14-25)36(44)40-16-15-39(20-29(40)22-47-23-34(42)43)28-17-26-7-4-5-10-31(26)32(19-28)37(45)46/h4-14,17-19,21,29H,3,15-16,20,22-23H2,1-2H3,(H,42,43)(H,45,46)/t29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50343703
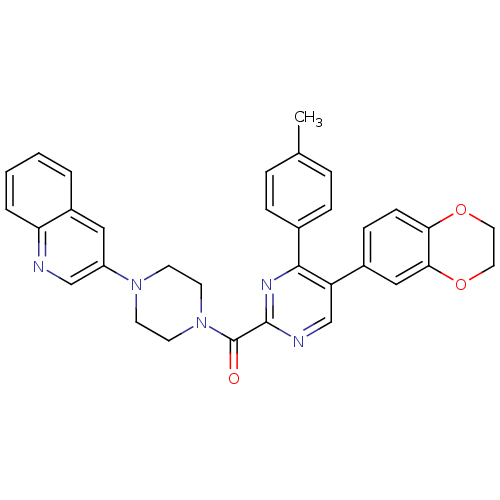
((5-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-4-p-tolyl...)Show SMILES Cc1ccc(cc1)-c1nc(ncc1-c1ccc2OCCOc2c1)C(=O)N1CCN(CC1)c1cnc2ccccc2c1 Show InChI InChI=1S/C33H29N5O3/c1-22-6-8-23(9-7-22)31-27(24-10-11-29-30(19-24)41-17-16-40-29)21-35-32(36-31)33(39)38-14-12-37(13-15-38)26-18-25-4-2-3-5-28(25)34-20-26/h2-11,18-21H,12-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 21: 2911-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.069
BindingDB Entry DOI: 10.7270/Q2TB177X |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263186
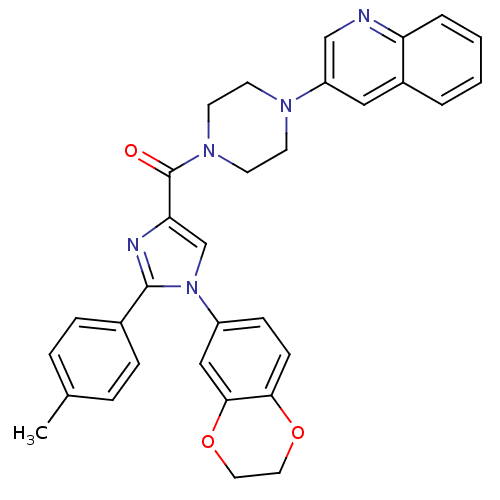
((1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-p-tolyl...)Show SMILES Cc1ccc(cc1)-c1nc(cn1-c1ccc2OCCOc2c1)C(=O)N1CCN(CC1)c1cnc2ccccc2c1 Show InChI InChI=1S/C32H29N5O3/c1-22-6-8-23(9-7-22)31-34-28(21-37(31)25-10-11-29-30(19-25)40-17-16-39-29)32(38)36-14-12-35(13-15-36)26-18-24-4-2-3-5-27(24)33-20-26/h2-11,18-21H,12-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245199
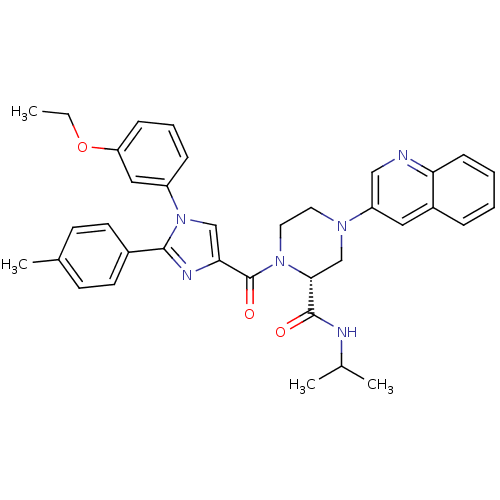
((2R)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazole-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1C(=O)NC(C)C)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C36H38N6O3/c1-5-45-30-11-8-10-28(20-30)42-22-32(39-34(42)26-15-13-25(4)14-16-26)36(44)41-18-17-40(23-33(41)35(43)38-24(2)3)29-19-27-9-6-7-12-31(27)37-21-29/h6-16,19-22,24,33H,5,17-18,23H2,1-4H3,(H,38,43)/t33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50343721
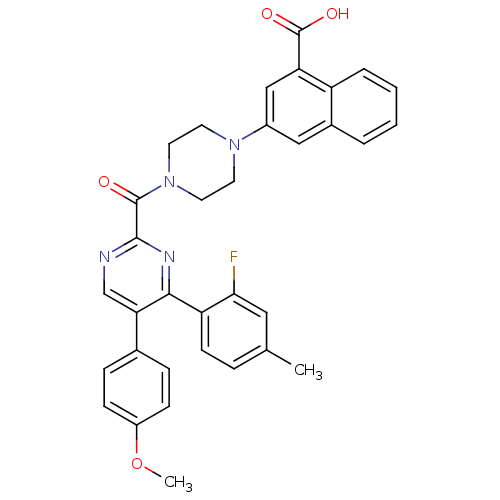
