 Found 4258 hits with Last Name = 'quan' and Initial = 'ml'
Found 4258 hits with Last Name = 'quan' and Initial = 'ml' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096105
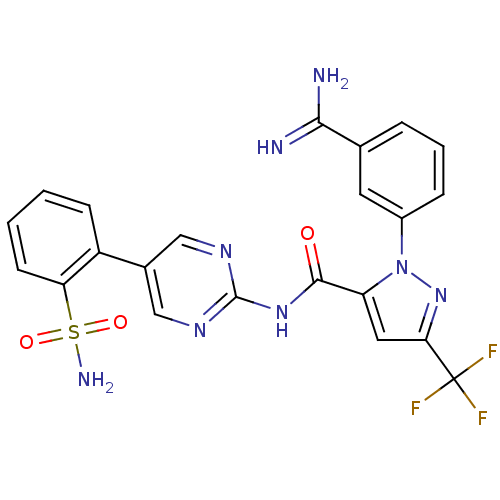
(2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...)Show SMILES NC(=N)c1cccc(c1)-n1nc(cc1C(=O)Nc1ncc(cn1)-c1ccccc1S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C22H17F3N8O3S/c23-22(24,25)18-9-16(33(32-18)14-5-3-4-12(8-14)19(26)27)20(34)31-21-29-10-13(11-30-21)15-6-1-2-7-17(15)37(28,35)36/h1-11H,(H3,26,27)(H2,28,35,36)(H,29,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096099
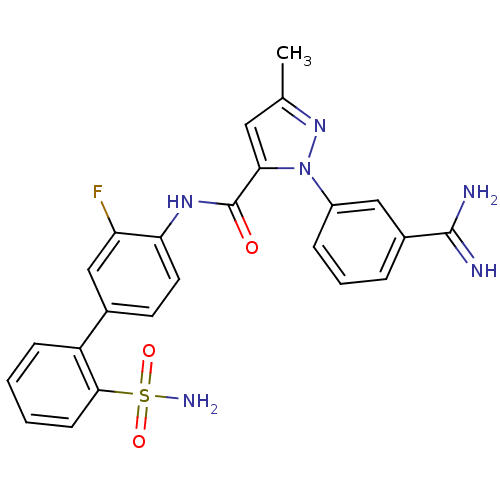
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H21FN6O3S/c1-14-11-21(31(30-14)17-6-4-5-16(12-17)23(26)27)24(32)29-20-10-9-15(13-19(20)25)18-7-2-3-8-22(18)35(28,33)34/h2-13H,1H3,(H3,26,27)(H,29,32)(H2,28,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096101
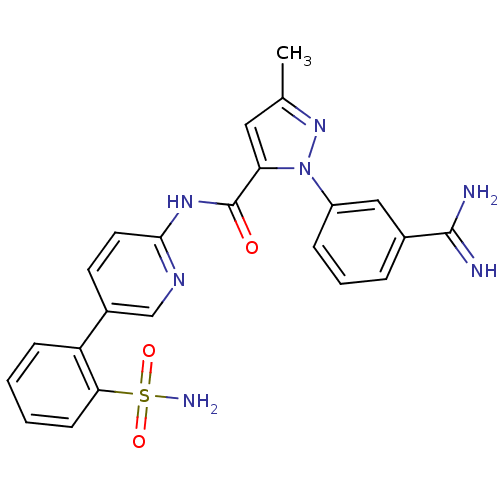
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cn2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C23H21N7O3S/c1-14-11-19(30(29-14)17-6-4-5-15(12-17)22(24)25)23(31)28-21-10-9-16(13-27-21)18-7-2-3-8-20(18)34(26,32)33/h2-13H,1H3,(H3,24,25)(H2,26,32,33)(H,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096110
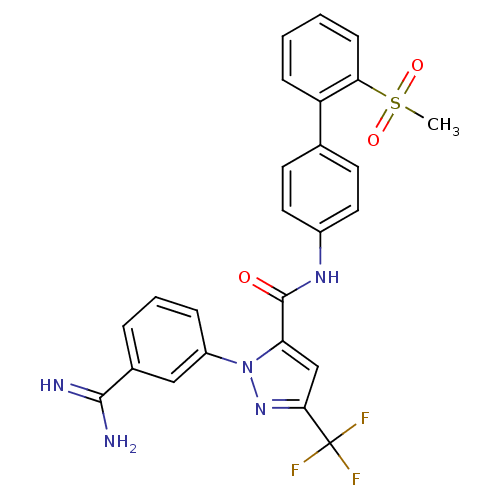
(2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)c2cc(nn2-c2cccc(c2)C(N)=N)C(F)(F)F)cc1 Show InChI InChI=1S/C25H20F3N5O3S/c1-37(35,36)21-8-3-2-7-19(21)15-9-11-17(12-10-15)31-24(34)20-14-22(25(26,27)28)32-33(20)18-6-4-5-16(13-18)23(29)30/h2-14H,1H3,(H3,29,30)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096091
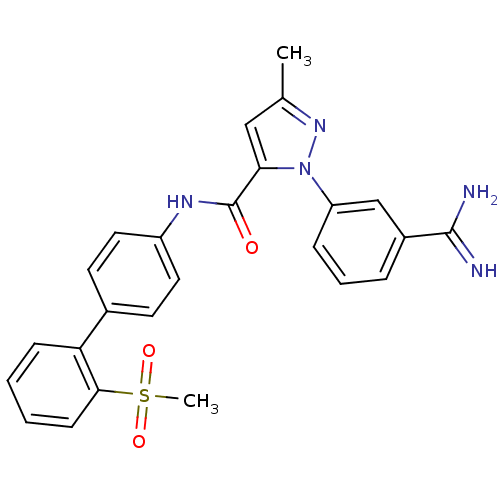
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(C)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C25H23N5O3S/c1-16-14-22(30(29-16)20-7-5-6-18(15-20)24(26)27)25(31)28-19-12-10-17(11-13-19)21-8-3-4-9-23(21)34(2,32)33/h3-15H,1-2H3,(H3,26,27)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096108
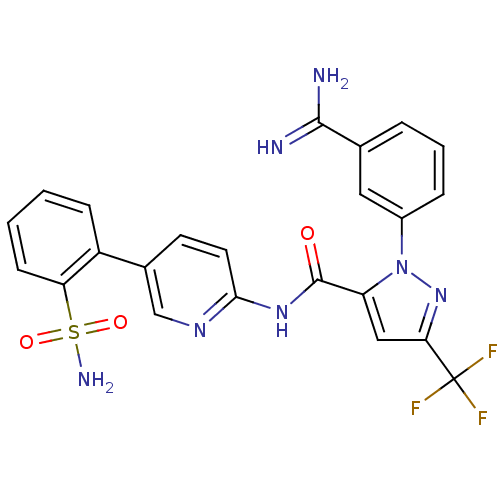
(2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...)Show SMILES NC(=N)c1cccc(c1)-n1nc(cc1C(=O)Nc1ccc(cn1)-c1ccccc1S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C23H18F3N7O3S/c24-23(25,26)19-11-17(33(32-19)15-5-3-4-13(10-15)21(27)28)22(34)31-20-9-8-14(12-30-20)16-6-1-2-7-18(16)37(29,35)36/h1-12H,(H3,27,28)(H2,29,35,36)(H,30,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096085
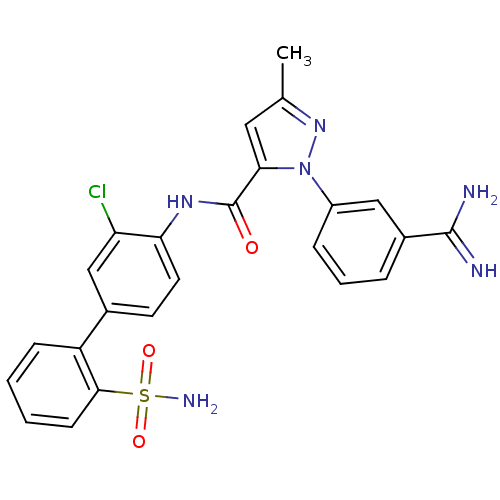
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2Cl)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H21ClN6O3S/c1-14-11-21(31(30-14)17-6-4-5-16(12-17)23(26)27)24(32)29-20-10-9-15(13-19(20)25)18-7-2-3-8-22(18)35(28,33)34/h2-13H,1H3,(H3,26,27)(H,29,32)(H2,28,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096098
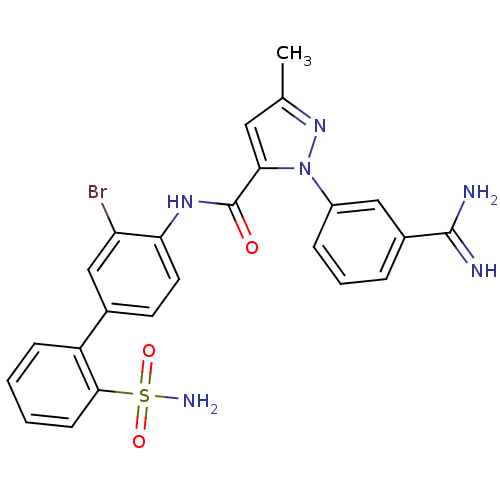
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2Br)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H21BrN6O3S/c1-14-11-21(31(30-14)17-6-4-5-16(12-17)23(26)27)24(32)29-20-10-9-15(13-19(20)25)18-7-2-3-8-22(18)35(28,33)34/h2-13H,1H3,(H3,26,27)(H,29,32)(H2,28,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374877
