 Found 389 hits with Last Name = 'rankine' and Initial = 'n'
Found 389 hits with Last Name = 'rankine' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Procathepsin L
(Homo sapiens (Human)) | BDBM50414644
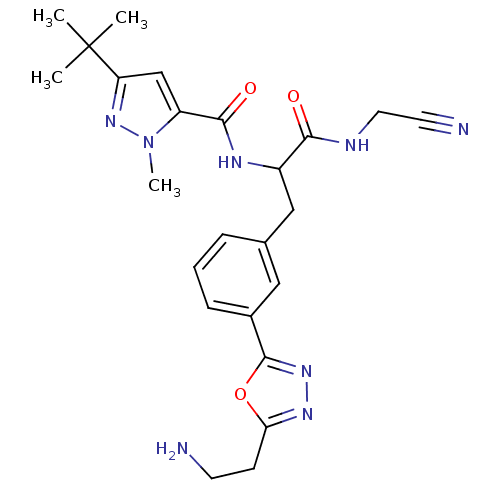
(CHEMBL555122)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nnc(CCN)o1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C24H30N8O3/c1-24(2,3)19-14-18(32(4)31-19)22(34)28-17(21(33)27-11-10-26)13-15-6-5-7-16(12-15)23-30-29-20(35-23)8-9-25/h5-7,12,14,17H,8-9,11,13,25H2,1-4H3,(H,27,33)(H,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50414643

(CHEMBL557455)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nnc(CN)o1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C23H28N8O3/c1-23(2,3)18-12-17(31(4)30-18)21(33)27-16(20(32)26-9-8-24)11-14-6-5-7-15(10-14)22-29-28-19(13-25)34-22/h5-7,10,12,16H,9,11,13,25H2,1-4H3,(H,26,32)(H,27,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM50414641
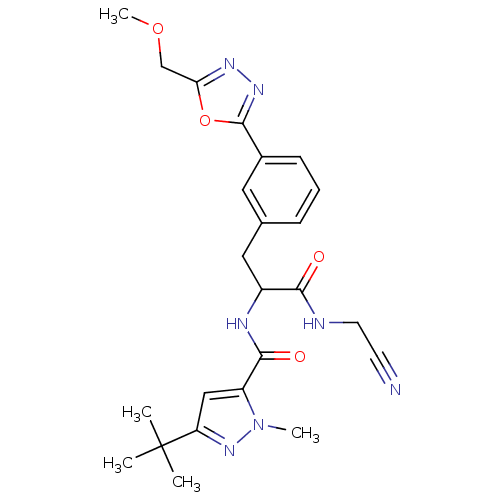
(CHEMBL554065)Show SMILES COCc1nnc(o1)-c1cccc(CC(NC(=O)c2cc(nn2C)C(C)(C)C)C(=O)NCC#N)c1 Show InChI InChI=1S/C24H29N7O4/c1-24(2,3)19-13-18(31(4)30-19)22(33)27-17(21(32)26-10-9-25)12-15-7-6-8-16(11-15)23-29-28-20(35-23)14-34-5/h6-8,11,13,17H,10,12,14H2,1-5H3,(H,26,32)(H,27,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L2 assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50414636
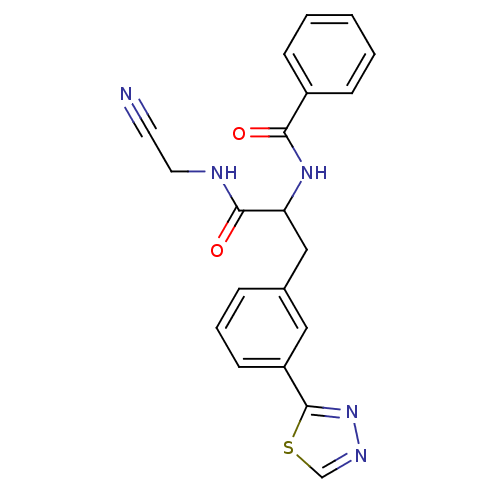
(CHEMBL559880)Show SMILES O=C(NCC#N)C(Cc1cccc(c1)-c1nncs1)NC(=O)c1ccccc1 Show InChI InChI=1S/C20H17N5O2S/c21-9-10-22-19(27)17(24-18(26)15-6-2-1-3-7-15)12-14-5-4-8-16(11-14)20-25-23-13-28-20/h1-8,11,13,17H,10,12H2,(H,22,27)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM50414640