(3-(4-(4-(2-fluoro-4-methylphenyl)-5-(4-methoxyphen...)Show SMILES COc1ccc(cc1)-c1cnc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(CC1)c1cc(C(O)=O)c2ccccc2c1 Show InChI InChI=1S/C34H29FN4O4/c1-21-7-12-27(30(35)17-21)31-29(22-8-10-25(43-2)11-9-22)20-36-32(37-31)33(40)39-15-13-38(14-16-39)24-18-23-5-3-4-6-26(23)28(19-24)34(41)42/h3-12,17-20H,13-16H2,1-2H3,(H,41,42) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 21: 2911-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.069
BindingDB Entry DOI: 10.7270/Q2TB177X |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245204

((2S)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazole-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1C(=O)NC(C)C)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C36H38N6O3/c1-5-45-30-11-8-10-28(20-30)42-22-32(39-34(42)26-15-13-25(4)14-16-26)36(44)41-18-17-40(23-33(41)35(43)38-24(2)3)29-19-27-9-6-7-12-31(27)37-21-29/h6-16,19-22,24,33H,5,17-18,23H2,1-4H3,(H,38,43)/t33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50263185
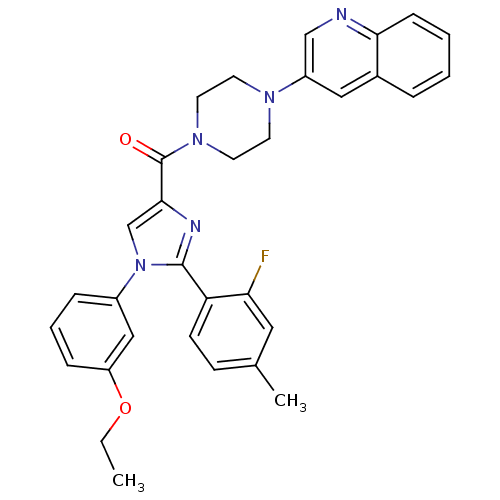
((1-(3-ethoxyphenyl)-2-(2-fluoro-4-methylphenyl)-1H...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(CC1)c1cnc2ccccc2c1 Show InChI InChI=1S/C32H30FN5O2/c1-3-40-26-9-6-8-24(19-26)38-21-30(35-31(38)27-12-11-22(2)17-28(27)33)32(39)37-15-13-36(14-16-37)25-18-23-7-4-5-10-29(23)34-20-25/h4-12,17-21H,3,13-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245194
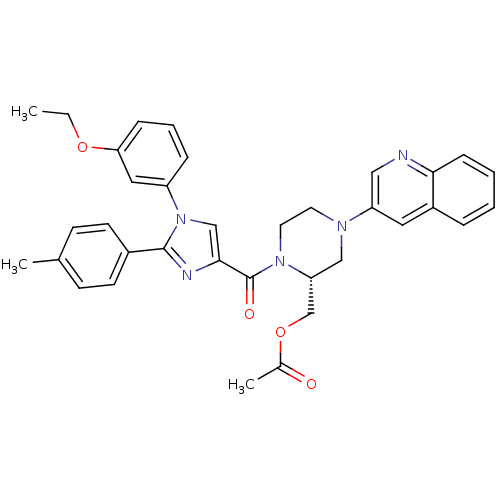
(((R)-1-(1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazole-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1COC(C)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H35N5O4/c1-4-43-31-10-7-9-28(19-31)40-22-33(37-34(40)26-14-12-24(2)13-15-26)35(42)39-17-16-38(21-30(39)23-44-25(3)41)29-18-27-8-5-6-11-32(27)36-20-29/h5-15,18-20,22,30H,4,16-17,21,23H2,1-3H3/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245196