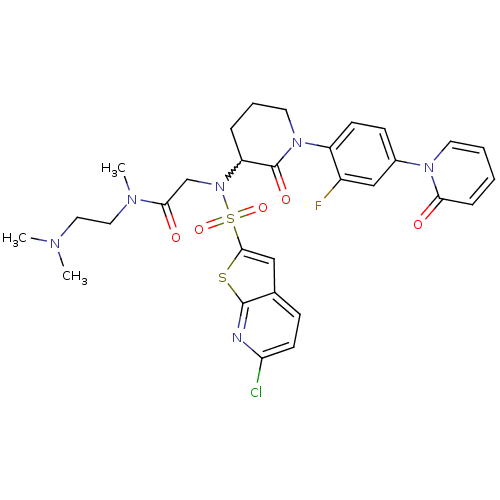
(CHEMBL270221)Show SMILES CN(C)CCN(C)C(=O)CN(C1CCCN(C1=O)c1ccc(cc1F)-n1ccccc1=O)S(=O)(=O)c1cc2ccc(Cl)nc2s1 |w:11.10| Show InChI InChI=1S/C30H32ClFN6O5S2/c1-34(2)15-16-35(3)27(40)19-38(45(42,43)28-17-20-9-12-25(31)33-29(20)44-28)24-7-6-14-37(30(24)41)23-11-10-21(18-22(23)32)36-13-5-4-8-26(36)39/h4-5,8-13,17-18,24H,6-7,14-16,19H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2428-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.054
BindingDB Entry DOI: 10.7270/Q2RX9CZ8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096111
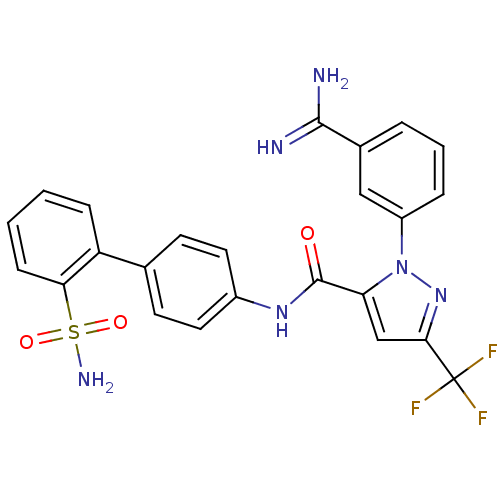
(2-(3-Carbamimidoyl-phenyl)-5-trifluoromethyl-2H-py...)Show SMILES NC(=N)c1cccc(c1)-n1nc(cc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C24H19F3N6O3S/c25-24(26,27)21-13-19(33(32-21)17-5-3-4-15(12-17)22(28)29)23(34)31-16-10-8-14(9-11-16)18-6-1-2-7-20(18)37(30,35)36/h1-13H,(H3,28,29)(H,31,34)(H2,30,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374879
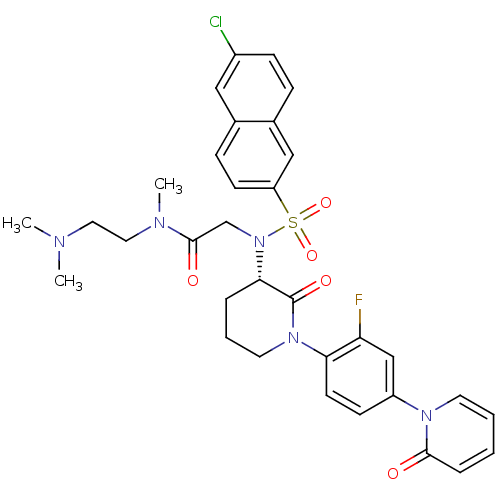
(CHEMBL401958)Show SMILES CN(C)CCN(C)C(=O)CN([C@H]1CCCN(C1=O)c1ccc(cc1F)-n1ccccc1=O)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C33H35ClFN5O5S/c1-36(2)17-18-37(3)32(42)22-40(46(44,45)27-13-10-23-19-25(34)11-9-24(23)20-27)30-7-6-16-39(33(30)43)29-14-12-26(21-28(29)35)38-15-5-4-8-31(38)41/h4-5,8-15,19-21,30H,6-7,16-18,22H2,1-3H3/t30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2428-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.054
BindingDB Entry DOI: 10.7270/Q2RX9CZ8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12852
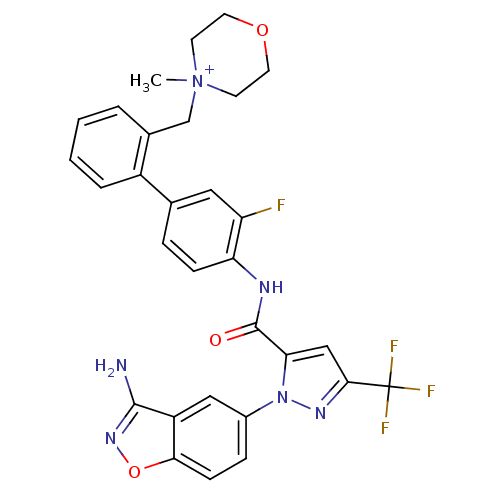
(4-{[2-(4-{[1-(3-amino-1,2-benzoxazol-5-yl)-3-(trif...)Show SMILES C[N+]1(Cc2ccccc2-c2ccc(NC(=O)c3cc(nn3-c3ccc4onc(N)c4c3)C(F)(F)F)c(F)c2)CCOCC1 Show InChI InChI=1S/C30H26F4N6O3/c1-40(10-12-42-13-11-40)17-19-4-2-3-5-21(19)18-6-8-24(23(31)14-18)36-29(41)25-16-27(30(32,33)34)37-39(25)20-7-9-26-22(15-20)28(35)38-43-26/h2-9,14-16H,10-13,17H2,1H3,(H2-,35,36,38,41)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | -60.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 1795-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.010
BindingDB Entry DOI: 10.7270/Q2FB515N |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50260646
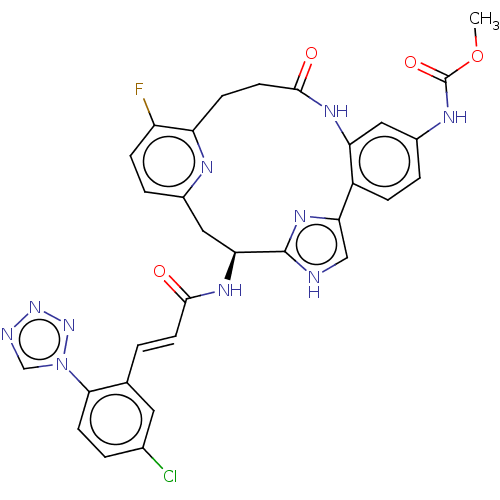
(CHEMBL4096251)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](Cc3ccc(F)c(CCC(=O)Nc2c1)n3)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r| Show InChI InChI=1S/C31H26ClFN10O4/c1-47-31(46)37-20-4-6-21-24(13-20)38-29(45)11-8-23-22(33)7-5-19(36-23)14-25(30-34-15-26(21)40-30)39-28(44)10-2-17-12-18(32)3-9-27(17)43-16-35-41-42-43/h2-7,9-10,12-13,15-16,25H,8,11,14H2,1H3,(H,34,40)(H,37,46)(H,38,45)(H,39,44)/b10-2+/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company, Research and Development, 350 Carter Road, Hopewell, NJ 08540 United States.
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using peptide substrate by spectrophotometry |
Bioorg Med Chem Lett 27: 4056-4060 (2017)
Article DOI: 10.1016/j.bmcl.2017.07.048
BindingDB Entry DOI: 10.7270/Q2TB19B3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374878
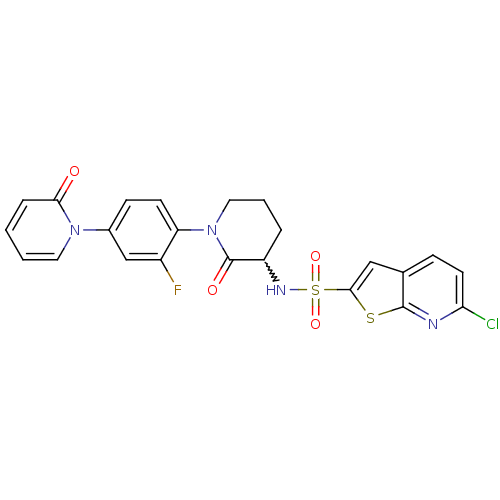
