 Found 1069 hits with Last Name = 'rana' and Initial = 's'
Found 1069 hits with Last Name = 'rana' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cysteine protease
(Trypanosoma brucei rhodesiense) | BDBM50509389

(CHEMBL4566436)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(F)cc1F |r| Show InChI InChI=1S/C29H28F2N2O3/c1-20(34)12-15-24(16-13-21-8-4-2-5-9-21)32-29(36)27(18-22-10-6-3-7-11-22)33-28(35)25-17-14-23(30)19-26(25)31/h2-12,14-15,17,19,24,27H,13,16,18H2,1H3,(H,32,36)(H,33,35)/b15-12+/t24-,27+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.000600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Cysteine protease
(Trypanosoma brucei rhodesiense) | BDBM50509387

(CHEMBL4475066)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C29H29FN2O3/c1-21(33)12-18-26(19-13-22-8-4-2-5-9-22)31-29(35)27(20-23-10-6-3-7-11-23)32-28(34)24-14-16-25(30)17-15-24/h2-12,14-18,26-27H,13,19-20H2,1H3,(H,31,35)(H,32,34)/b18-12+/t26-,27+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Cysteine protease
(Trypanosoma brucei rhodesiense) | BDBM50509386
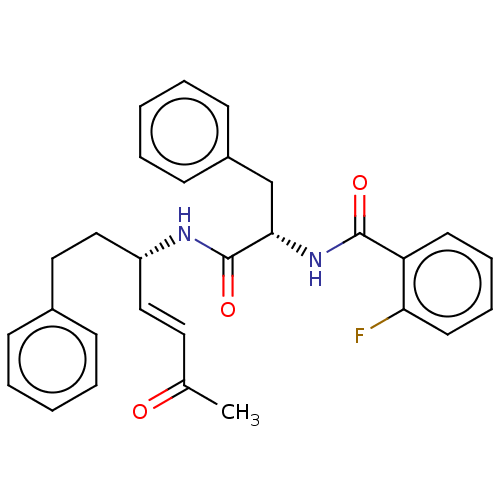
(CHEMBL4515298)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1F |r| Show InChI InChI=1S/C29H29FN2O3/c1-21(33)16-18-24(19-17-22-10-4-2-5-11-22)31-29(35)27(20-23-12-6-3-7-13-23)32-28(34)25-14-8-9-15-26(25)30/h2-16,18,24,27H,17,19-20H2,1H3,(H,31,35)(H,32,34)/b18-16+/t24-,27+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Cysteine protease
(Trypanosoma brucei rhodesiense) | BDBM50509388

(CHEMBL4587965)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C30H29F3N2O3/c1-21(36)12-18-26(19-13-22-8-4-2-5-9-22)34-29(38)27(20-23-10-6-3-7-11-23)35-28(37)24-14-16-25(17-15-24)30(31,32)33/h2-12,14-18,26-27H,13,19-20H2,1H3,(H,34,38)(H,35,37)/b18-12+/t26-,27+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Cysteine protease
(Trypanosoma brucei rhodesiense) | BDBM50509383
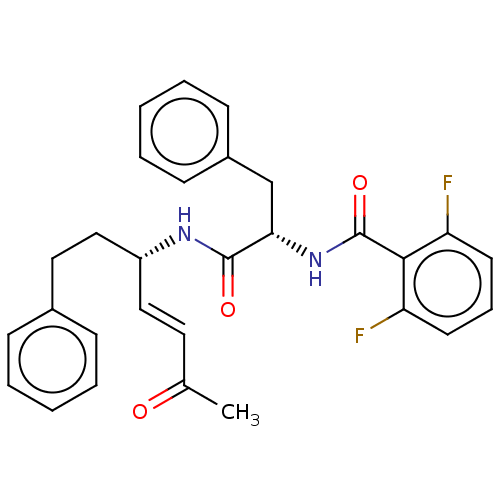
(CHEMBL4441635)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1c(F)cccc1F |r| Show InChI InChI=1S/C29H28F2N2O3/c1-20(34)15-17-23(18-16-21-9-4-2-5-10-21)32-28(35)26(19-22-11-6-3-7-12-22)33-29(36)27-24(30)13-8-14-25(27)31/h2-15,17,23,26H,16,18-19H2,1H3,(H,32,35)(H,33,36)/b17-15+/t23-,26+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Cysteine protease
(Trypanosoma brucei rhodesiense) | BDBM50509390

