

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50004178 (Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.00130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]nociceptin from human recombinant NOP receptor expressed in HEK293 cells by scintillation counting analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00256 BindingDB Entry DOI: 10.7270/Q2VX0MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50098576 (5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Human cathepsin K | J Med Chem 44: 1380-95 (2001) BindingDB Entry DOI: 10.7270/Q2QR4WDC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19770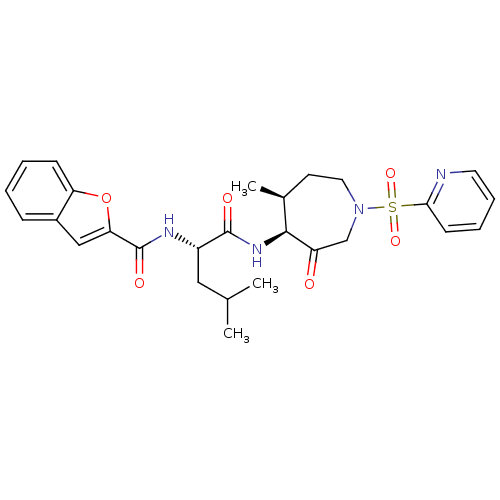 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00990 | -62.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Neuropeptide FF receptor 1 (Homo sapiens (Human)) | BDBM50029188 (CHEMBL2165920) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-1DMeNPFF from recombinant human NPFF1 receptor expressed in CHO cells by TopCount scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00256 BindingDB Entry DOI: 10.7270/Q2VX0MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide FF receptor 2 (Homo sapiens (Human)) | BDBM50037557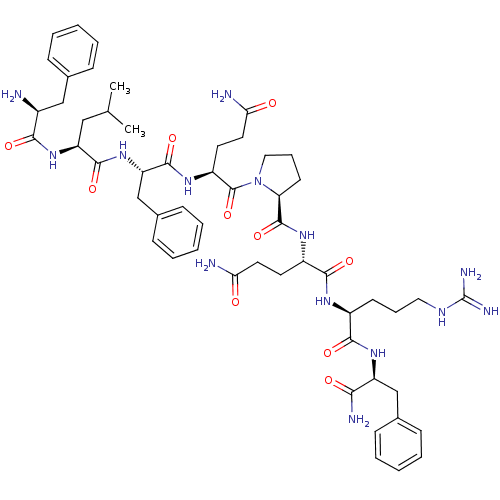 (2-{[1-(2-{2-[2-(2-Amino-3-phenyl-propionylamino)-4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-1DMeNPFF from recombinant human NPFF2 receptor expressed in CHO cells by TopCount scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00256 BindingDB Entry DOI: 10.7270/Q2VX0MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50451113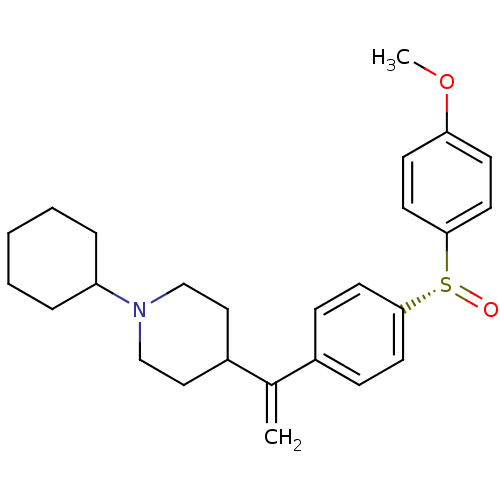 (CHEMBL2114068) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2209-12 (2001) BindingDB Entry DOI: 