(CHEMBL270862)Show SMILES Fc1cc(ccc1N1CCCC(NS(=O)(=O)c2cc3ccc(Cl)nc3s2)C1=O)-n1ccccc1=O |w:11.12| Show InChI InChI=1S/C23H18ClFN4O4S2/c24-19-9-6-14-12-21(34-22(14)26-19)35(32,33)27-17-4-3-11-29(23(17)31)18-8-7-15(13-16(18)25)28-10-2-1-5-20(28)30/h1-2,5-10,12-13,17,27H,3-4,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2428-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.054
BindingDB Entry DOI: 10.7270/Q2RX9CZ8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374879

(CHEMBL401958)Show SMILES CN(C)CCN(C)C(=O)CN([C@H]1CCCN(C1=O)c1ccc(cc1F)-n1ccccc1=O)S(=O)(=O)c1ccc2cc(Cl)ccc2c1 Show InChI InChI=1S/C33H35ClFN5O5S/c1-36(2)17-18-37(3)32(42)22-40(46(44,45)27-13-10-23-19-25(34)11-9-24(23)20-27)30-7-6-16-39(33(30)43)29-14-12-26(21-28(29)35)38-15-5-4-8-31(38)41/h4-5,8-15,19-21,30H,6-7,16-18,22H2,1-3H3/t30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2428-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.054
BindingDB Entry DOI: 10.7270/Q2RX9CZ8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096096
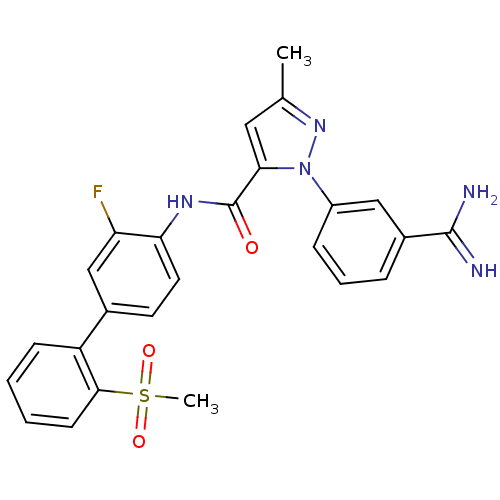
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2F)-c2ccccc2S(C)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C25H22FN5O3S/c1-15-12-22(31(30-15)18-7-5-6-17(13-18)24(27)28)25(32)29-21-11-10-16(14-20(21)26)19-8-3-4-9-23(19)35(2,33)34/h3-14H,1-2H3,(H3,27,28)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50097624
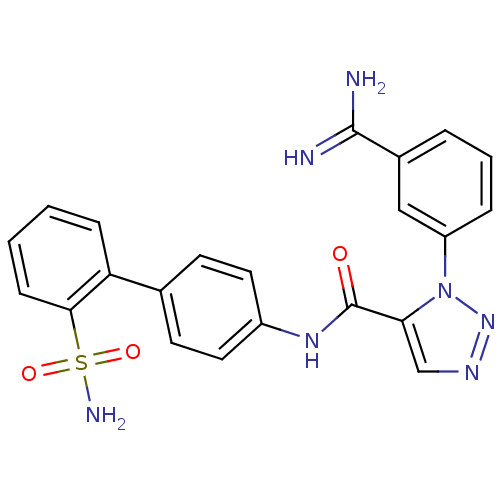
(3-(3-Carbamimidoyl-phenyl)-3H-[1,2,3]triazole-4-ca...)Show SMILES NC(=N)c1cccc(c1)-n1nncc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C22H19N7O3S/c23-21(24)15-4-3-5-17(12-15)29-19(13-26-28-29)22(30)27-16-10-8-14(9-11-16)18-6-1-2-7-20(18)33(25,31)32/h1-13H,(H3,23,24)(H,27,30)(H2,25,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human Serine protease FXa |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Oryctolagus cuniculus) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human trypsin |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
In vitro activity against rabbit FXa. |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50230326
(CHEMBL4060950)Show SMILES COC(=O)Nc1ccc2-c3nc([nH]c3Cl)[C@H](C\C=C\CCC(=O)Nc2c1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r,t:18| Show InChI InChI=1S/C28H25Cl2N9O4/c1-43-28(42)32-18-9-10-19-21(14-18)34-23(40)6-4-2-3-5-20(27-35-25(19)26(30)36-27)33-24(41)12-7-16-13-17(29)8-11-22(16)39-15-31-37-38-39/h2-3,7-15,20H,4-6H2,1H3,(H,32,42)(H,33,41)(H,34,40)(H,35,36)/b3-2+,12-7+/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 27: 3833-3839 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.058
BindingDB Entry DOI: 10.7270/Q2GT5QPS |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50230326

(CHEMBL4060950)Show SMILES COC(=O)Nc1ccc2-c3nc([nH]c3Cl)[C@H](C\C=C\CCC(=O)Nc2c1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r,t:18| Show InChI InChI=1S/C28H25Cl2N9O4/c1-43-28(42)32-18-9-10-19-21(14-18)34-23(40)6-4-2-3-5-20(27-35-25(19)26(30)36-27)33-24(41)12-7-16-13-17(29)8-11-22(16)39-15-31-37-38-39/h2-3,7-15,20H,4-6H2,1H3,(H,32,42)(H,33,41)(H,34,40)(H,35,36)/b3-2+,12-7+/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method |