(CHEMBL562844)Show SMILES Cc1nnc(s1)-c1cccc(CC(NC(=O)c2cc(nn2C)C(C)(C)C)C(=O)NCC#N)c1 Show InChI InChI=1S/C23H27N7O2S/c1-14-27-28-22(33-14)16-8-6-7-15(11-16)12-17(20(31)25-10-9-24)26-21(32)18-13-19(23(2,3)4)29-30(18)5/h6-8,11,13,17H,10,12H2,1-5H3,(H,25,31)(H,26,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L2 assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50414641

(CHEMBL554065)Show SMILES COCc1nnc(o1)-c1cccc(CC(NC(=O)c2cc(nn2C)C(C)(C)C)C(=O)NCC#N)c1 Show InChI InChI=1S/C24H29N7O4/c1-24(2,3)19-13-18(31(4)30-19)22(33)27-17(21(32)26-10-9-25)12-15-7-6-8-16(11-15)23-29-28-20(35-23)14-34-5/h6-8,11,13,17H,10,12,14H2,1-5H3,(H,26,32)(H,27,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50414638
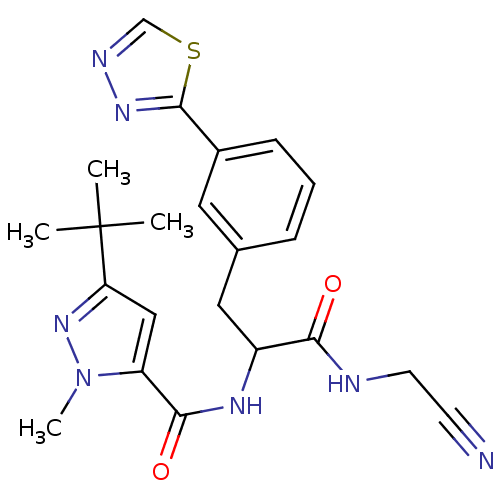
(CHEMBL549378)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nncs1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C22H25N7O2S/c1-22(2,3)18-12-17(29(4)28-18)20(31)26-16(19(30)24-9-8-23)11-14-6-5-7-15(10-14)21-27-25-13-32-21/h5-7,10,12-13,16H,9,11H2,1-4H3,(H,24,30)(H,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50414639
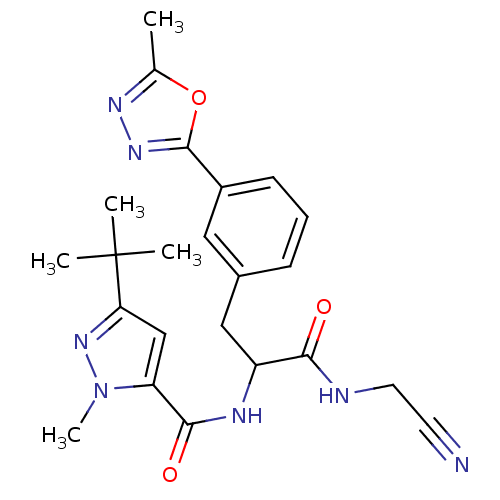
(CHEMBL550872)Show SMILES Cc1nnc(o1)-c1cccc(CC(NC(=O)c2cc(nn2C)C(C)(C)C)C(=O)NCC#N)c1 Show InChI InChI=1S/C23H27N7O3/c1-14-27-28-22(33-14)16-8-6-7-15(11-16)12-17(20(31)25-10-9-24)26-21(32)18-13-19(23(2,3)4)29-30(18)5/h6-8,11,13,17H,10,12H2,1-5H3,(H,25,31)(H,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50414642