(CHEMBL4436812)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C29H28F2N2O3/c1-20(34)12-14-26(15-13-21-8-4-2-5-9-21)32-29(36)27(16-22-10-6-3-7-11-22)33-28(35)23-17-24(30)19-25(31)18-23/h2-12,14,17-19,26-27H,13,15-16H2,1H3,(H,32,36)(H,33,35)/b14-12+/t26-,27+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Cysteine protease
(Trypanosoma brucei rhodesiense) | BDBM50509381
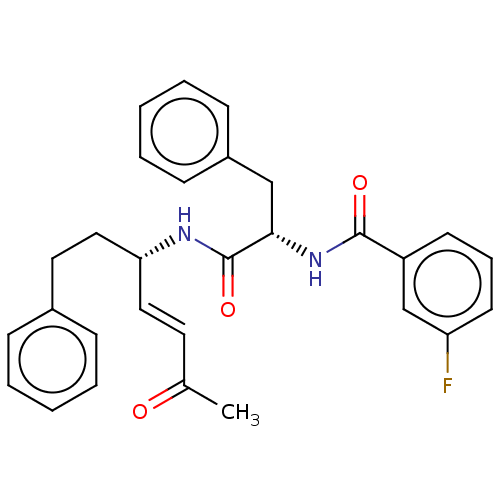
(CHEMBL4453415)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C29H29FN2O3/c1-21(33)15-17-26(18-16-22-9-4-2-5-10-22)31-29(35)27(19-23-11-6-3-7-12-23)32-28(34)24-13-8-14-25(30)20-24/h2-15,17,20,26-27H,16,18-19H2,1H3,(H,31,35)(H,32,34)/b17-15+/t26-,27+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Cysteine protease
(Trypanosoma brucei rhodesiense) | BDBM50509394

(CHEMBL4584291)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C29H29ClN2O3/c1-21(33)12-18-26(19-13-22-8-4-2-5-9-22)31-29(35)27(20-23-10-6-3-7-11-23)32-28(34)24-14-16-25(30)17-15-24/h2-12,14-18,26-27H,13,19-20H2,1H3,(H,31,35)(H,32,34)/b18-12+/t26-,27+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Cysteine protease
(Trypanosoma brucei rhodesiense) | BDBM50509395

(CHEMBL4437914)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C30H30N2O5/c1-21(33)12-15-25(16-13-22-8-4-2-5-9-22)31-30(35)26(18-23-10-6-3-7-11-23)32-29(34)24-14-17-27-28(19-24)37-20-36-27/h2-12,14-15,17,19,25-26H,13,16,18,20H2,1H3,(H,31,35)(H,32,34)/b15-12+/t25-,26+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM81441
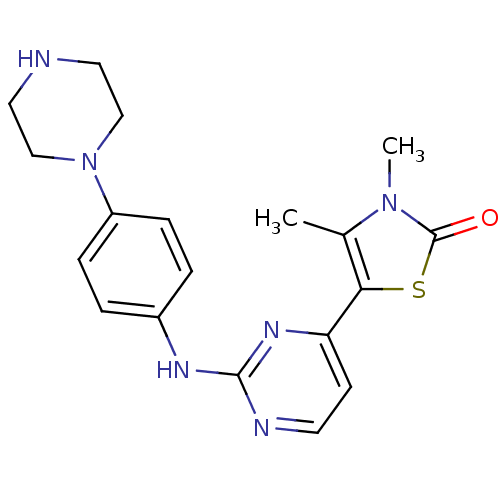
(CDK Inhibitor, 14)Show SMILES Cc1c(sc(=O)n1C)-c1ccnc(Nc2ccc(cc2)N2CCNCC2)n1 Show InChI InChI=1S/C19H22N6OS/c1-13-17(27-19(26)24(13)2)16-7-8-21-18(23-16)22-14-3-5-15(6-4-14)25-11-9-20-10-12-25/h3-8,20H,9-12H2,1-2H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged CDK9/cyclin T1 expressed in baculovirus infected sf9 cells using (biotinyl-Ahx-(Tyr-Ser-ProThr-Ser-Pro-Ser... |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | |
Cysteine protease
(Trypanosoma brucei rhodesiense) | BDBM50509391
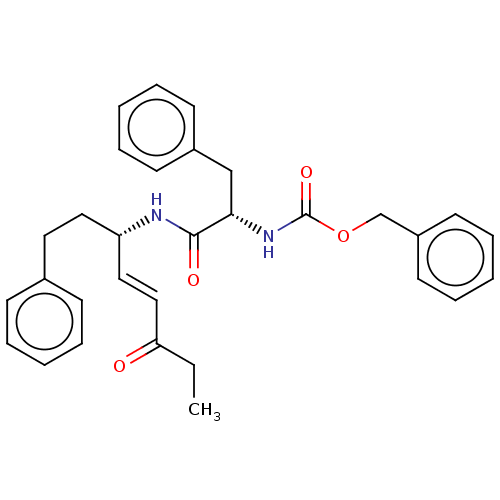
(CHEMBL4459413)Show SMILES CCC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C31H34N2O4/c1-2-28(34)21-20-27(19-18-24-12-6-3-7-13-24)32-30(35)29(22-25-14-8-4-9-15-25)33-31(36)37-23-26-16-10-5-11-17-26/h3-17,20-21,27,29H,2,18-19,22-23H2,1H3,(H,32,35)(H,33,36)/b21-20+/t27-,29-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50509394