10.7270/Q24T6HMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50110539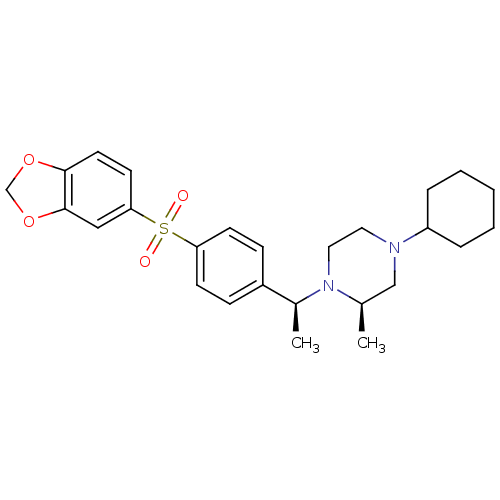 (1-{1-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against human cloned Muscarinic acetylcholine receptor M2. | Bioorg Med Chem Lett 12: 791-4 (2002) BindingDB Entry DOI: 10.7270/Q2VH5N5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50110523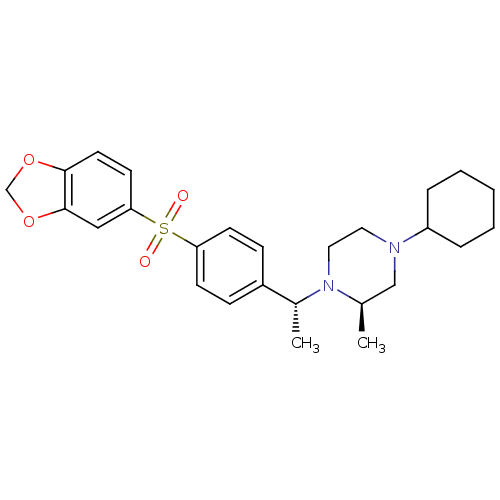 (1-{1-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against human cloned Muscarinic acetylcholine receptor M2. | Bioorg Med Chem Lett 12: 791-4 (2002) BindingDB Entry DOI: 10.7270/Q2VH5N5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19770 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50110534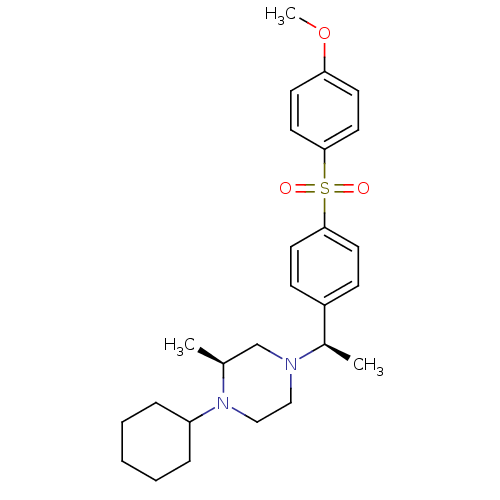 (1-Cyclohexyl-4-{1-[4-(4-methoxy-benzenesulfonyl)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against human cloned Muscarinic acetylcholine receptor M2. | Bioorg Med Chem Lett 12: 791-4 (2002) BindingDB Entry DOI: 10.7270/Q2VH5N5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0410 | -58.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50451114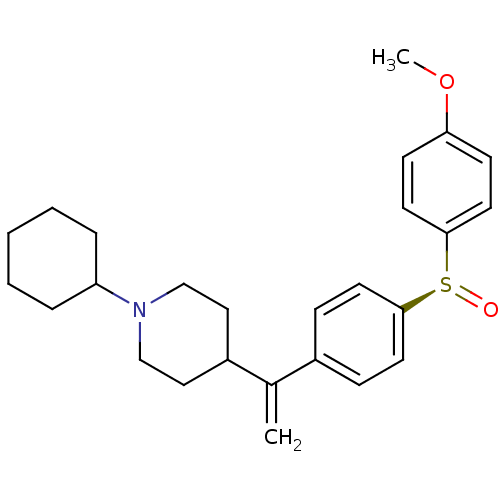 (CHEMBL2115128) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2209-12 (2001) BindingDB Entry DOI: 10.7270/Q24T6HMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide FF receptor 1 (Homo sapiens (Human)) | BDBM50037557 (2-{[1-(2-{2-[2-(2-Amino-3-phenyl-propionylamino)-4...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-1DMeNPFF from recombinant human NPFF1 receptor expressed in CHO cells by TopCount scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00256 BindingDB Entry DOI: 10.7270/Q2VX0MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| KiSS-1 receptor (Homo sapiens (Human)) | BDBM50045513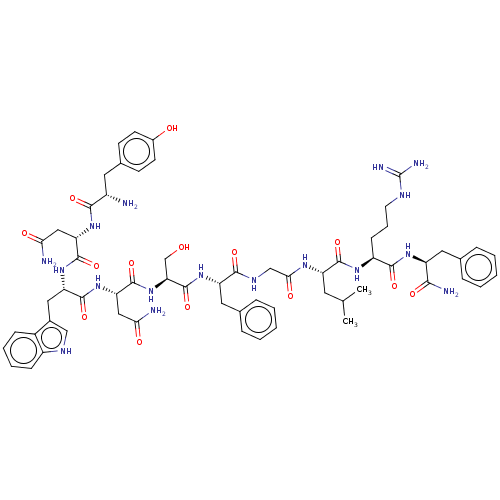 (Kisspeptin-10) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [125I]-Kp-10 from human Kiss1 receptor by TopCount scintillation counting method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00256 BindingDB Entry DOI: 10.7270/Q2VX0MDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin L2 (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0680 | -57.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50092313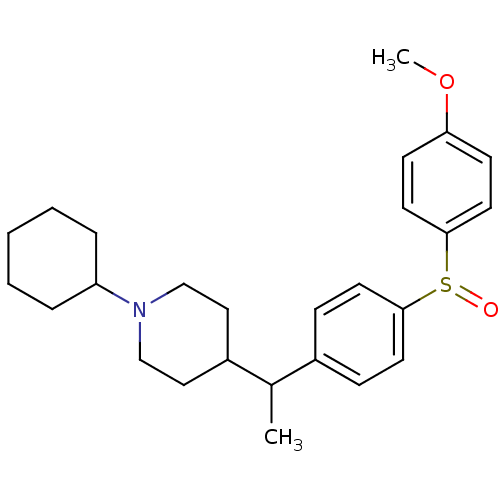 (1-Cyclohexyl-4-{1-[4-(4-methoxy-benzenesulfinyl)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M4 | Bioorg Med Chem Lett 10: 2209-12 (2001) BindingDB Entry DOI: 10.7270/Q24T6HMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50038096 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of squalene synthase in rat liver. | Bioorg Med Chem Lett 3: 2029-2034 (1993) Article DOI: 10.1016/S0960-894X(01)81008-8 BindingDB Entry DOI: 10.7270/Q22J6BSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50451113 (CHEMBL2114068) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M4 | Bioorg Med Chem Lett 10: 2209-12 (2001) BindingDB Entry DOI: 10.7270/Q24T6HMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50092959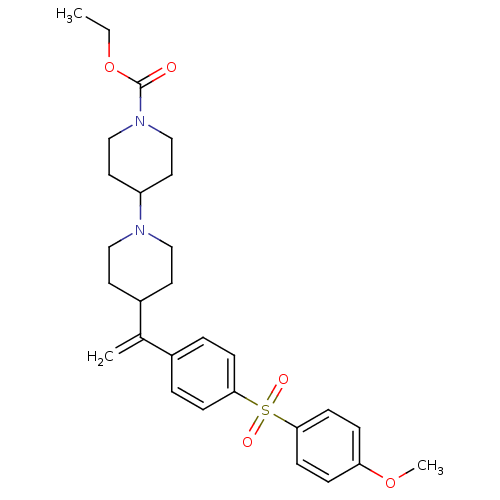 (4-{1-[4-(4-Methoxy-benzenesulfonyl)-phenyl]-vinyl}...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M2 stably expressed in CHO-K1 cells. | Bioorg Med Chem Lett 10: 2247-50 (2001) BindingDB Entry DOI: 10.7270/Q2Z60NBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM29525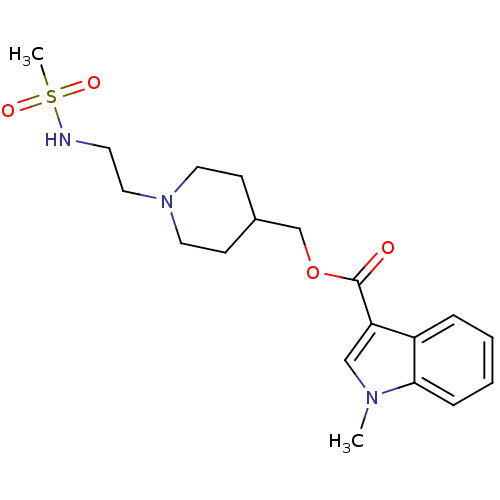 (3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