(CHEMBL549791)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nnc(CO)o1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C23H27N7O4/c1-23(2,3)18-12-17(30(4)29-18)21(33)26-16(20(32)25-9-8-24)11-14-6-5-7-15(10-14)22-28-27-19(13-31)34-22/h5-7,10,12,16,31H,9,11,13H2,1-4H3,(H,25,32)(H,26,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM50414639

(CHEMBL550872)Show SMILES Cc1nnc(o1)-c1cccc(CC(NC(=O)c2cc(nn2C)C(C)(C)C)C(=O)NCC#N)c1 Show InChI InChI=1S/C23H27N7O3/c1-14-27-28-22(33-14)16-8-6-7-15(11-16)12-17(20(31)25-10-9-24)26-21(32)18-13-19(23(2,3)4)29-30(18)5/h6-8,11,13,17H,10,12H2,1-5H3,(H,25,31)(H,26,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L2 assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM50414638

(CHEMBL549378)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nncs1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C22H25N7O2S/c1-22(2,3)18-12-17(29(4)28-18)20(31)26-16(19(30)24-9-8-23)11-14-6-5-7-15(10-14)21-27-25-13-32-21/h5-7,10,12-13,16H,9,11H2,1-4H3,(H,24,30)(H,26,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L2 assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM50414642

(CHEMBL549791)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nnc(CO)o1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C23H27N7O4/c1-23(2,3)18-12-17(30(4)29-18)21(33)26-16(20(32)25-9-8-24)11-14-6-5-7-15(10-14)22-28-27-19(13-31)34-22/h5-7,10,12,16,31H,9,11,13H2,1-4H3,(H,25,32)(H,26,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L2 assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50414633
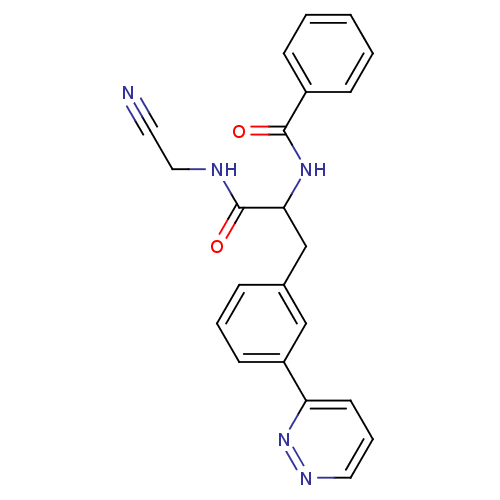
(CHEMBL556436)Show SMILES O=C(NCC#N)C(Cc1cccc(c1)-c1cccnn1)NC(=O)c1ccccc1 Show InChI InChI=1S/C22H19N5O2/c23-11-13-24-22(29)20(26-21(28)17-7-2-1-3-8-17)15-16-6-4-9-18(14-16)19-10-5-12-25-27-19/h1-10,12,14,20H,13,15H2,(H,24,29)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V
(Homo sapiens (Human)) | BDBM50296598
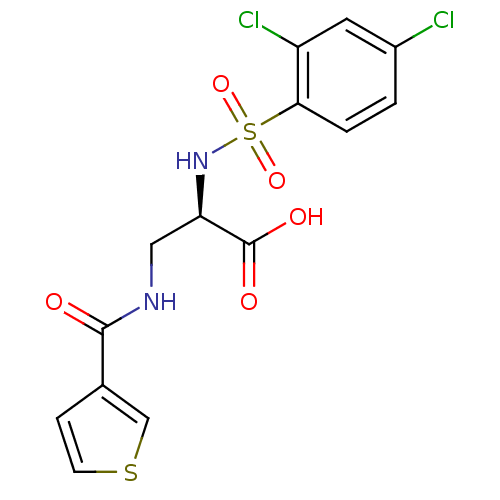
((R)-2-(2,4-dichlorophenylsulfonamido)-3-(thiophene...)Show SMILES OC(=O)[C@@H](CNC(=O)c1ccsc1)NS(=O)(=O)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C14H12Cl2N2O5S2/c15-9-1-2-12(10(16)5-9)25(22,23)18-11(14(20)21)6-17-13(19)8-3-4-24-7-8/h1-5,7,11,18H,6H2,(H,17,19)(H,20,21)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of fibrinogen binding to alphavbeta3 integrin |
Bioorg Med Chem Lett 19: 4832-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.041
BindingDB Entry DOI: 10.7270/Q2CJ8DJQ |
More data for this
Ligand-Target Pair | |
Integrin alpha-V
(Homo sapiens (Human)) | BDBM50296610

