

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50296336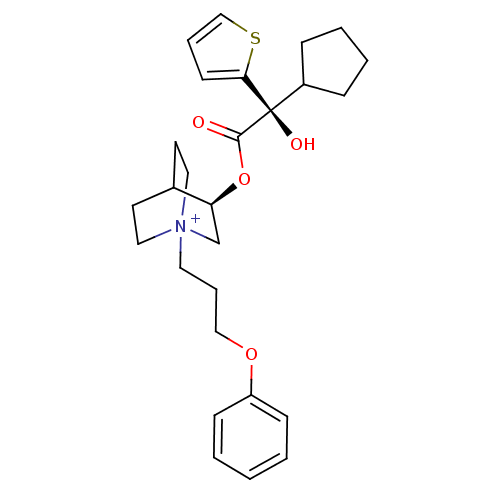 ((3R)-3-{[(2S)-2-Cyclopentyl-2-hydroxy-2-(2-thienyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M2 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296336 ((3R)-3-{[(2S)-2-Cyclopentyl-2-hydroxy-2-(2-thienyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M3 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296331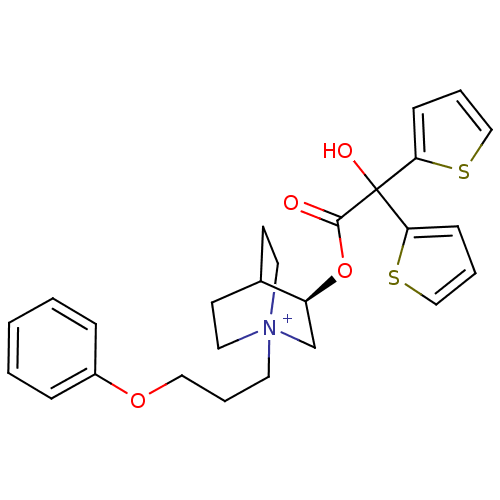 ((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M3 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50296331 ((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phe...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M1 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50296329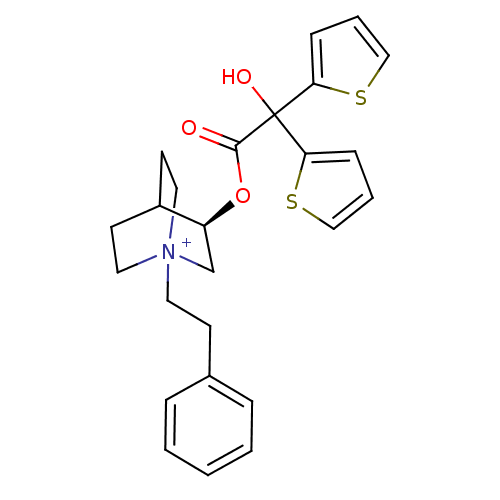 ((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(2-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M2 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296329 ((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(2-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M3 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50296329 ((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(2-phe...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M1 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50296331 ((3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M2 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50296336 ((3R)-3-{[(2S)-2-Cyclopentyl-2-hydroxy-2-(2-thienyl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M1 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M2 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M1 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M3 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50378083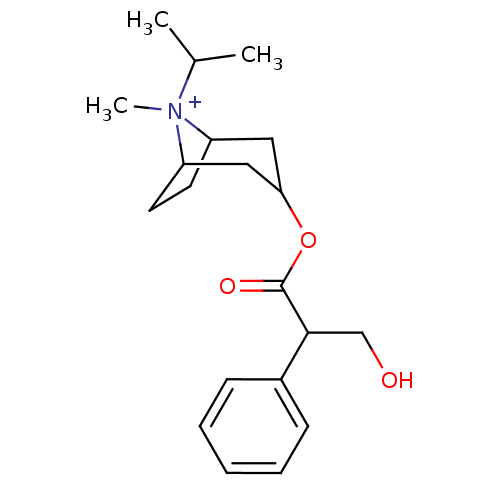 (Atrovent HFA | IPRATROPIUM BROMIDE) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M3 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50378083 (Atrovent HFA | IPRATROPIUM BROMIDE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | 1.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M2 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50378083 (Atrovent HFA | IPRATROPIUM BROMIDE) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | 1.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Binding affinity to muscarinic M1 receptor | J Med Chem 52: 5076-92 (2010) Article DOI: 10.1021/jm900132z BindingDB Entry DOI: 10.7270/Q2SX6F5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483809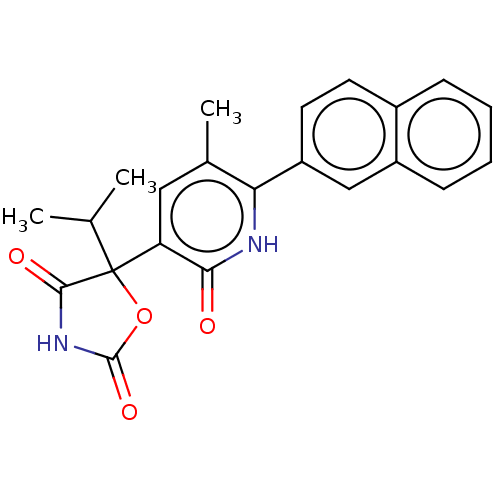 (CHEMBL1770337) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483814 (CHEMBL1770319) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483823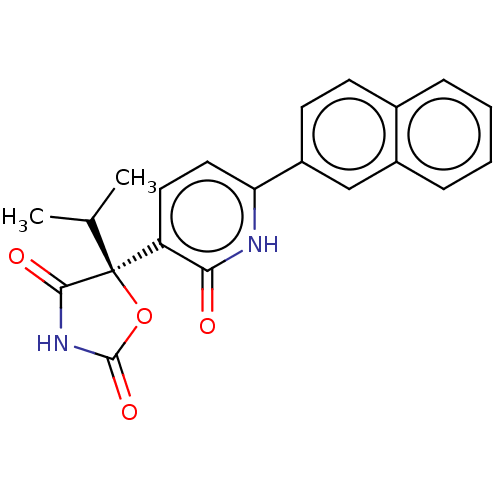 (CHEMBL1770341) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483821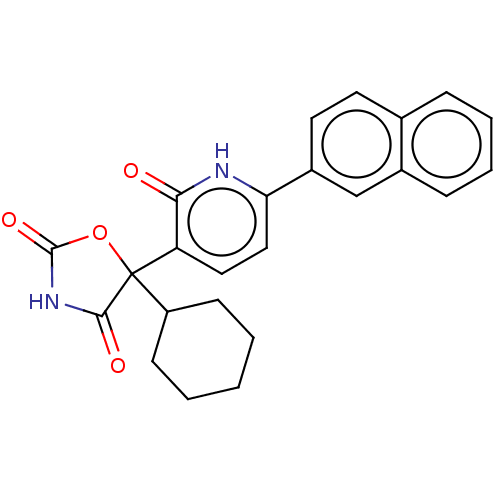 (CHEMBL1770320) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50384443 (CHEMBL1770317) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483827 (CHEMBL1770322) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483811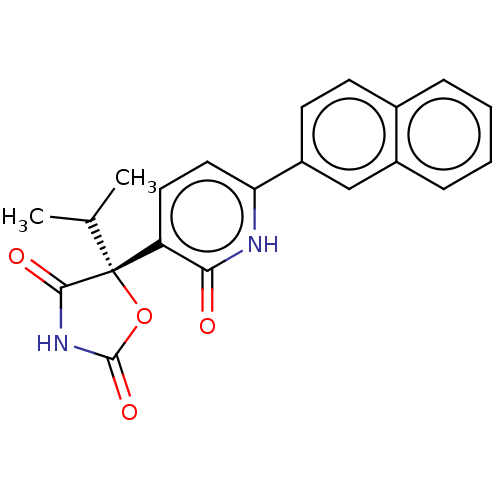 (CHEMBL1770340) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483812 (CHEMBL1770316) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483815 (CHEMBL1770321) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483818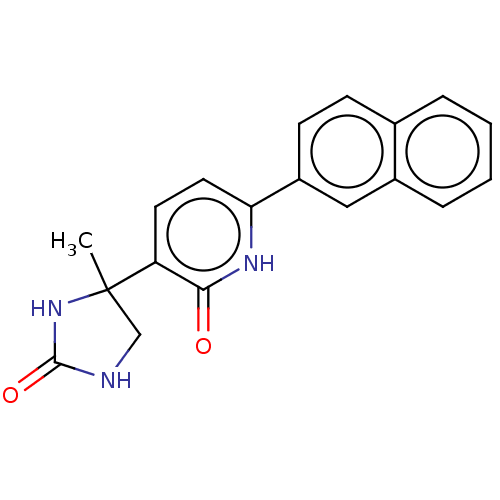 (CHEMBL1770328) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483808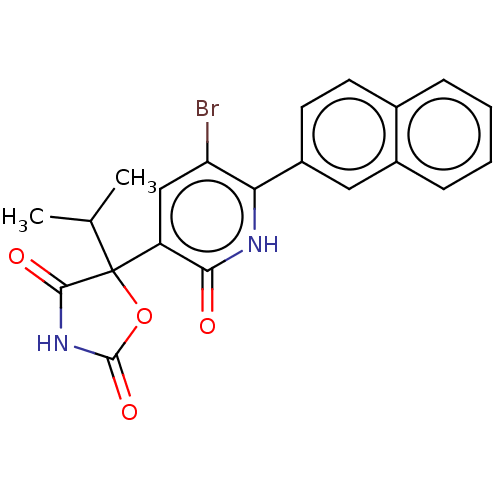 (CHEMBL1770336) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483829 (CHEMBL1770335) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483813 (CHEMBL1770318) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50245983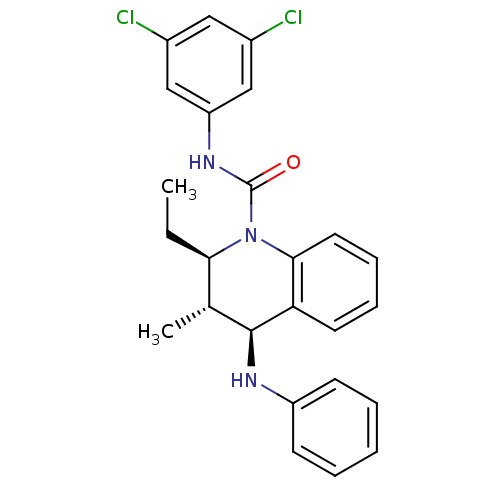 ((2R,3S,4S)-2-Ethyl-3-methyl-N-[3,5-(chloro)phenyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483822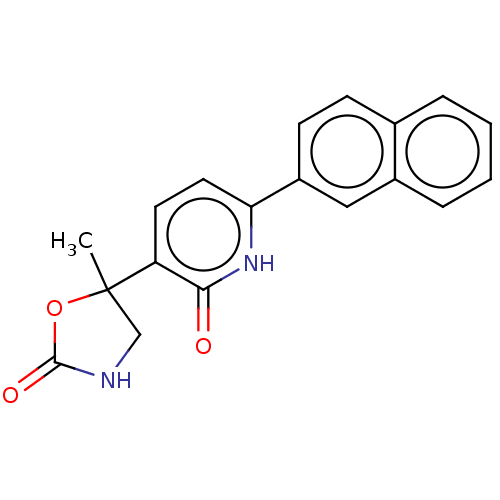 (CHEMBL1770329) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50245937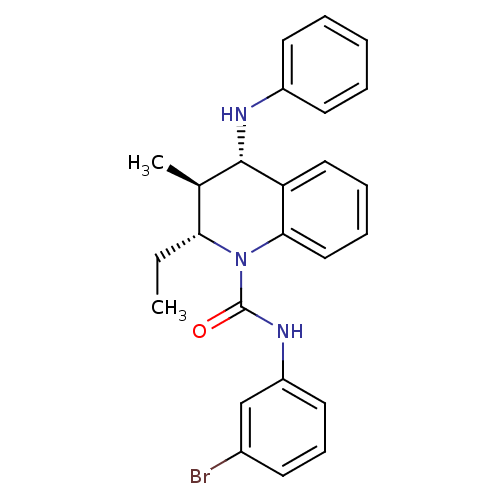 ((2R,3S,4S)-N-(3-bromophenyl)-2-ethyl-3-methyl-4-(p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50246518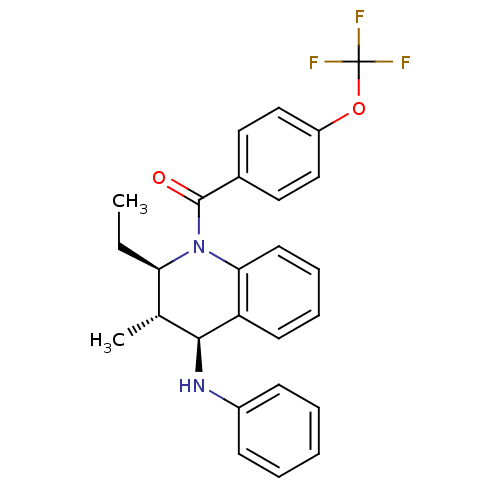 (((2R,3S,4S)-2-ethyl-3-methyl-4-(phenylamino)-3,4-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50245772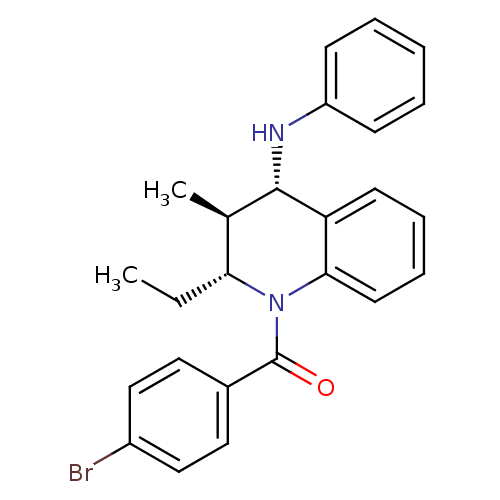 ((4-bromophenyl)((2R,3S,4S)-2-ethyl-3-methyl-4-(phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483824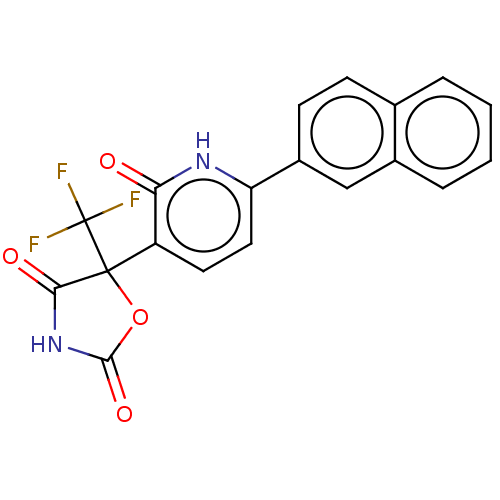 (CHEMBL1770323) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50245982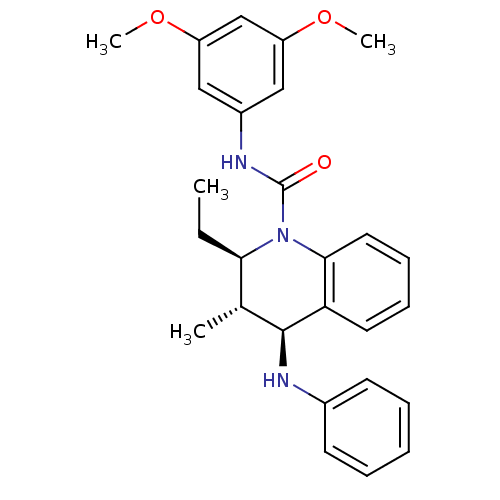 ((2R,3S,4S)-N-(3,5-dimethoxyphenyl)-2-ethyl-3-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483816 (CHEMBL1770324) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50245835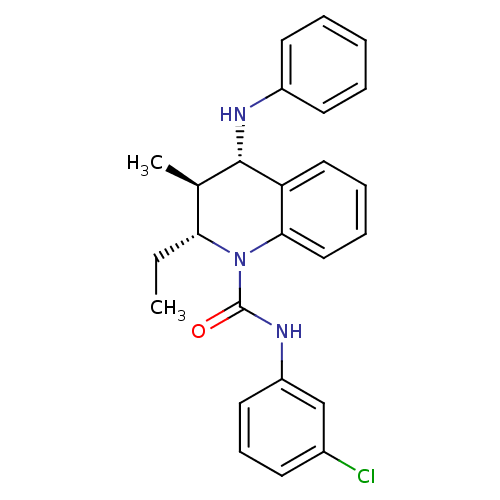 ((2R,3S,4S)-N-(3-chlorophenyl)-2-ethyl-3-methyl-4-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50245934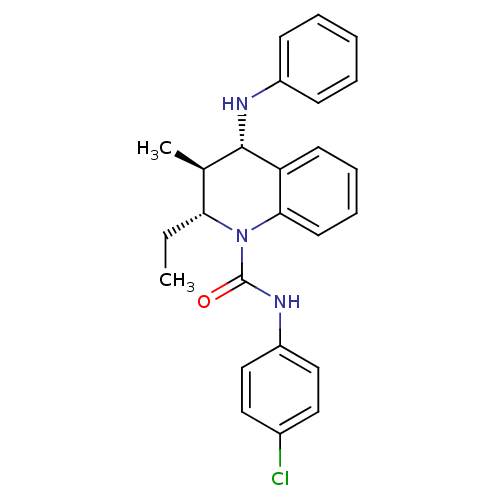 ((2R,3S,4S)-N-(4-chlorophenyl)-2-ethyl-3-methyl-4-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483825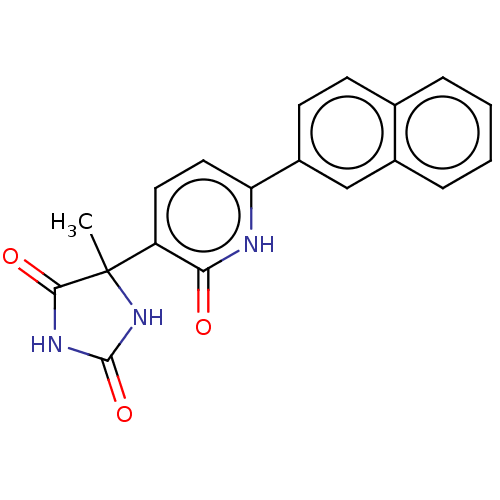 (CHEMBL1770327) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50245981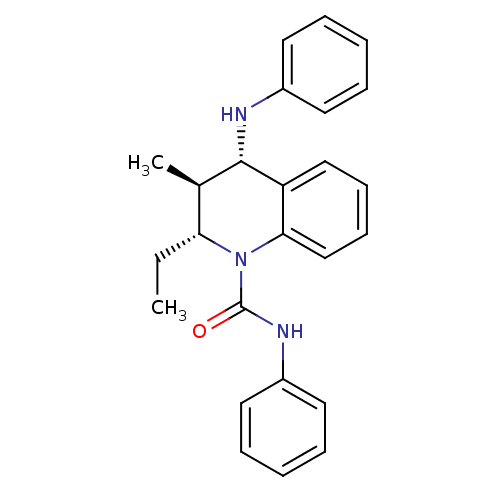 ((2R,3S,4S)-2-ethyl-3-methyl-N-phenyl-4-(phenylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50246517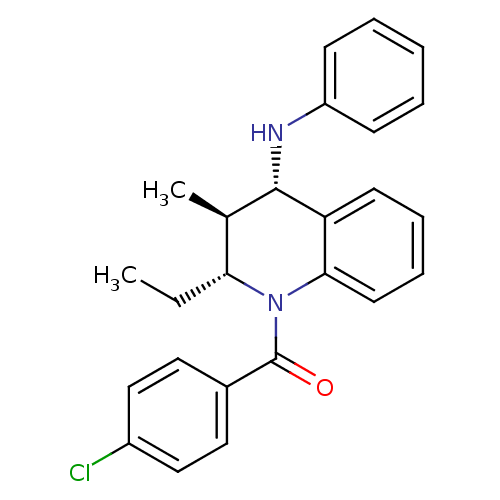 ((4-chlorophenyl)((2R,3S,4S)-2-ethyl-3-methyl-4-(ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50246516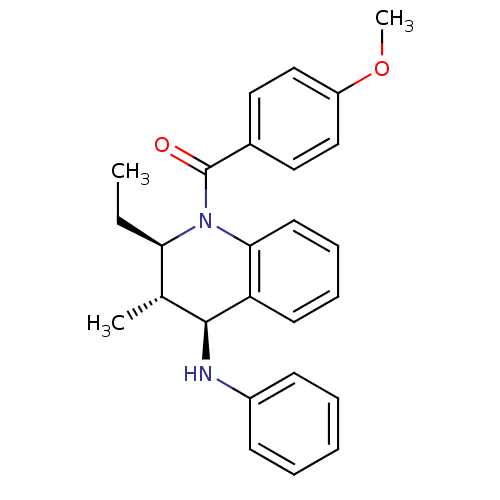 (((2R,3S,4S)-2-ethyl-3-methyl-4-(phenylamino)-3,4-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50245935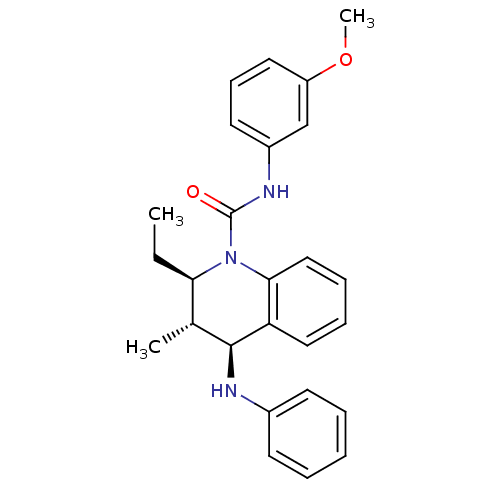 ((2R,3S,4S)-2-ethyl-N-(3-methoxyphenyl)-3-methyl-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50246468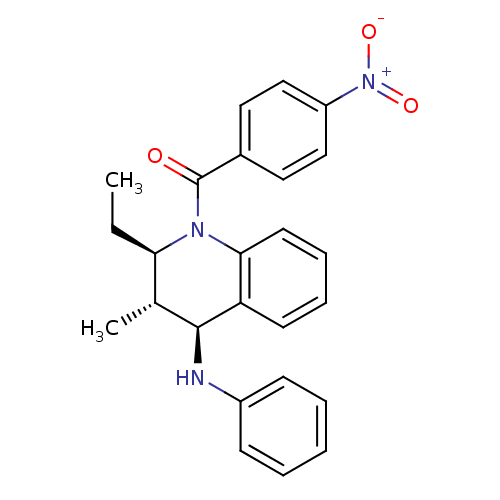 (((2R,3S,4S)-2-ethyl-3-methyl-4-(phenylamino)-3,4-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483817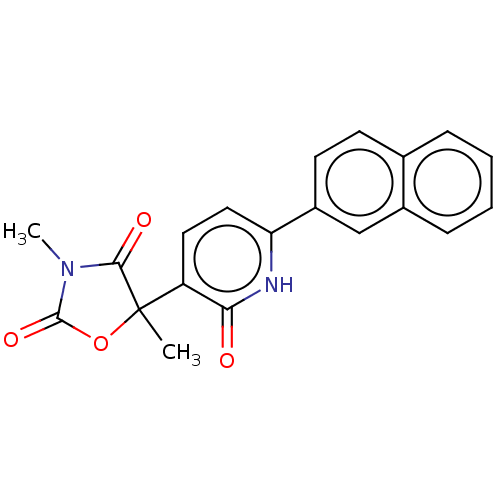 (CHEMBL1770325) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483810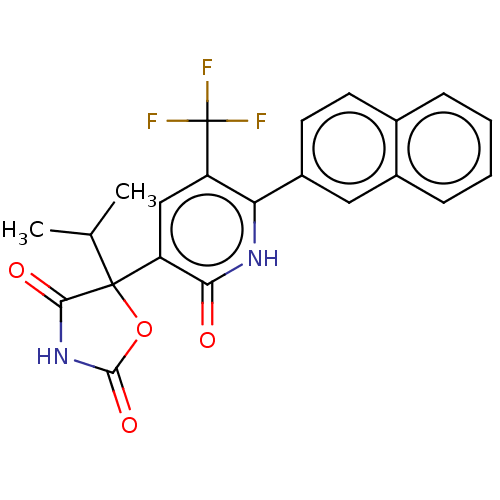 (CHEMBL1770339) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483807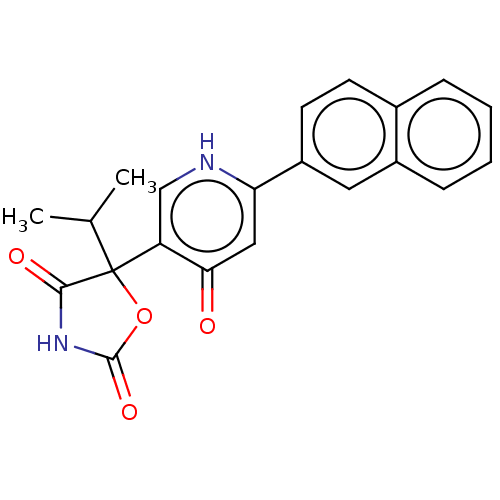 (CHEMBL1770333) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50245984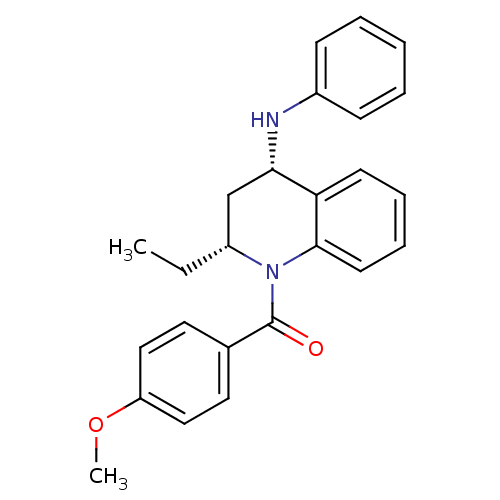 (CHEMBL516508 | cis-(2(R)-ethyl-4-(phenylamino)-3,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483831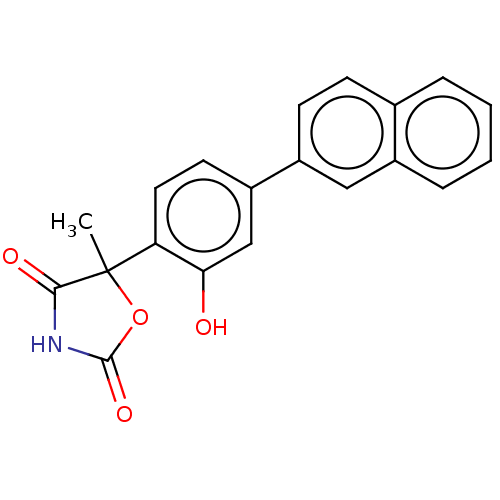 (CHEMBL1770331) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483828 (CHEMBL1770326) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 345 total ) | Next | Last >> |