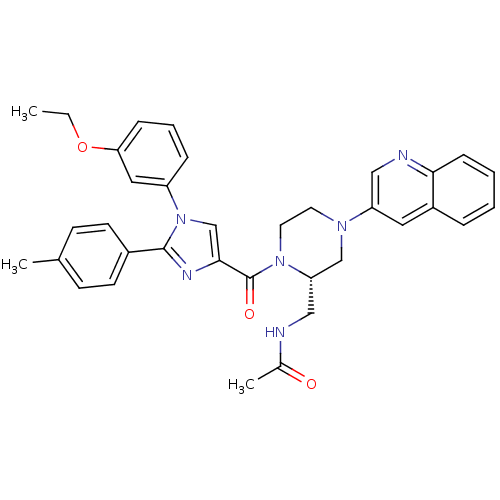
(CHEMBL450443 | N-(((S)-1-(1-(3-ethoxyphenyl)-2-p-t...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNC(C)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H36N6O3/c1-4-44-31-10-7-9-28(19-31)41-23-33(38-34(41)26-14-12-24(2)13-15-26)35(43)40-17-16-39(22-30(40)21-36-25(3)42)29-18-27-8-5-6-11-32(27)37-20-29/h5-15,18-20,23,30H,4,16-17,21-22H2,1-3H3,(H,36,42)/t30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50343711
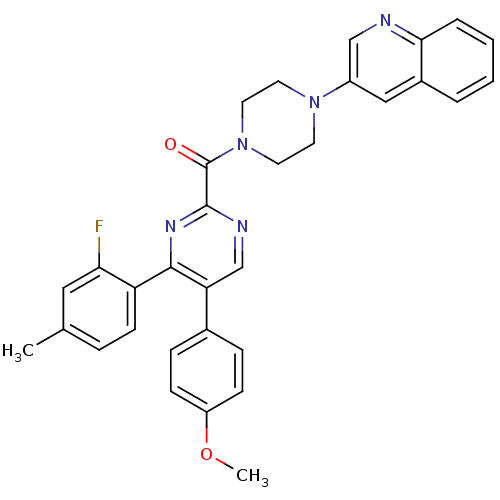
((4-(2-fluoro-4-methylphenyl)-5-(4-methoxyphenyl)py...)Show SMILES COc1ccc(cc1)-c1cnc(nc1-c1ccc(C)cc1F)C(=O)N1CCN(CC1)c1cnc2ccccc2c1 Show InChI InChI=1S/C32H28FN5O2/c1-21-7-12-26(28(33)17-21)30-27(22-8-10-25(40-2)11-9-22)20-35-31(36-30)32(39)38-15-13-37(14-16-38)24-18-23-5-3-4-6-29(23)34-19-24/h3-12,17-20H,13-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Inc.
Curated by ChEMBL
| Assay Description
Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation counting |
Bioorg Med Chem Lett 21: 2911-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.069
BindingDB Entry DOI: 10.7270/Q2TB177X |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245202
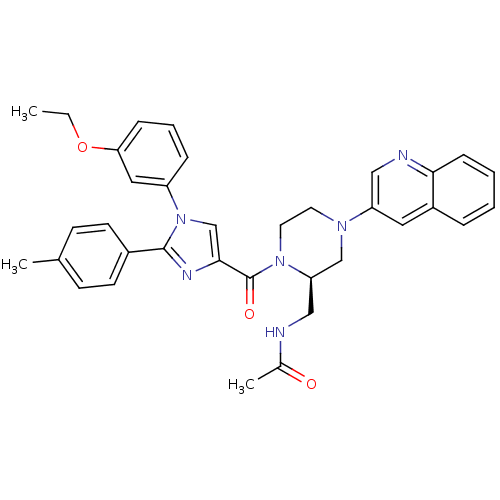
(CHEMBL503331 | N-(((R)-1-(1-(3-ethoxyphenyl)-2-p-t...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@H]1CNC(C)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C35H36N6O3/c1-4-44-31-10-7-9-28(19-31)41-23-33(38-34(41)26-14-12-24(2)13-15-26)35(43)40-17-16-39(22-30(40)21-36-25(3)42)29-18-27-8-5-6-11-32(27)37-20-29/h5-15,18-20,23,30H,4,16-17,21-22H2,1-3H3,(H,36,42)/t30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50262973