J Med Chem 60: 1060-1075 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01460
BindingDB Entry DOI: 10.7270/Q25D8V31 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12751

(1-(3-carbamimidoylphenyl)-3-methyl-N-[4-(2-sulfamo...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H22N6O3S/c1-15-13-21(30(29-15)19-6-4-5-17(14-19)23(25)26)24(31)28-18-11-9-16(10-12-18)20-7-2-3-8-22(20)34(27,32)33/h2-14H,1H3,(H3,25,26)(H,28,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards Rabbit Coagulation factor X in a rabbit arterio-venous (A-V) shunt model |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12693
(1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...)Show SMILES Nc1noc2ccc(cc12)-n1nc(c2CCN(C(=O)c12)c1ccc(cc1)-c1ccccc1CN1CC[C@@H](O)C1)C(F)(F)F |r| Show InChI InChI=1S/C31H27F3N6O3/c32-31(33,34)28-24-12-14-39(30(42)27(24)40(36-28)21-9-10-26-25(15-21)29(35)37-43-26)20-7-5-18(6-8-20)23-4-2-1-3-19(23)16-38-13-11-22(41)17-38/h1-10,15,22,41H,11-14,16-17H2,(H2,35,37)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0300 | -59.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 4141-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.069
BindingDB Entry DOI: 10.7270/Q26T0JW1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12693

(1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...)Show SMILES Nc1noc2ccc(cc12)-n1nc(c2CCN(C(=O)c12)c1ccc(cc1)-c1ccccc1CN1CC[C@@H](O)C1)C(F)(F)F |r| Show InChI InChI=1S/C31H27F3N6O3/c32-31(33,34)28-24-12-14-39(30(42)27(24)40(36-28)21-9-10-26-25(15-21)29(35)37-43-26)20-7-5-18(6-8-20)23-4-2-1-3-19(23)16-38-13-11-22(41)17-38/h1-10,15,22,41H,11-14,16-17H2,(H2,35,37)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0300 | -59.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5584-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.027
BindingDB Entry DOI: 10.7270/Q2Z899NQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50097626
(1-(3-Carbamimidoyl-phenyl)-1H-tetrazole-5-carboxyl...)Show SMILES NC(=N)c1cccc(c1)-n1nnnc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C21H18N8O3S/c22-19(23)14-4-3-5-16(12-14)29-20(26-27-28-29)21(30)25-15-10-8-13(9-11-15)17-6-1-2-7-18(17)33(24,31)32/h1-12H,(H3,22,23)(H,25,30)(H2,24,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of human purified factor Xa |
Bioorg Med Chem Lett 13: 369-73 (2003)
BindingDB Entry DOI: 10.7270/Q2BG2NC3 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50097626

(1-(3-Carbamimidoyl-phenyl)-1H-tetrazole-5-carboxyl...)Show SMILES NC(=N)c1cccc(c1)-n1nnnc1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C21H18N8O3S/c22-19(23)14-4-3-5-16(12-14)29-20(26-27-28-29)21(30)25-15-10-8-13(9-11-15)17-6-1-2-7-18(17)33(24,31)32/h1-12H,(H3,22,23)(H,25,30)(H2,24,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human Serine protease FXa |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374876
(CHEMBL270034)Show SMILES CNC(=O)CN(C1CCCN(C1=O)c1ccc(cc1F)-n1ccccc1=O)S(=O)(=O)c1cc2ccc(Cl)nc2s1 |w:6.5| Show InChI InChI=1S/C26H23ClFN5O5S2/c1-29-22(34)15-33(40(37,38)24-13-16-7-10-21(27)30-25(16)39-24)20-5-4-12-32(26(20)36)19-9-8-17(14-18(19)28)31-11-3-2-6-23(31)35/h2-3,6-11,13-14,20H,4-5,12,15H2,1H3,(H,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2428-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.054