(CHEMBL4584291)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C29H29ClN2O3/c1-21(33)12-18-26(19-13-22-8-4-2-5-9-22)31-29(35)27(20-23-10-6-3-7-11-23)32-28(34)24-14-16-25(30)17-15-24/h2-12,14-18,26-27H,13,19-20H2,1H3,(H,31,35)(H,32,34)/b18-12+/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin L assessed as inhibition constant using Cbz-Phe-Arg-AMC as substrate by fluorescence assay |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Cysteine protease
(Trypanosoma brucei rhodesiense) | BDBM50509392

(CHEMBL4456010)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C31H32N2O5/c1-22(34)12-15-26(16-13-23-8-4-2-5-9-23)32-31(36)27(20-24-10-6-3-7-11-24)33-30(35)25-14-17-28-29(21-25)38-19-18-37-28/h2-12,14-15,17,21,26-27H,13,16,18-20H2,1H3,(H,32,36)(H,33,35)/b15-12+/t26-,27+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 29-35 (2002)
Article DOI: 10.1124/jpet.102.036376
BindingDB Entry DOI: 10.7270/Q20R9N0G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 29-35 (2002)
Article DOI: 10.1124/jpet.102.036376
BindingDB Entry DOI: 10.7270/Q20R9N0G |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50509387

(CHEMBL4475066)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(F)cc1 |r| Show InChI InChI=1S/C29H29FN2O3/c1-21(33)12-18-26(19-13-22-8-4-2-5-9-22)31-29(35)27(20-23-10-6-3-7-11-23)32-28(34)24-14-16-25(30)17-15-24/h2-12,14-18,26-27H,13,19-20H2,1H3,(H,31,35)(H,32,34)/b18-12+/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin L assessed as inhibition constant using Cbz-Phe-Arg-AMC as substrate by fluorescence assay |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Cysteine protease
(Trypanosoma brucei rhodesiense) | BDBM50509384