FAES, S.A. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 4 receptor in guinea pig striatum using [3H]GR-113808 as radioligand | J Med Chem 42: 2870-80 (1999) Article DOI: 10.1021/jm981098j BindingDB Entry DOI: 10.7270/Q24X58GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50092959 (4-{1-[4-(4-Methoxy-benzenesulfonyl)-phenyl]-vinyl}...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Muscarinic acetylcholine receptor M2 stably expressed in CHO-K1 cells using [3H]-QNB as radioligand | Bioorg Med Chem Lett 11: 891-4 (2001) BindingDB Entry DOI: 10.7270/Q2SN087R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50451113 (CHEMBL2114068) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M3 | Bioorg Med Chem Lett 10: 2209-12 (2001) BindingDB Entry DOI: 10.7270/Q24T6HMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19775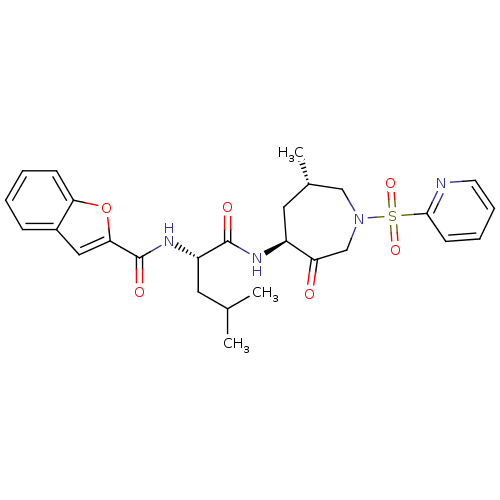 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | -55.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50092313 (1-Cyclohexyl-4-{1-[4-(4-methoxy-benzenesulfinyl)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2209-12 (2001) BindingDB Entry DOI: 10.7270/Q24T6HMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50110537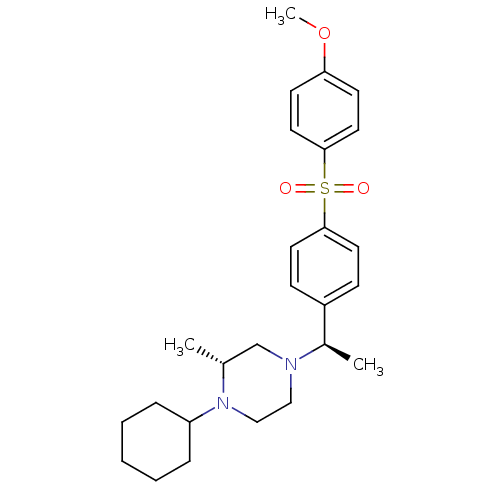 (1-Cyclohexyl-4-{1-[4-(4-methoxy-benzenesulfonyl)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against human cloned Muscarinic acetylcholine receptor M2. | Bioorg Med Chem Lett 12: 791-4 (2002) BindingDB Entry DOI: 10.7270/Q2VH5N5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50110538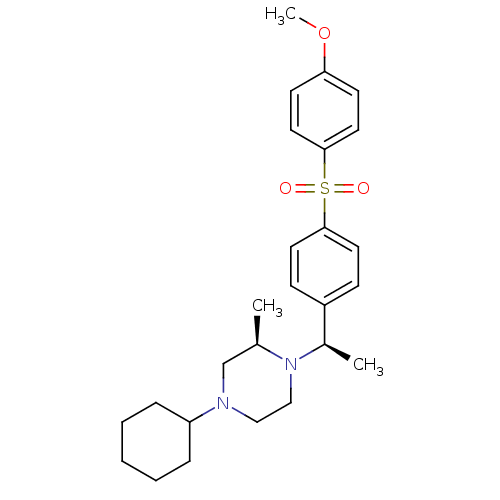 (4-Cyclohexyl-1-{1-[4-(4-methoxy-benzenesulfonyl)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against human cloned Muscarinic acetylcholine receptor M2. | Bioorg Med Chem Lett 12: 791-4 (2002) BindingDB Entry DOI: 10.7270/Q2VH5N5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50092961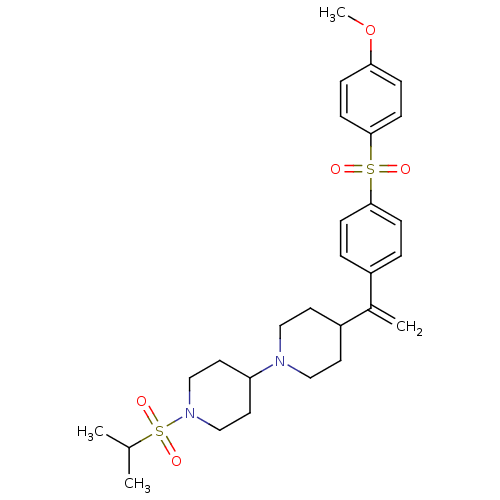 (1-(isopropylsulfonyl)-4-(4-(1-(4-(4-methoxyphenyls...