BindingDB Entry DOI: 10.7270/Q2RX9CZ8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12681
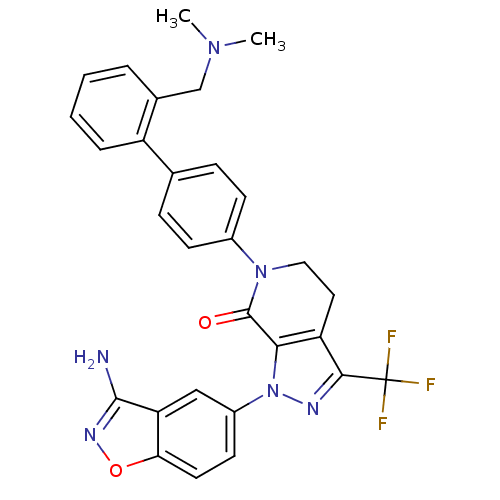
(1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...)Show SMILES CN(C)Cc1ccccc1-c1ccc(cc1)N1CCc2c(nn(c2C1=O)-c1ccc2onc(N)c2c1)C(F)(F)F Show InChI InChI=1S/C29H25F3N6O2/c1-36(2)16-18-5-3-4-6-21(18)17-7-9-19(10-8-17)37-14-13-22-25(28(37)39)38(34-26(22)29(30,31)32)20-11-12-24-23(15-20)27(33)35-40-24/h3-12,15H,13-14,16H2,1-2H3,(H2,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 4141-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.069
BindingDB Entry DOI: 10.7270/Q26T0JW1 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50096792
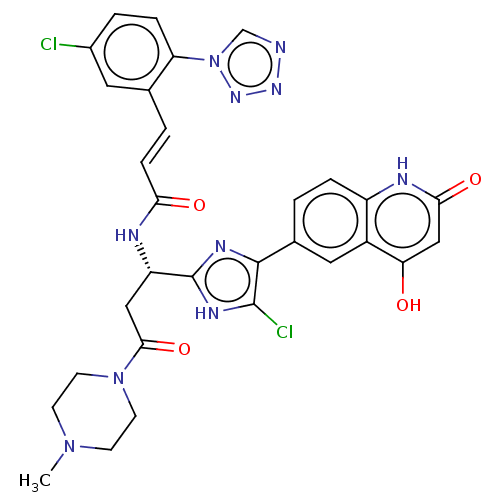
(CHEMBL3580759)Show SMILES CN1CCN(CC1)C(=O)C[C@H](NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1)c1nc(c(Cl)[nH]1)-c1ccc2[nH]c(=O)cc(O)c2c1 |r| Show InChI InChI=1S/C30H26Cl2N10O4/c1-40-8-10-41(11-9-40)27(46)14-22(35-25(44)7-3-17-12-19(31)4-6-23(17)42-16-33-38-39-42)30-36-28(29(32)37-30)18-2-5-21-20(13-18)24(43)15-26(45)34-21/h2-7,12-13,15-16,22,43H,8-11,14H2,1H3,(H,35,44)/b7-3+,28-18-/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometry |
ACS Med Chem Lett 6: 590-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00066
BindingDB Entry DOI: 10.7270/Q2B27X24 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50288406
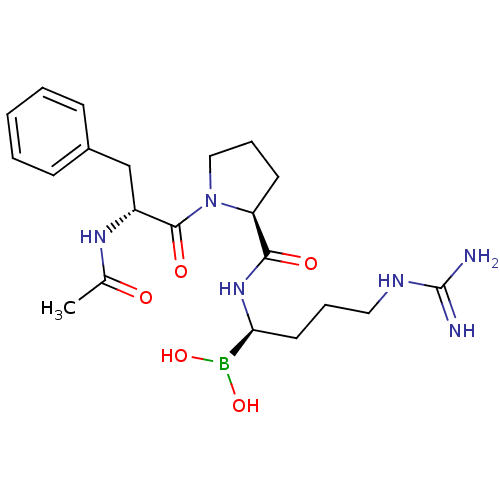
(1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CC(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)B(O)O Show InChI InChI=1S/C21H33BN6O5/c1-14(29)26-16(13-15-7-3-2-4-8-15)20(31)28-12-6-9-17(28)19(30)27-18(22(32)33)10-5-11-25-21(23)24/h2-4,7-8,16-18,32-33H,5-6,9-13H2,1H3,(H,26,29)(H,27,30)(H4,23,24,25)/t16-,17+,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50369433
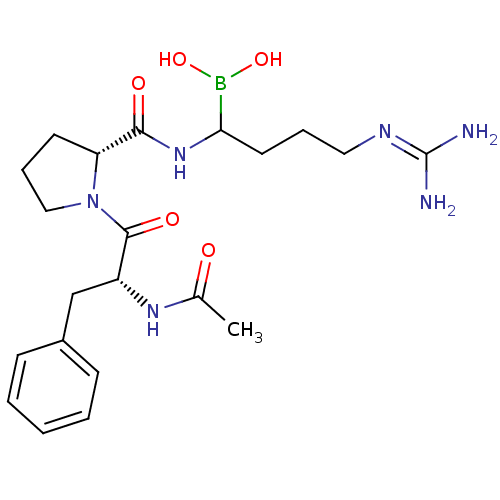
(CHEMBL1202108)Show SMILES [#6]-[#6](=O)-[#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#5](-[#8])-[#8] |r| Show InChI InChI=1S/C21H33BN6O5/c1-14(29)26-16(13-15-7-3-2-4-8-15)20(31)28-12-6-9-17(28)19(30)27-18(22(32)33)10-5-11-25-21(23)24/h2-4,7-8,16-18,32-33H,5-6,9-13H2,1H3,(H,26,29)(H,27,30)(H4,23,24,25)/t16-,17-,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity to purified human coagulation factor Xa (FXa) |
J Med Chem 42: 2752-9 (1999)
Article DOI: 10.1021/jm980405i
BindingDB Entry DOI: 10.7270/Q28P616C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096094
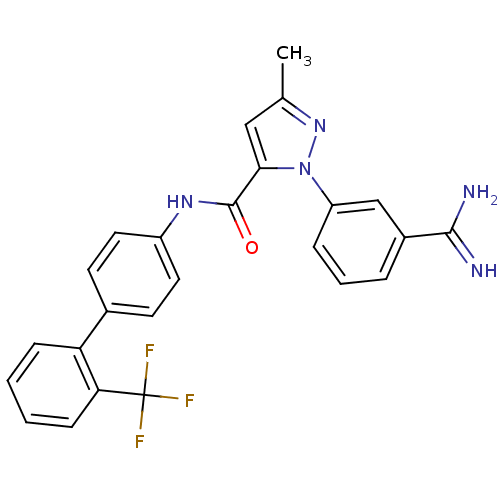
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2C(F)(F)F)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C25H20F3N5O/c1-15-13-22(33(32-15)19-6-4-5-17(14-19)23(29)30)24(34)31-18-11-9-16(10-12-18)20-7-2-3-8-21(20)25(26,27)28/h2-14H,1H3,(H3,29,30)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12681

(1-(3-amino-1,2-benzoxazol-5-yl)-6-(4-{2-[(dimethyl...)Show SMILES CN(C)Cc1ccccc1-c1ccc(cc1)N1CCc2c(nn(c2C1=O)-c1ccc2onc(N)c2c1)C(F)(F)F Show InChI InChI=1S/C29H25F3N6O2/c1-36(2)16-18-5-3-4-6-21(18)17-7-9-19(10-8-17)37-14-13-22-25(28(37)39)38(34-26(22)29(30,31)32)20-11-12-24-23(15-20)27(33)35-40-24/h3-12,15H,13-14,16H2,1-2H3,(H2,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 5584-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.027