(CHEMBL4456727)Show SMILES CN1CCN(CC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)\C=C\C(C)=O |r| Show InChI InChI=1S/C28H36N4O3/c1-22(33)13-15-25(16-14-23-9-5-3-6-10-23)29-27(34)26(21-24-11-7-4-8-12-24)30-28(35)32-19-17-31(2)18-20-32/h3-13,15,25-26H,14,16-21H2,1-2H3,(H,29,34)(H,30,35)/b15-13+/t25-,26+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50110183
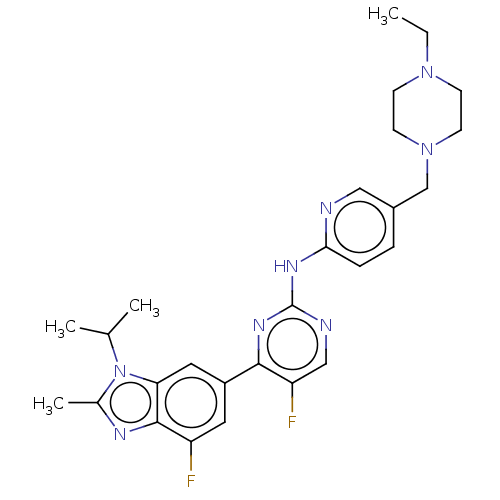
(Abemaciclib | LY-2835219 | US10626107, Example LY2...)Show SMILES CCN1CCN(Cc2ccc(Nc3ncc(F)c(n3)-c3cc(F)c4nc(C)n(C(C)C)c4c3)nc2)CC1 Show InChI InChI=1S/C27H32F2N8/c1-5-35-8-10-36(11-9-35)16-19-6-7-24(30-14-19)33-27-31-15-22(29)25(34-27)20-12-21(28)26-23(13-20)37(17(2)3)18(4)32-26/h6-7,12-15,17H,5,8-11,16H2,1-4H3,(H,30,31,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged CDK4 (4 to 303 residues)/cyclin D1 (4 to 295 residues) expressed in sf9 cells using C-terminal ... |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50229973
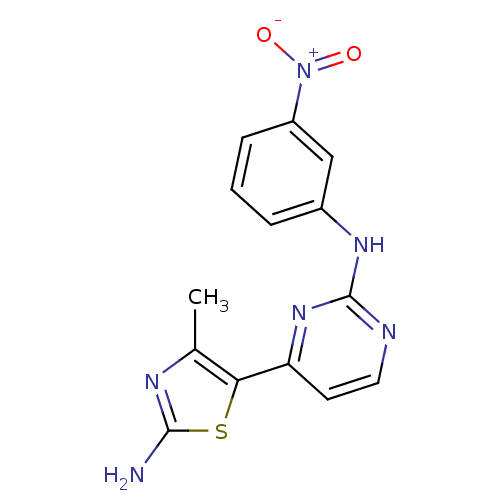
((4-(2-Amino-4-methylthiazol-5-yl)pyrimidin-2-yl)-(...)Show SMILES Cc1nc(N)sc1-c1ccnc(Nc2cccc(c2)[N+]([O-])=O)n1 Show InChI InChI=1S/C14H12N6O2S/c1-8-12(23-13(15)17-8)11-5-6-16-14(19-11)18-9-3-2-4-10(7-9)20(21)22/h2-7H,1H3,(H2,15,17)(H,16,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arkansas
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 303: 29-35 (2002)
Article DOI: 10.1124/jpet.102.036376
BindingDB Entry DOI: 10.7270/Q20R9N0G |
More data for this
Ligand-Target Pair | |
Cysteine protease
(Trypanosoma brucei rhodesiense) | BDBM50509385
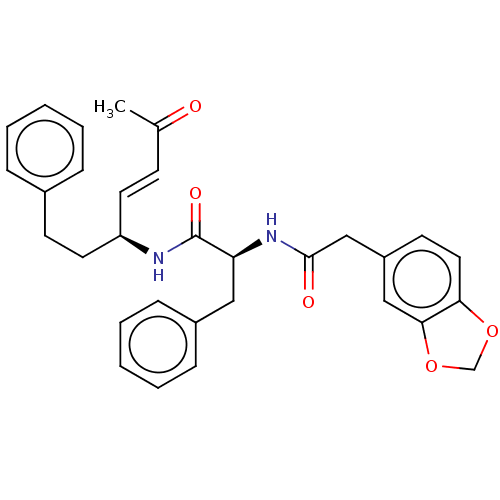
(CHEMBL4454883)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)Cc1ccc2OCOc2c1 |r| Show InChI InChI=1S/C31H32N2O5/c1-22(34)12-15-26(16-13-23-8-4-2-5-9-23)32-31(36)27(18-24-10-6-3-7-11-24)33-30(35)20-25-14-17-28-29(19-25)38-21-37-28/h2-12,14-15,17,19,26-27H,13,16,18,20-21H2,1H3,(H,32,36)(H,33,35)/b15-12+/t26-,27+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 7
(Homo sapiens (Human)) | BDBM81441

(CDK Inhibitor, 14)Show SMILES Cc1c(sc(=O)n1C)-c1ccnc(Nc2ccc(cc2)N2CCNCC2)n1 Show InChI InChI=1S/C19H22N6OS/c1-13-17(27-19(26)24(13)2)16-7-8-21-18(23-16)22-14-3-5-15(6-4-14)25-11-9-20-10-12-25/h3-8,20H,9-12H2,1-2H3,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK7 (unknown origin) using (biotinyl-Ahx-(Tyr-Ser-ProThr-Ser-Pro-Ser)4-NH2 as substrate after 45 mins by [gamma-32P]ATP based microbet... |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | |
Cysteine protease
(Trypanosoma brucei rhodesiense) | BDBM50322349
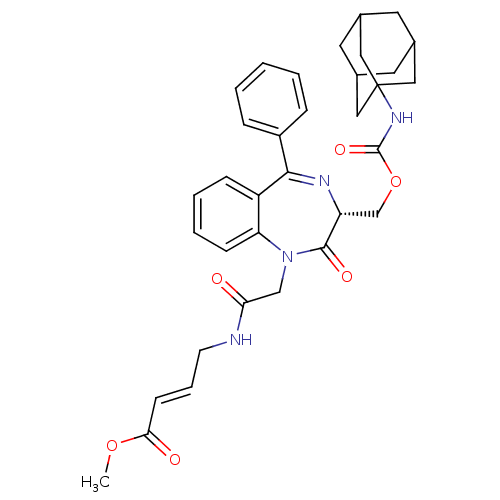
((R)-Methyl4-(2-(3-((adamantan-1-ylcarbamoyloxy)met...)Show SMILES COC(=O)\C=C\CNC(=O)CN1c2ccccc2C(=N[C@H](COC(=O)NC23CC4CC(CC(C4)C2)C3)C1=O)c1ccccc1 |r,c:19,TLB:25:26:29:33.32.31,THB:27:28:31:35.26.34,27:26:29.28.33:31,34:26:29:33.32.31,34:32:29:35.27.26,25:26:29.28.33:31| Show InChI InChI=1S/C34H38N4O6/c1-43-30(40)12-7-13-35-29(39)20-38-28-11-6-5-10-26(28)31(25-8-3-2-4-9-25)36-27(32(38)41)21-44-33(42)37-34-17-22-14-23(18-34)16-24(15-22)19-34/h2-12,22-24,27H,13-21H2,1H3,(H,35,39)(H,37,42)/b12-7+/t22?,23?,24?,27-,34?/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei rhodesiense rhodesain |
Bioorg Med Chem Lett 26: 3453-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.06.047
BindingDB Entry DOI: 10.7270/Q26D5VXZ |
More data for this
Ligand-Target Pair | |
Cysteine protease
(Trypanosoma brucei rhodesiense) | BDBM50509382