((R)-3-(1-methyl-1H-pyrazole-5-carboxamido)-2-(2,4,...)Show SMILES Cc1cc(C)c(c(C)c1)S(=O)(=O)N[C@H](CNC(=O)c1ccnn1C)C(O)=O |r| Show InChI InChI=1S/C17H22N4O5S/c1-10-7-11(2)15(12(3)8-10)27(25,26)20-13(17(23)24)9-18-16(22)14-5-6-19-21(14)4/h5-8,13,20H,9H2,1-4H3,(H,18,22)(H,23,24)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of fibrinogen binding to alphavbeta3 integrin |
Bioorg Med Chem Lett 19: 4832-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.041
BindingDB Entry DOI: 10.7270/Q2CJ8DJQ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM31992
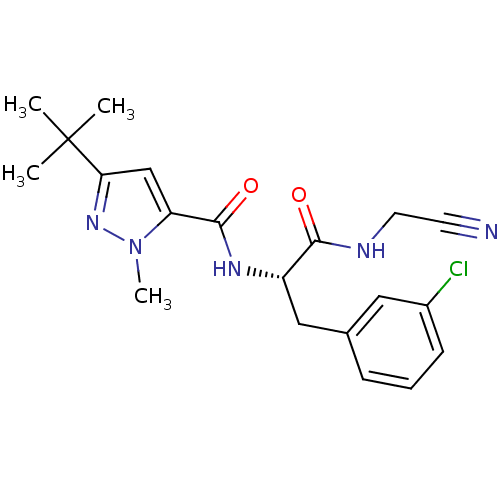
(Dipeptidyl nitrile inhibitor, 25)Show SMILES Cn1nc(cc1C(=O)N[C@@H](Cc1cccc(Cl)c1)C(=O)NCC#N)C(C)(C)C |r| Show InChI InChI=1S/C20H24ClN5O2/c1-20(2,3)17-12-16(26(4)25-17)19(28)24-15(18(27)23-9-8-22)11-13-6-5-7-14(21)10-13/h5-7,10,12,15H,9,11H2,1-4H3,(H,23,27)(H,24,28)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM31993

(Dipeptidyl nitrile inhibitor, 26)Show SMILES Cc1cccc(C[C@H](NC(=O)c2cc(nn2C)C(C)(C)C)C(=O)NCC#N)c1 |r| Show InChI InChI=1S/C21H27N5O2/c1-14-7-6-8-15(11-14)12-16(19(27)23-10-9-22)24-20(28)17-13-18(21(2,3)4)25-26(17)5/h6-8,11,13,16H,10,12H2,1-5H3,(H,23,27)(H,24,28)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Procathepsin L [114-333,T223A]
(Homo sapiens (Human)) | BDBM31993