BindingDB Entry DOI: 10.7270/Q2Z899NQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096112
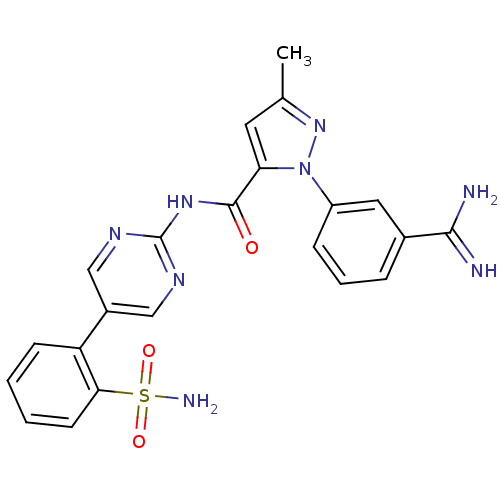
(2-(3-Carbamimidoyl-phenyl)-5-methyl-2H-pyrazole-3-...)Show SMILES Cc1cc(C(=O)Nc2ncc(cn2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C22H20N8O3S/c1-13-9-18(30(29-13)16-6-4-5-14(10-16)20(23)24)21(31)28-22-26-11-15(12-27-22)17-7-2-3-8-19(17)34(25,32)33/h2-12H,1H3,(H3,23,24)(H2,25,32,33)(H,26,27,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374871
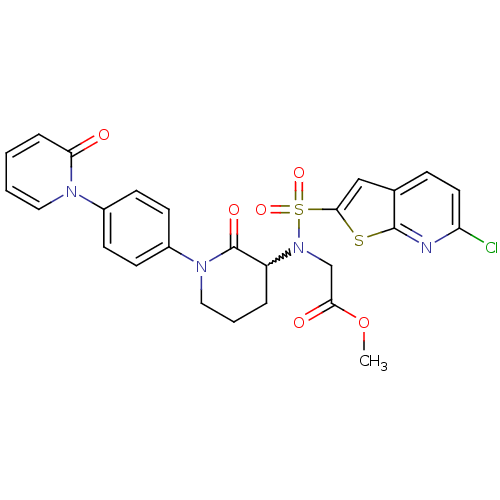
(CHEMBL258274)Show SMILES COC(=O)CN(C1CCCN(C1=O)c1ccc(cc1)-n1ccccc1=O)S(=O)(=O)c1cc2ccc(Cl)nc2s1 |w:6.5| Show InChI InChI=1S/C26H23ClN4O6S2/c1-37-23(33)16-31(39(35,36)24-15-17-7-12-21(27)28-25(17)38-24)20-5-4-14-30(26(20)34)19-10-8-18(9-11-19)29-13-3-2-6-22(29)32/h2-3,6-13,15,20H,4-5,14,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2428-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.054
BindingDB Entry DOI: 10.7270/Q2RX9CZ8 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50288406

(1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CC(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)B(O)O Show InChI InChI=1S/C21H33BN6O5/c1-14(29)26-16(13-15-7-3-2-4-8-15)20(31)28-12-6-9-17(28)19(30)27-18(22(32)33)10-5-11-25-21(23)24/h2-4,7-8,16-18,32-33H,5-6,9-13H2,1H3,(H,26,29)(H,27,30)(H4,23,24,25)/t16-,17+,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 7: 1595-1600 (1997)
Article DOI: 10.1016/S0960-894X(97)00254-0
BindingDB Entry DOI: 10.7270/Q2VQ32PJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50369433

(CHEMBL1202108)Show SMILES [#6]-[#6](=O)-[#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#5](-[#8])-[#8] |r| Show InChI InChI=1S/C21H33BN6O5/c1-14(29)26-16(13-15-7-3-2-4-8-15)20(31)28-12-6-9-17(28)19(30)27-18(22(32)33)10-5-11-25-21(23)24/h2-4,7-8,16-18,32-33H,5-6,9-13H2,1H3,(H,26,29)(H,27,30)(H4,23,24,25)/t16-,17-,18?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
The binding affinity towards thrombin obtained from human purified enzymes. |
J Med Chem 42: 2752-9 (1999)
Article DOI: 10.1021/jm980405i
BindingDB Entry DOI: 10.7270/Q28P616C |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50374875
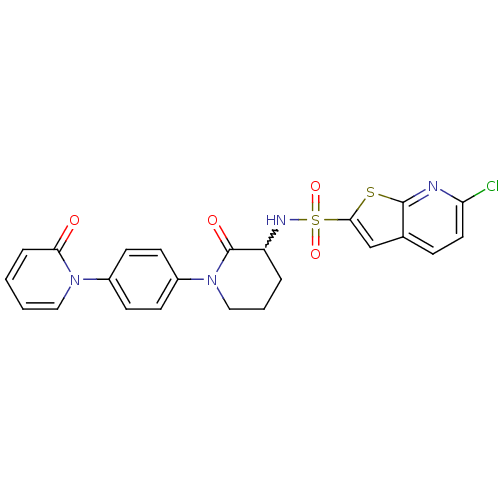
(CHEMBL269955)Show SMILES Clc1ccc2cc(sc2n1)S(=O)(=O)NC1CCCN(C1=O)c1ccc(cc1)-n1ccccc1=O |w:14.15| Show InChI InChI=1S/C23H19ClN4O4S2/c24-19-11-6-15-14-21(33-22(15)25-19)34(31,32)26-18-4-3-13-28(23(18)30)17-9-7-16(8-10-17)27-12-2-1-5-20(27)29/h1-2,5-12,14,18,26H,3-4,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2428-33 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.054
BindingDB Entry DOI: 10.7270/Q2RX9CZ8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12853
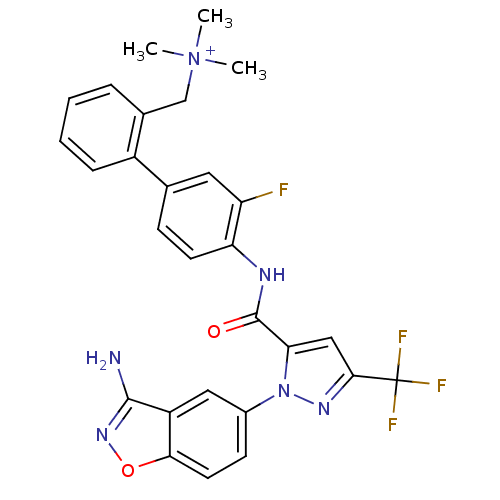
(Aminobenzisoxazole 2v | {[2-(4-{[1-(3-amino-1,2-be...)Show SMILES C[N+](C)(C)Cc1ccccc1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C28H24F4N6O2/c1-38(2,3)15-17-6-4-5-7-19(17)16-8-10-22(21(29)12-16)34-27(39)23-14-25(28(30,31)32)35-37(23)18-9-11-24-20(13-18)26(33)36-40-24/h4-14H,15H2,1-3H3,(H2-,33,34,36,39)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | -58.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 1795-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.010