(CHEMBL4464737)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)N1CCOCC1 |r| Show InChI InChI=1S/C27H33N3O4/c1-21(31)12-14-24(15-13-22-8-4-2-5-9-22)28-26(32)25(20-23-10-6-3-7-11-23)29-27(33)30-16-18-34-19-17-30/h2-12,14,24-25H,13,15-20H2,1H3,(H,28,32)(H,29,33)/b14-12+/t24-,25+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50229973

((4-(2-Amino-4-methylthiazol-5-yl)pyrimidin-2-yl)-(...)Show SMILES Cc1nc(N)sc1-c1ccnc(Nc2cccc(c2)[N+]([O-])=O)n1 Show InChI InChI=1S/C14H12N6O2S/c1-8-12(23-13(15)17-8)11-5-6-16-14(19-11)18-9-3-2-4-10(7-9)20(21)22/h2-7H,1H3,(H2,15,17)(H,16,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK9/cyclin T1 (unknown origin) using (biotinyl-Ahx-(Tyr-Ser-ProThr-Ser-Pro-Ser)4-NH2 as substrate after 45 mins by [gamma-32P]ATP base... |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50509388

(CHEMBL4587965)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C30H29F3N2O3/c1-21(36)12-18-26(19-13-22-8-4-2-5-9-22)34-29(38)27(20-23-10-6-3-7-11-23)35-28(37)24-14-16-25(17-15-24)30(31,32)33/h2-12,14-18,26-27H,13,19-20H2,1H3,(H,34,38)(H,35,37)/b18-12+/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin L assessed as inhibition constant using Cbz-Phe-Arg-AMC as substrate by fluorescence assay |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50509386

(CHEMBL4515298)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccccc1F |r| Show InChI InChI=1S/C29H29FN2O3/c1-21(33)16-18-24(19-17-22-10-4-2-5-11-22)31-29(35)27(20-23-12-6-3-7-13-23)32-28(34)25-14-8-9-15-26(25)30/h2-16,18,24,27H,17,19-20H2,1H3,(H,31,35)(H,32,34)/b18-16+/t24-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin L assessed as inhibition constant using Cbz-Phe-Arg-AMC as substrate by fluorescence assay |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Cysteine protease
(Trypanosoma brucei rhodesiense) | BDBM50509393

(CHEMBL4438444)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](CCc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C31H34N2O4/c1-24(34)17-20-28(21-18-25-11-5-2-6-12-25)32-30(35)29(22-19-26-13-7-3-8-14-26)33-31(36)37-23-27-15-9-4-10-16-27/h2-17,20,28-29H,18-19,21-23H2,1H3,(H,32,35)(H,33,36)/b20-17+/t28-,29+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma brucei rhodesiense rhodesain expressed in Pichia pastoris assessed as inhibition constant measured for 30 mins using Cbz-Ph... |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Escherichia coli (strain K12)) | BDBM50226181
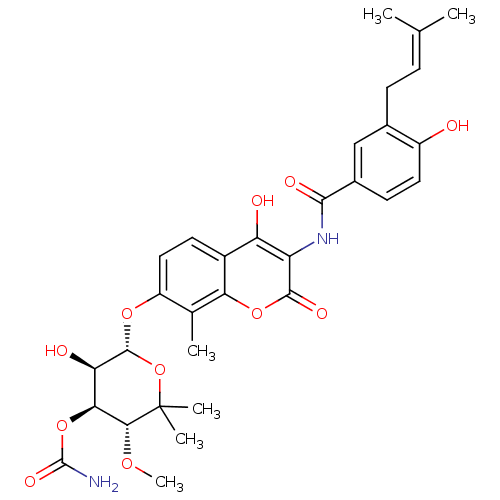
((3R,4S,5R,6R)-5-hydroxy-6-(4-hydroxy-3-(4-hydroxy-...)Show SMILES [#6]-[#8]-[#6@@H]1-[#6@@H](-[#8]-[#6](-[#7])=O)-[#6@@H](-[#8])-[#6@H](-[#8]-c2ccc3c(-[#8])c(-[#7]-[#6](=O)-c4ccc(-[#8])c(-[#6]\[#6]=[#6](/[#6])-[#6])c4)c(=O)oc3c2-[#6])-[#8]C1([#6])[#6] |r| Show InChI InChI=1S/C31H36N2O11/c1-14(2)7-8-16-13-17(9-11-19(16)34)27(37)33-21-22(35)18-10-12-20(15(3)24(18)42-28(21)38)41-29-23(36)25(43-30(32)39)26(40-6)31(4,5)44-29/h7,9-13,23,25-26,29,34-36H,8H2,1-6H3,(H2,32,39)(H,33,37)/t23-,25+,26-,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. C. Patel Institute of Pharmaceutical Education and Research
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli H560 DNA gyrase B ATPase activity after 30 mins by ammonium molybdate/malachite green-based phosphate detection assay |
Eur J Med Chem 124: 160-185 (2016)
Article DOI: 10.1016/j.ejmech.2016.08.034
BindingDB Entry DOI: 10.7270/Q2K64M38 |
More data for this
Ligand-Target Pair | |
Trace amine-associated receptor 1
(Homo sapiens (Human)) | BDBM85807