(Dipeptidyl nitrile inhibitor, 26)Show SMILES Cc1cccc(C[C@H](NC(=O)c2cc(nn2C)C(C)(C)C)C(=O)NCC#N)c1 |r| Show InChI InChI=1S/C21H27N5O2/c1-14-7-6-8-15(11-14)12-16(19(27)23-10-9-22)24-20(28)17-13-18(21(2,3)4)25-26(17)5/h6-8,11,13,16H,10,12H2,1-5H3,(H,23,27)(H,24,28)/t16-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca
| Assay Description
IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. |
Bioorg Med Chem Lett 19: 4280-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.071
BindingDB Entry DOI: 10.7270/Q2TT4PBM |
More data for this
Ligand-Target Pair | |
Procathepsin L [114-333,T223A]
(Homo sapiens (Human)) | BDBM31992

(Dipeptidyl nitrile inhibitor, 25)Show SMILES Cn1nc(cc1C(=O)N[C@@H](Cc1cccc(Cl)c1)C(=O)NCC#N)C(C)(C)C |r| Show InChI InChI=1S/C20H24ClN5O2/c1-20(2,3)17-12-16(26(4)25-17)19(28)24-15(18(27)23-9-8-22)11-13-6-5-7-14(21)10-13/h5-7,10,12,15H,9,11H2,1-4H3,(H,23,27)(H,24,28)/t15-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca
| Assay Description
IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. |
Bioorg Med Chem Lett 19: 4280-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.071
BindingDB Entry DOI: 10.7270/Q2TT4PBM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Cavia porcellus) | BDBM50003386
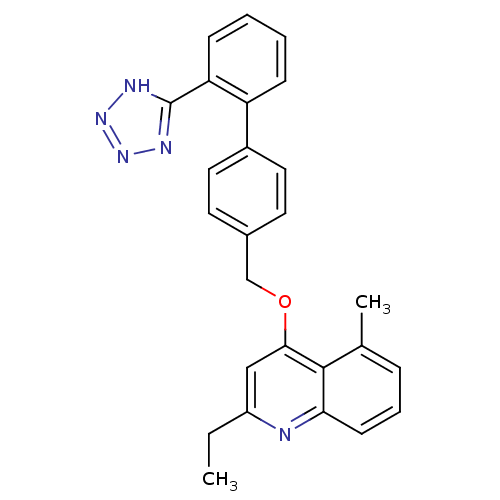
(2-Ethyl-5-methyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl...)Show SMILES CCc1cc(OCc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c2c(C)cccc2n1 Show InChI InChI=1S/C26H23N5O/c1-3-20-15-24(25-17(2)7-6-10-23(25)27-20)32-16-18-11-13-19(14-12-18)21-8-4-5-9-22(21)26-28-30-31-29-26/h4-15H,3,16H2,1-2H3,(H,28,29,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation |
J Med Chem 35: 4027-38 (1992)
BindingDB Entry DOI: 10.7270/Q2G44P8Q |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50414637
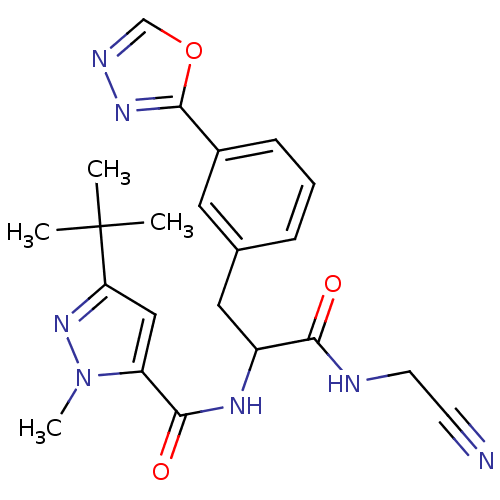
(CHEMBL562915)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nnco1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C22H25N7O3/c1-22(2,3)18-12-17(29(4)28-18)20(31)26-16(19(30)24-9-8-23)11-14-6-5-7-15(10-14)21-27-25-13-32-21/h5-7,10,12-13,16H,9,11H2,1-4H3,(H,24,30)(H,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50414638