BindingDB Entry DOI: 10.7270/Q2FB515N |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096090
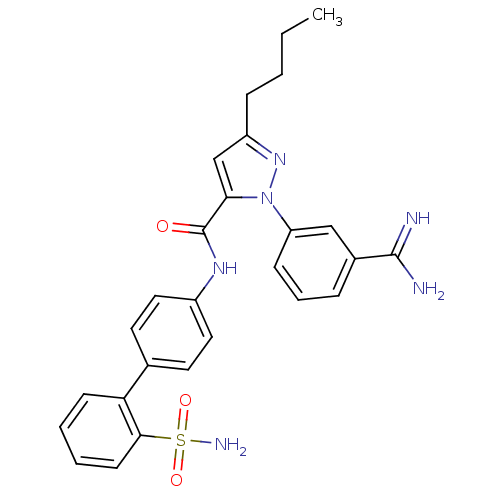
(5-Butyl-2-(3-carbamimidoyl-phenyl)-2H-pyrazole-3-c...)Show SMILES CCCCc1cc(C(=O)Nc2ccc(cc2)-c2ccccc2S(N)(=O)=O)n(n1)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C27H28N6O3S/c1-2-3-8-21-17-24(33(32-21)22-9-6-7-19(16-22)26(28)29)27(34)31-20-14-12-18(13-15-20)23-10-4-5-11-25(23)37(30,35)36/h4-7,9-17H,2-3,8H2,1H3,(H3,28,29)(H,31,34)(H2,30,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 566-78 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5VQK |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50269207
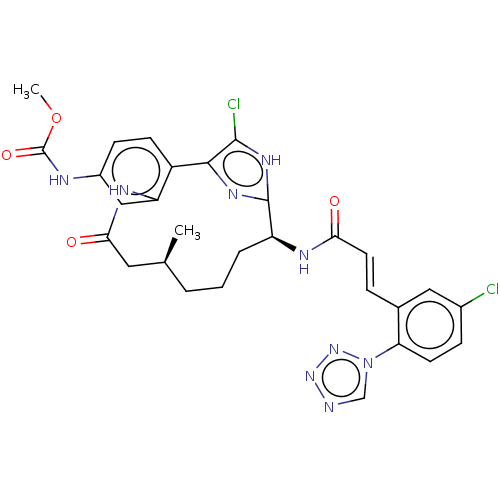
(CHEMBL4097522)Show SMILES COC(=O)Nc1ccc2-c3nc([nH]c3Cl)[C@H](CCC[C@H](C)CC(=O)Nc2c1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r| Show InChI InChI=1S/C29H29Cl2N9O4/c1-16-4-3-5-21(34-24(41)11-6-17-13-18(30)7-10-23(17)40-15-32-38-39-40)28-36-26(27(31)37-28)20-9-8-19(33-29(43)44-2)14-22(20)35-25(42)12-16/h6-11,13-16,21H,3-5,12H2,1-2H3,(H,33,43)(H,34,41)(H,35,42)(H,36,37)/b11-6+/t16-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 27: 3833-3839 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.058
BindingDB Entry DOI: 10.7270/Q2GT5QPS |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50250493
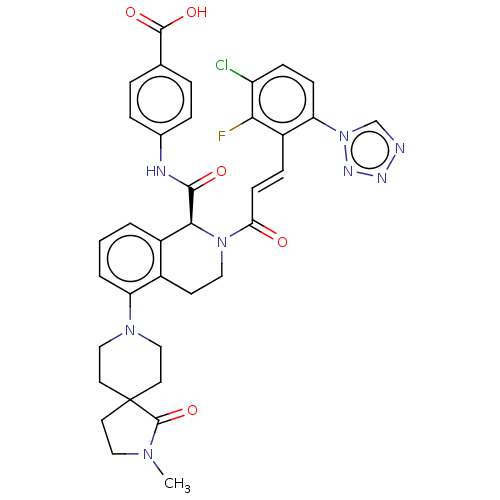
(CHEMBL4068445)Show SMILES CN1CCC2(CCN(CC2)c2cccc3[C@H](N(CCc23)C(=O)\C=C\c2c(F)c(Cl)ccc2-n2cnnn2)C(=O)Nc2ccc(cc2)C(O)=O)C1=O |r| Show InChI InChI=1S/C36H34ClFN8O5/c1-43-18-14-36(35(43)51)15-19-44(20-16-36)28-4-2-3-25-24(28)13-17-45(32(25)33(48)40-23-7-5-22(6-8-23)34(49)50)30(47)12-9-26-29(46-21-39-41-42-46)11-10-27(37)31(26)38/h2-12,21,32H,13-20H2,1H3,(H,40,48)(H,49,50)/b12-9+/t32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method |
J Med Chem 60: 9703-9723 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01171
BindingDB Entry DOI: 10.7270/Q2K64MHN |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50269199
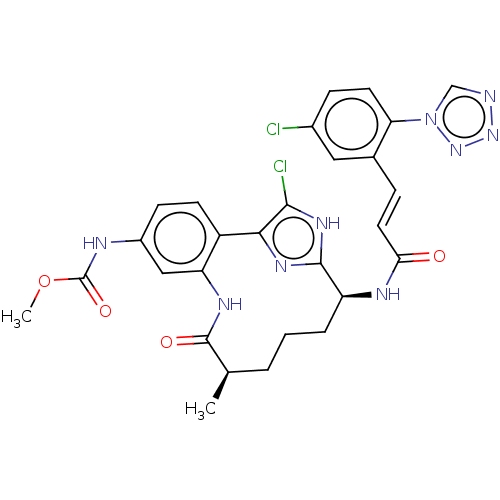
(CHEMBL4083769)Show SMILES COC(=O)Nc1ccc2-c3nc([nH]c3Cl)[C@H](CCC[C@@H](C)C(=O)Nc2c1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r| Show InChI InChI=1S/C28H27Cl2N9O4/c1-15-4-3-5-20(33-23(40)11-6-16-12-17(29)7-10-22(16)39-14-31-37-38-39)26-35-24(25(30)36-26)19-9-8-18(32-28(42)43-2)13-21(19)34-27(15)41/h6-15,20H,3-5H2,1-2H3,(H,32,42)(H,33,40)(H,34,41)(H,35,36)/b11-6+/t15-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 27: 3833-3839 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.058
BindingDB Entry DOI: 10.7270/Q2GT5QPS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
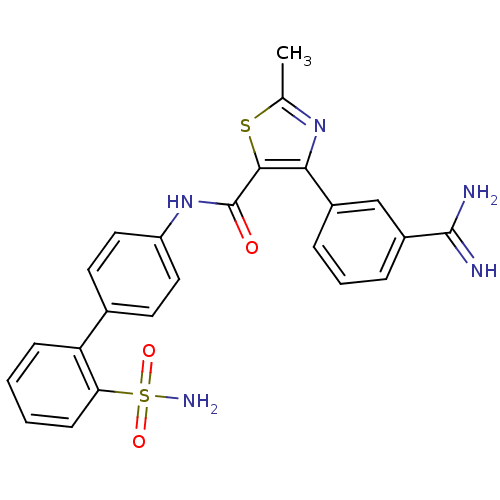
(Oryctolagus cuniculus) | BDBM50097625
(4-(3-Carbamimidoyl-phenyl)-2-methyl-thiazole-5-car...)Show SMILES Cc1nc(c(s1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H21N5O3S2/c1-14-28-21(16-5-4-6-17(13-16)23(25)26)22(33-14)24(30)29-18-11-9-15(10-12-18)19-7-2-3-8-20(19)34(27,31)32/h2-13H,1H3,(H3,25,26)(H,29,30)(H2,27,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards human trypsin |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50097625