(Beta-PEA | CAS_60-12-8 | NSC_6054)Show InChI InChI=1S/C8H10O/c9-7-6-8-4-2-1-3-5-8/h1-5,9H,6-7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 8966-71 (2001)
Article DOI: 10.1073/pnas.151105198
BindingDB Entry DOI: 10.7270/Q2G15ZD5 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 6/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50110183

(Abemaciclib | LY-2835219 | US10626107, Example LY2...)Show SMILES CCN1CCN(Cc2ccc(Nc3ncc(F)c(n3)-c3cc(F)c4nc(C)n(C(C)C)c4c3)nc2)CC1 Show InChI InChI=1S/C27H32F2N8/c1-5-35-8-10-36(11-9-35)16-19-6-7-24(30-14-19)33-27-31-15-22(29)25(34-27)20-12-21(28)26-23(13-20)37(17(2)3)18(4)32-26/h6-7,12-15,17H,5,8-11,16H2,1-4H3,(H,30,31,33,34) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human N-terminal GST-tagged CDK6 (1 to 326 residues)/cyclin D1 (4 to 295 residues) expressed in sf9 cells using... |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Procathepsin L
(Homo sapiens (Human)) | BDBM50509395

(CHEMBL4437914)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C30H30N2O5/c1-21(33)12-15-25(16-13-22-8-4-2-5-9-22)31-30(35)26(18-23-10-6-3-7-11-23)32-29(34)24-14-17-27-28(19-24)37-20-36-27/h2-12,14-15,17,19,25-26H,13,16,18,20H2,1H3,(H,31,35)(H,32,34)/b15-12+/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin L assessed as inhibition constant using Cbz-Phe-Arg-AMC as substrate by fluorescence assay |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50509390

(CHEMBL4436812)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C29H28F2N2O3/c1-20(34)12-14-26(15-13-21-8-4-2-5-9-21)32-29(36)27(16-22-10-6-3-7-11-22)33-28(35)23-17-24(30)19-25(31)18-23/h2-12,14,17-19,26-27H,13,15-16H2,1H3,(H,32,36)(H,33,35)/b14-12+/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin L assessed as inhibition constant using Cbz-Phe-Arg-AMC as substrate by fluorescence assay |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50193090
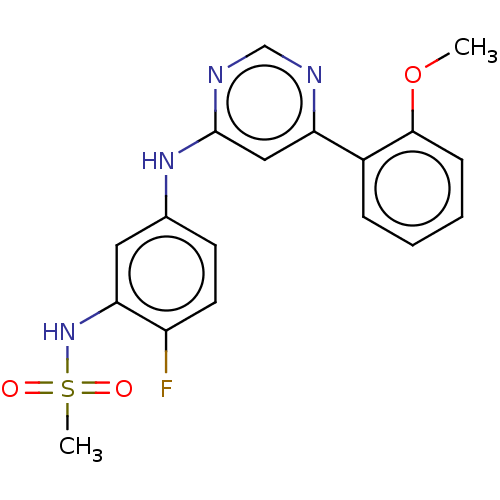
(CHEMBL3895785)Show SMILES COc1ccccc1-c1cc(Nc2ccc(F)c(NS(C)(=O)=O)c2)ncn1 Show InChI InChI=1S/C18H17FN4O3S/c1-26-17-6-4-3-5-13(17)15-10-18(21-11-20-15)22-12-7-8-14(19)16(9-12)23-27(2,24)25/h3-11,23H,1-2H3,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of human CDK9 (unknown origin) |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5 activator 1
(Homo sapiens (Human)) | BDBM50113281