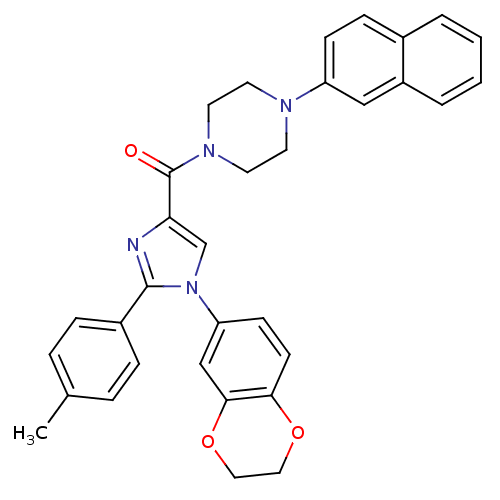
((1-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-2-p-tolyl...)Show SMILES Cc1ccc(cc1)-c1nc(cn1-c1ccc2OCCOc2c1)C(=O)N1CCN(CC1)c1ccc2ccccc2c1 Show InChI InChI=1S/C33H30N4O3/c1-23-6-8-25(9-7-23)32-34-29(22-37(32)28-12-13-30-31(21-28)40-19-18-39-30)33(38)36-16-14-35(15-17-36)27-11-10-24-4-2-3-5-26(24)20-27/h2-13,20-22H,14-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245197
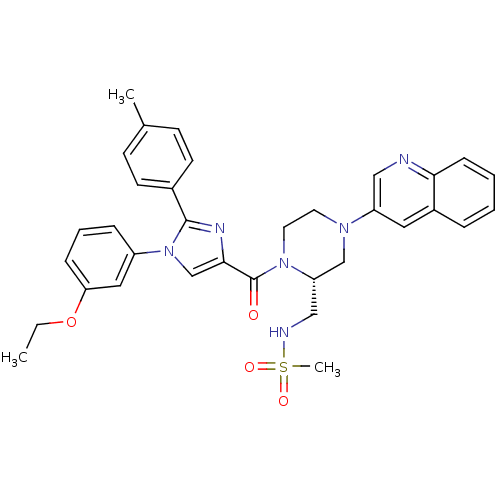
(CHEMBL449725 | N-(((R)-1-(1-(3-ethoxyphenyl)-2-p-t...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(C[C@@H]1CNS(C)(=O)=O)c1cnc2ccccc2c1 |r| Show InChI InChI=1S/C34H36N6O4S/c1-4-44-30-10-7-9-27(19-30)40-23-32(37-33(40)25-14-12-24(2)13-15-25)34(41)39-17-16-38(22-29(39)21-36-45(3,42)43)28-18-26-8-5-6-11-31(26)35-20-28/h5-15,18-20,23,29,36H,4,16-17,21-22H2,1-3H3/t29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4833-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.083
BindingDB Entry DOI: 10.7270/Q2RJ4J94 |
More data for this
Ligand-Target Pair | |
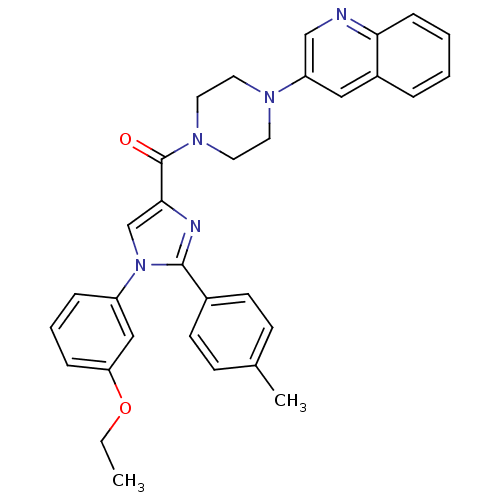
Cholecystokinin receptor type A
(Homo sapiens (Human)) | BDBM50245193
((1-(3-ethoxyphenyl)-2-p-tolyl-1H-imidazol-4-yl)(4-...)Show SMILES CCOc1cccc(c1)-n1cc(nc1-c1ccc(C)cc1)C(=O)N1CCN(CC1)c1cnc2ccccc2c1 Show InChI InChI=1S/C32H31N5O2/c1-3-39-28-9-6-8-26(20-28)37-22-30(34-31(37)24-13-11-23(2)12-14-24)32(38)36-17-15-35(16-18-36)27-19-25-7-4-5-10-29(25)33-21-27/h4-14,19-22H,3,15-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human CCK1 receptor |
Bioorg Med Chem Lett 18: 4393-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.057
BindingDB Entry DOI: 10.7270/Q2HH6JW0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data