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M2 stably expressed in CHO-K1 cells. | Bioorg Med Chem Lett 10: 2247-50 (2001) BindingDB Entry DOI: 10.7270/Q2Z60NBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Apparent inhibitory constant against human cathepsin K | J Med Chem 48: 6870-8 (2005) Article DOI: 10.1021/jm0502079 BindingDB Entry DOI: 10.7270/Q2WM1CZC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50092313 (1-Cyclohexyl-4-{1-[4-(4-methoxy-benzenesulfinyl)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M3 | Bioorg Med Chem Lett 10: 2209-12 (2001) BindingDB Entry DOI: 10.7270/Q24T6HMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Human cathepsin K | J Med Chem 44: 1380-95 (2001) BindingDB Entry DOI: 10.7270/Q2QR4WDC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50110536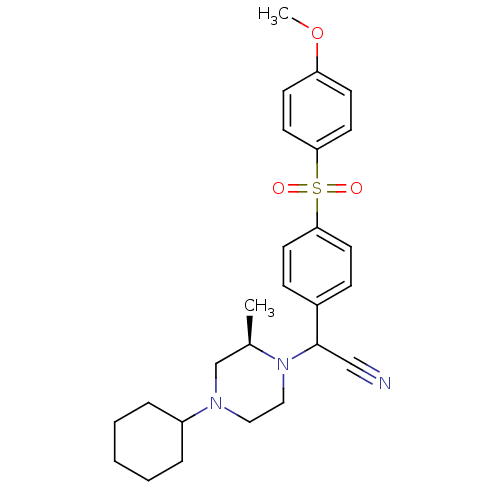 ((4-Cyclohexyl-2-methyl-piperazin-1-yl)-[4-(4-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Compound was tested for its binding affinity against cloned human Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 12: 791-4 (2002) BindingDB Entry DOI: 10.7270/Q2VH5N5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50092321 (1-Cyclohexyl-4-{1-[4-(4-methoxy-benzenesulfonyl)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2209-12 (2001) BindingDB Entry DOI: 10.7270/Q24T6HMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50092321 (1-Cyclohexyl-4-{1-[4-(4-methoxy-benzenesulfonyl)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M2 stably expressed in CHO-K1 cells. | Bioorg Med Chem Lett 10: 2247-50 (2001) BindingDB Entry DOI: 10.7270/Q2Z60NBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50148325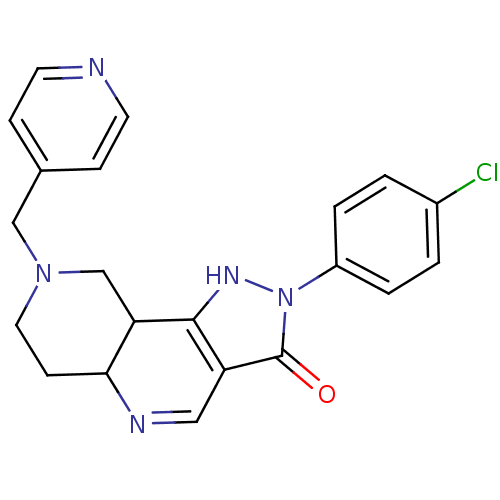 (2-(4-Chloro-phenyl)-8-pyridin-4-ylmethyl-2,5,5a,6,...) | GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity against Gamma-aminobutyric acid A receptor, alpha 3 expressed in L(tk) cells by displacement of [3H]-Ro-15-1788 | Bioorg Med Chem Lett 14: 3441-4 (2004) Article DOI: 10.1016/j.bmcl.2004.04.085 BindingDB Entry DOI: 10.7270/Q2NP23VM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50162192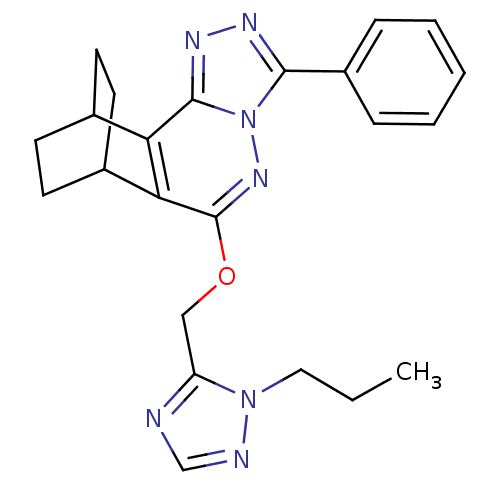 (6-phenyl-4,5,7,8-tetraazatetracyclo[9.2.2.02,10.03...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-Ro-15-1788 binding to recombinant human gamma-aminobutyric-acid A receptor alpha5-beta3-gamma2 subtype expressed in L (tk-) cells | J Med Chem 48: 1367-83 (2005) Article DOI: 10.1021/jm040883v BindingDB Entry DOI: 10.7270/Q2ZW1KD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50148320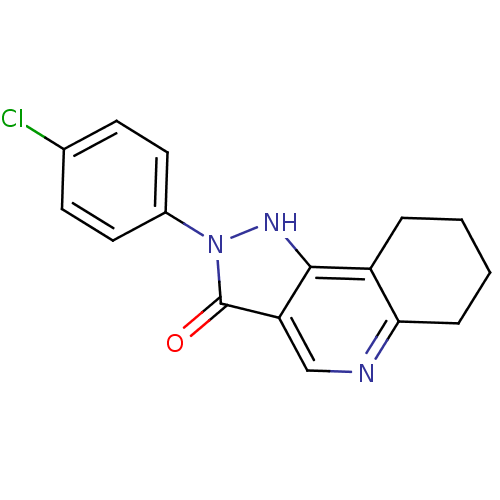 (2-(4-Chloro-phenyl)-2,5,6,7,8,9-hexahydro-pyrazolo...) | GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity against gamma-aminobutyric acid A receptor, alpha 1 expressed in L(tk) cells by displacement of [3H]-Ro-15-1788 | Bioorg Med Chem Lett 14: 3441-4 (2004) Article DOI: 10.1016/j.bmcl.2004.04.085 BindingDB Entry DOI: 10.7270/Q2NP23VM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50098086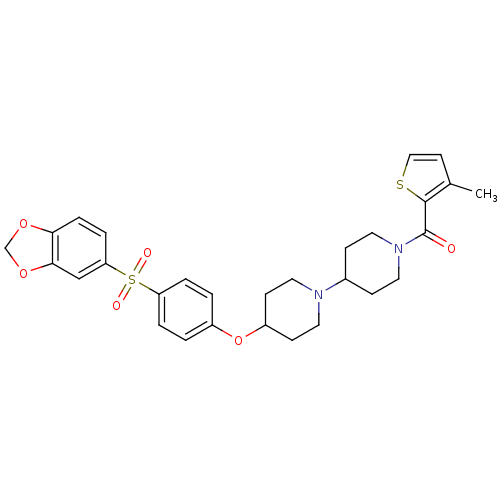 (CHEMBL174070 | {4-[4-(Benzo[1,3]dioxole-5-sulfonyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards Muscarinic acetylcholine receptor M2 stably expressed in CHO-K1 cells using [3H]-QNB as radioligand | Bioorg Med Chem Lett 11: 891-4 (2001) BindingDB Entry DOI: 10.7270/Q2SN087R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50092989 (1-[1-(4-Benzenesulfonyl-phenyl)-ethyl]-4-cyclohexy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity towards the cloned human Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 10: 2255-7 (2001) BindingDB Entry DOI: 10.7270/Q2TD9WMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50162207 (3,5-dimethyl-2-pyridyl 6-phenyl-4,5,7,8-tetraazate...