(AT-7519)Show SMILES Clc1cccc(Cl)c1C(=O)Nc1c[nH]nc1C(=O)NC1CCNCC1 Show InChI InChI=1S/C16H17Cl2N5O2/c17-10-2-1-3-11(18)13(10)15(24)22-12-8-20-23-14(12)16(25)21-9-4-6-19-7-5-9/h1-3,8-9,19H,4-7H2,(H,20,23)(H,21,25)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length N-terminal His6-tagged CDK5/N-terminal GST-tagged p25 expressed in baculovirus infected Sf21 insect cells... |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | |
Trace amine-associated receptor 1
(Homo sapiens (Human)) | BDBM29135

(CHEMBL11608 | cid_5610 | p-Tyramine | tyramine)Show InChI InChI=1S/C8H11NO/c9-6-5-7-1-3-8(10)4-2-7/h1-4,10H,5-6,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 8966-71 (2001)
Article DOI: 10.1073/pnas.151105198
BindingDB Entry DOI: 10.7270/Q2G15ZD5 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50509389

(CHEMBL4566436)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc(F)cc1F |r| Show InChI InChI=1S/C29H28F2N2O3/c1-20(34)12-15-24(16-13-21-8-4-2-5-9-21)32-29(36)27(18-22-10-6-3-7-11-22)33-28(35)25-17-14-23(30)19-26(25)31/h2-12,14-15,17,19,24,27H,13,16,18H2,1H3,(H,32,36)(H,33,35)/b15-12+/t24-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin L assessed as inhibition constant using Cbz-Phe-Arg-AMC as substrate by fluorescence assay |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50113281

(AT-7519)Show SMILES Clc1cccc(Cl)c1C(=O)Nc1c[nH]nc1C(=O)NC1CCNCC1 Show InChI InChI=1S/C16H17Cl2N5O2/c17-10-2-1-3-11(18)13(10)15(24)22-12-8-20-23-14(12)16(25)21-9-4-6-19-7-5-9/h1-3,8-9,19H,4-7H2,(H,20,23)(H,21,25)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of human full length C-terminal His6-tagged CDK2/N-terminal GST-tagged cyclin A expressed in baculovirus infected Sf21 insect cells using ... |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Procathepsin L
(Homo sapiens (Human)) | BDBM50509381

(CHEMBL4453415)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cccc(F)c1 |r| Show InChI InChI=1S/C29H29FN2O3/c1-21(33)15-17-26(18-16-22-9-4-2-5-10-22)31-29(35)27(19-23-11-6-3-7-12-23)32-28(34)24-13-8-14-25(30)20-24/h2-15,17,20,26-27H,16,18-19H2,1H3,(H,31,35)(H,32,34)/b17-15+/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin L assessed as inhibition constant using Cbz-Phe-Arg-AMC as substrate by fluorescence assay |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50110183

(Abemaciclib | LY-2835219 | US10626107, Example LY2...)Show SMILES CCN1CCN(Cc2ccc(Nc3ncc(F)c(n3)-c3cc(F)c4nc(C)n(C(C)C)c4c3)nc2)CC1 Show InChI InChI=1S/C27H32F2N8/c1-5-35-8-10-36(11-9-35)16-19-6-7-24(30-14-19)33-27-31-15-22(29)25(34-27)20-12-21(28)26-23(13-20)37(17(2)3)18(4)32-26/h6-7,12-15,17H,5,8-11,16H2,1-4H3,(H,30,31,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK9/cyclin T (unknown origin) |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50113281

(AT-7519)Show SMILES Clc1cccc(Cl)c1C(=O)Nc1c[nH]nc1C(=O)NC1CCNCC1 Show InChI InChI=1S/C16H17Cl2N5O2/c17-10-2-1-3-11(18)13(10)15(24)22-12-8-20-23-14(12)16(25)21-9-4-6-19-7-5-9/h1-3,8-9,19H,4-7H2,(H,20,23)(H,21,25)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged CDK4/cyclin D1 expressed in baculovirus infected sf cells |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50509392

(CHEMBL4456010)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C31H32N2O5/c1-22(34)12-15-26(16-13-23-8-4-2-5-9-23)32-31(36)27(20-24-10-6-3-7-11-24)33-30(35)25-14-17-28-29(21-25)38-19-18-37-28/h2-12,14-15,17,21,26-27H,13,16,18-20H2,1H3,(H,32,36)(H,33,35)/b15-12+/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin L assessed as inhibition constant using Cbz-Phe-Arg-AMC as substrate by fluorescence assay |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50509385