(4-(3-Carbamimidoyl-phenyl)-2-methyl-thiazole-5-car...)Show SMILES Cc1nc(c(s1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)-c1cccc(c1)C(N)=N Show InChI InChI=1S/C24H21N5O3S2/c1-14-28-21(16-5-4-6-17(13-16)23(25)26)22(33-14)24(30)29-18-11-9-15(10-12-18)19-7-2-3-8-20(19)34(27,31)32/h2-13H,1H3,(H3,25,26)(H,29,30)(H2,27,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company
Curated by ChEMBL
| Assay Description
Binding affinity towards Rabbit Coagulation factor X in a rabbit arterio-venous (A-V) shunt model |
Bioorg Med Chem Lett 11: 641-5 (2001)
BindingDB Entry DOI: 10.7270/Q2RV0MZ2 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM19023

(1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...)Show SMILES COc1ccc(cc1)-n1nc(C(N)=O)c2CCN(C(=O)c12)c1ccc(cc1)N1CCCCC1=O Show InChI InChI=1S/C25H25N5O4/c1-34-19-11-9-18(10-12-19)30-23-20(22(27-30)24(26)32)13-15-29(25(23)33)17-7-5-16(6-8-17)28-14-3-2-4-21(28)31/h5-12H,2-4,13-15H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 20: 1373-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.011
BindingDB Entry DOI: 10.7270/Q2FQ9WQG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12851
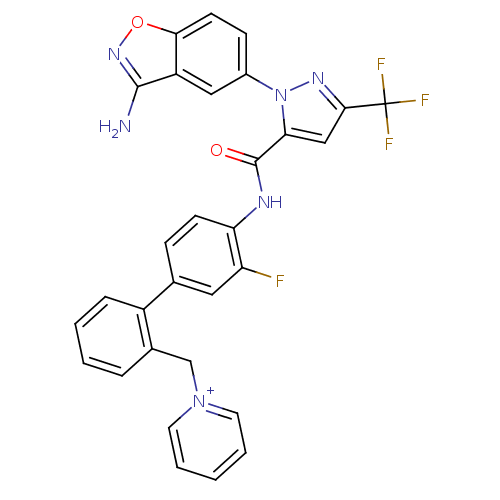
(1-{[2-(4-{[1-(3-amino-1,2-benzoxazol-5-yl)-3-(trif...)Show SMILES Nc1noc2ccc(cc12)-n1nc(cc1C(=O)Nc1ccc(cc1F)-c1ccccc1C[n+]1ccccc1)C(F)(F)F Show InChI InChI=1S/C30H20F4N6O2/c31-23-14-18(21-7-3-2-6-19(21)17-39-12-4-1-5-13-39)8-10-24(23)36-29(41)25-16-27(30(32,33)34)37-40(25)20-9-11-26-22(15-20)28(35)38-42-26/h1-16H,17H2,(H2-,35,36,38,41)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | -57.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
Bioorg Med Chem Lett 16: 1795-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.010
BindingDB Entry DOI: 10.7270/Q2FB515N |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12659
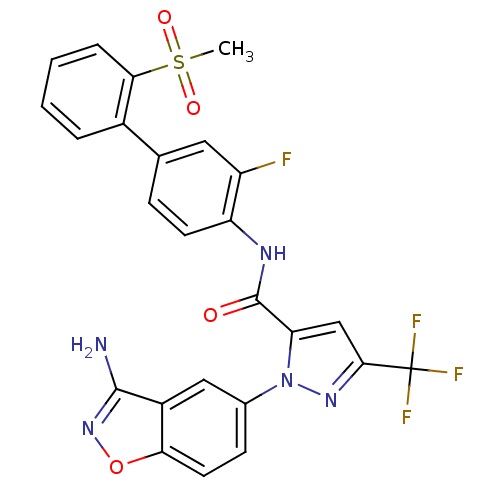
(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-5-[...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C25H17F4N5O4S/c1-39(36,37)21-5-3-2-4-15(21)13-6-8-18(17(26)10-13)31-24(35)19-12-22(25(27,28)29)32-34(19)14-7-9-20-16(11-14)23(30)33-38-20/h2-12H,1H3,(H2,30,33)(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | -56.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Ki values were obtained from human purified enzyme. All assays were run in microtiter plates. Plates were read for 30 min at 405 nm. Rates were deter... |
J Med Chem 48: 1729-44 (2005)
Article DOI: 10.1021/jm0497949
BindingDB Entry DOI: 10.7270/Q2BK19K9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12659

(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-5-[...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C25H17F4N5O4S/c1-39(36,37)21-5-3-2-4-15(21)13-6-8-18(17(26)10-13)31-24(35)19-12-22(25(27,28)29)32-34(19)14-7-9-20-16(11-14)23(30)33-38-20/h2-12H,1H3,(H2,30,33)(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 6481-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.091
BindingDB Entry DOI: 10.7270/Q2GX4B9Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data