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-Ro-15-1788 binding to recombinant human gamma-aminobutyric-acid A receptor alpha5-beta3-gamma2 subtype expressed in L (tk-) cells | J Med Chem 48: 1367-83 (2005) Article DOI: 10.1021/jm040883v BindingDB Entry DOI: 10.7270/Q2ZW1KD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376226 (CHEMBL259922) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant p38alpha-mediated myelin basic protein phosphorylation | Bioorg Med Chem Lett 18: 2652-7 (2008) Article DOI: 10.1016/j.bmcl.2008.03.019 BindingDB Entry DOI: 10.7270/Q2C53MR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50110529 (1-{1-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against human cloned Muscarinic acetylcholine receptor M2. | Bioorg Med Chem Lett 12: 791-4 (2002) BindingDB Entry DOI: 10.7270/Q2VH5N5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50376226 (CHEMBL259922) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant p38alpha-mediated myelin basic protein phosphorylation | Bioorg Med Chem Lett 18: 2652-7 (2008) Article DOI: 10.1016/j.bmcl.2008.03.019 BindingDB Entry DOI: 10.7270/Q2C53MR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50110528 (1-{1-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against human cloned Muscarinic acetylcholine receptor M2. | Bioorg Med Chem Lett 12: 791-4 (2002) BindingDB Entry DOI: 10.7270/Q2VH5N5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50162183 (1-benzyl-1H-2-imidazolyl[6-phenyl-4,5,7,8-tetraaza...