(CHEMBL4454883)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)Cc1ccc2OCOc2c1 |r| Show InChI InChI=1S/C31H32N2O5/c1-22(34)12-15-26(16-13-23-8-4-2-5-9-23)32-31(36)27(18-24-10-6-3-7-11-24)33-30(35)20-25-14-17-28-29(19-25)38-21-37-28/h2-12,14-15,17,19,26-27H,13,16,18,20-21H2,1H3,(H,32,36)(H,33,35)/b15-12+/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin L assessed as inhibition constant using Cbz-Phe-Arg-AMC as substrate by fluorescence assay |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Cyclin-T1/Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50113281

(AT-7519)Show SMILES Clc1cccc(Cl)c1C(=O)Nc1c[nH]nc1C(=O)NC1CCNCC1 Show InChI InChI=1S/C16H17Cl2N5O2/c17-10-2-1-3-11(18)13(10)15(24)22-12-8-20-23-14(12)16(25)21-9-4-6-19-7-5-9/h1-3,8-9,19H,4-7H2,(H,20,23)(H,21,25)(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length C-terminal His6-tagged CDK9/cyclin T1 expressed in baculovirus infected Sf21 insect cells using PDKtide a... |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50509391

(CHEMBL4459413)Show SMILES CCC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C31H34N2O4/c1-2-28(34)21-20-27(19-18-24-12-6-3-7-13-24)32-30(35)29(22-25-14-8-4-9-15-25)33-31(36)37-23-26-16-10-5-11-17-26/h3-17,20-21,27,29H,2,18-19,22-23H2,1H3,(H,32,35)(H,33,36)/b21-20+/t27-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 108 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin L assessed as inhibition constant using Cbz-Phe-Arg-AMC as substrate by fluorescence assay |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM81441

(CDK Inhibitor, 14)Show SMILES Cc1c(sc(=O)n1C)-c1ccnc(Nc2ccc(cc2)N2CCNCC2)n1 Show InChI InChI=1S/C19H22N6OS/c1-13-17(27-19(26)24(13)2)16-7-8-21-18(23-16)22-14-3-5-15(6-4-14)25-11-9-20-10-12-25/h3-8,20H,9-12H2,1-2H3,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
Article
PubMed
| 149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 (unknown origin) |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50509384

(CHEMBL4456727)Show SMILES CN1CCN(CC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCc1ccccc1)\C=C\C(C)=O |r| Show InChI InChI=1S/C28H36N4O3/c1-22(33)13-15-25(16-14-23-9-5-3-6-10-23)29-27(34)26(21-24-11-7-4-8-12-24)30-28(35)32-19-17-31(2)18-20-32/h3-13,15,25-26H,14,16-21H2,1-2H3,(H,29,34)(H,30,35)/b15-13+/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin L assessed as inhibition constant using Cbz-Phe-Arg-AMC as substrate by fluorescence assay |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50509382

(CHEMBL4464737)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)N1CCOCC1 |r| Show InChI InChI=1S/C27H33N3O4/c1-21(31)12-14-24(15-13-22-8-4-2-5-9-22)28-26(32)25(20-23-10-6-3-7-11-23)29-27(33)30-16-18-34-19-17-30/h2-12,14,24-25H,13,15-20H2,1H3,(H,28,32)(H,29,33)/b14-12+/t24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin L assessed as inhibition constant using Cbz-Phe-Arg-AMC as substrate by fluorescence assay |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1
(Homo sapiens (Human)) | BDBM50113281

(AT-7519)Show SMILES Clc1cccc(Cl)c1C(=O)Nc1c[nH]nc1C(=O)NC1CCNCC1 Show InChI InChI=1S/C16H17Cl2N5O2/c17-10-2-1-3-11(18)13(10)15(24)22-12-8-20-23-14(12)16(25)21-9-4-6-19-7-5-9/h1-3,8-9,19H,4-7H2,(H,20,23)(H,21,25)(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of human full length C-terminal His6-tagged CDK1/N-terminal GST-tagged cyclin B expressed in baculovirus infected Sf21 insect cells using ... |
J Med Chem 59: 8667-8684 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00150
BindingDB Entry DOI: 10.7270/Q2G73GP8 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50509383

(CHEMBL4441635)Show SMILES CC(=O)\C=C\[C@H](CCc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)c1c(F)cccc1F |r| Show InChI InChI=1S/C29H28F2N2O3/c1-20(34)15-17-23(18-16-21-9-4-2-5-10-21)32-28(35)26(19-22-11-6-3-7-12-22)33-29(36)27-24(30)13-8-14-25(27)31/h2-15,17,23,26H,16,18-19H2,1H3,(H,32,35)(H,33,36)/b17-15+/t23-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina
Curated by ChEMBL
| Assay Description
Inhibition of human Cathepsin L assessed as inhibition constant using Cbz-Phe-Arg-AMC as substrate by fluorescence assay |
J Med Chem 62: 10617-10629 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00908
BindingDB Entry DOI: 10.7270/Q2Z60SCT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data