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-Ro-15-1788 binding to recombinant human gamma-aminobutyric-acid A receptor alpha5-beta3-gamma2 subtype expressed in L (tk-) cells | J Med Chem 48: 1367-83 (2005) Article DOI: 10.1021/jm040883v BindingDB Entry DOI: 10.7270/Q2ZW1KD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (Homo sapiens (Human)) | BDBM50318494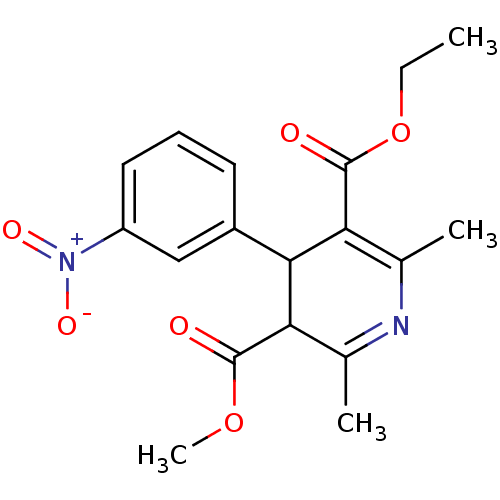 (3-ethyl 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to L-type calcium channel dihydropyridine site of porcine cardiac sarcolemma membrane vesicles | J Med Chem 30: 690-5 (1987) BindingDB Entry DOI: 10.7270/Q2BC404T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50092969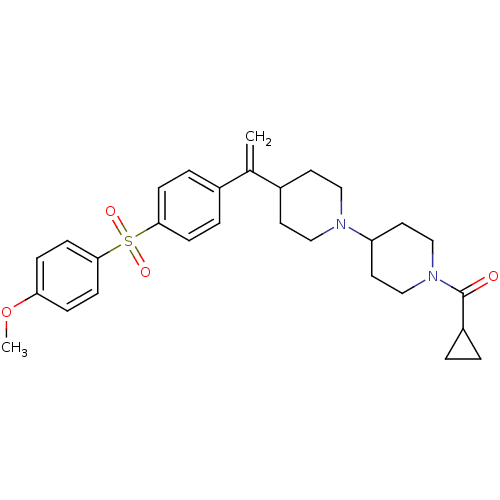 (CHEMBL74117 | Cyclopropyl-(4-{1-[4-(4-methoxy-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor M2 stably expressed in CHO-K1 cells. | Bioorg Med Chem Lett 10: 2247-50 (2001) BindingDB Entry DOI: 10.7270/Q2Z60NBQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50162232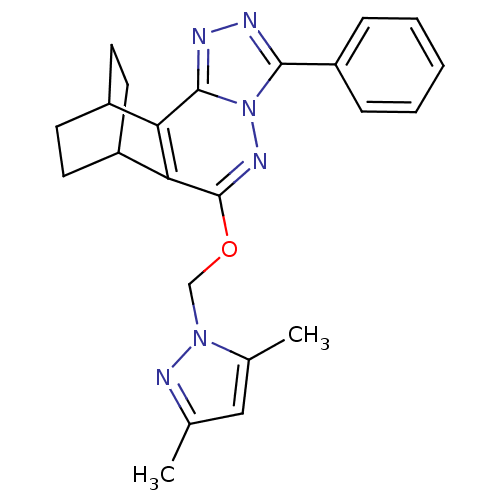 (3,5-dimethyl-1H-1-pyrazolyl[6-phenyl-4,5,7,8-tetra...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-Ro-15-1788 binding to recombinant human gamma-aminobutyric-acid A receptor alpha5-beta3-gamma2 subtype expressed in L (tk-) cells | J Med Chem 48: 1367-83 (2005) Article DOI: 10.1021/jm040883v BindingDB Entry DOI: 10.7270/Q2ZW1KD2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50084650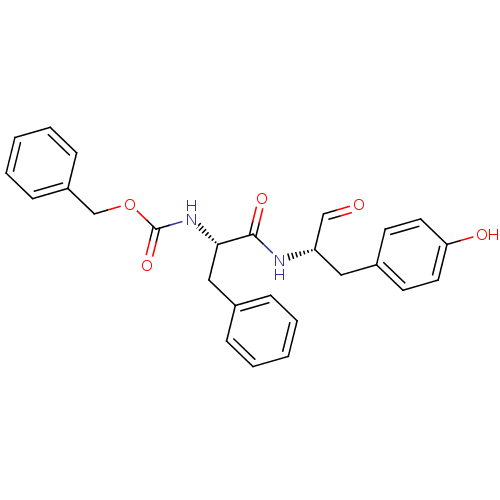 (CHEMBL177914 | {(S)-1-[(S)-1-Formyl-2-(4-hydroxy-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Apparent inhibitory constant against human cathepsin S | J Med Chem 48: 6870-8 (2005) Article DOI: 10.1021/jm0502079 BindingDB Entry DOI: 10.7270/Q2WM1CZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 9265 total ) | Next | Last >> |