(CHEMBL549378)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nncs1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C22H25N7O2S/c1-22(2,3)18-12-17(29(4)28-18)20(31)26-16(19(30)24-9-8-23)11-14-6-5-7-15(10-14)21-27-25-13-32-21/h5-7,10,12-13,16H,9,11H2,1-4H3,(H,24,30)(H,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50414629
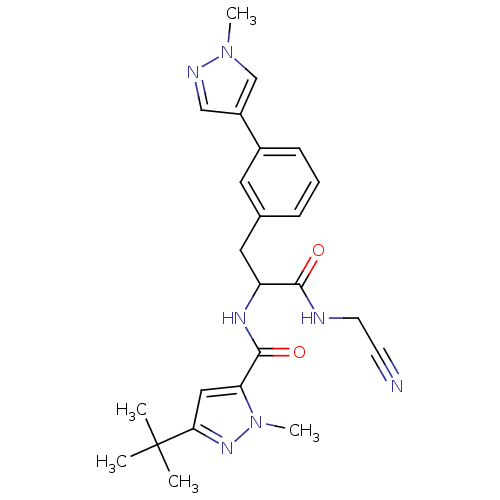
(CHEMBL563471)Show SMILES Cn1cc(cn1)-c1cccc(CC(NC(=O)c2cc(nn2C)C(C)(C)C)C(=O)NCC#N)c1 Show InChI InChI=1S/C24H29N7O2/c1-24(2,3)21-13-20(31(5)29-21)23(33)28-19(22(32)26-10-9-25)12-16-7-6-8-17(11-16)18-14-27-30(4)15-18/h6-8,11,13-15,19H,10,12H2,1-5H3,(H,26,32)(H,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Cavia porcellus) | BDBM50003392
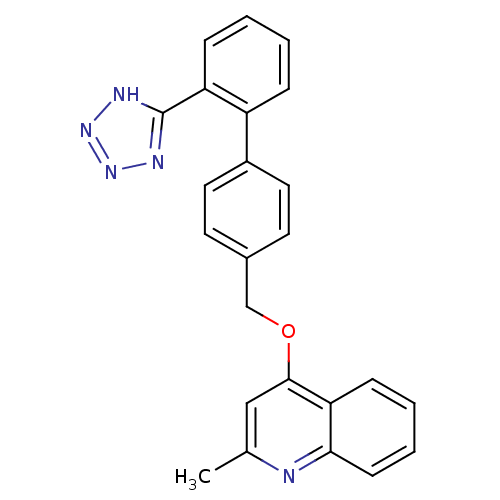
(2-Methyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES Cc1cc(OCc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c2ccccc2n1 Show InChI InChI=1S/C24H19N5O/c1-16-14-23(21-8-4-5-9-22(21)25-16)30-15-17-10-12-18(13-11-17)19-6-2-3-7-20(19)24-26-28-29-27-24/h2-14H,15H2,1H3,(H,26,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation |
J Med Chem 35: 4027-38 (1992)
BindingDB Entry DOI: 10.7270/Q2G44P8Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Cavia porcellus) | BDBM50406795
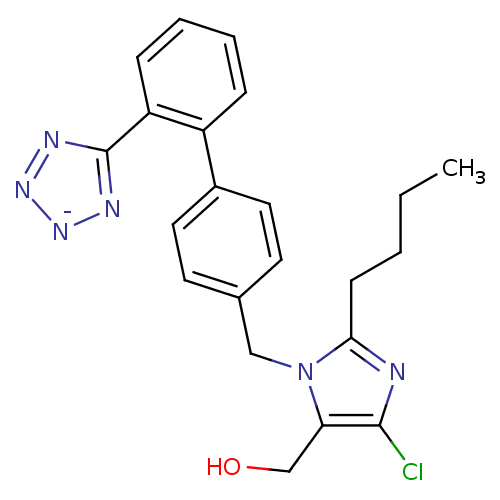
(Cozaar | LOSARTAN POTASSIUM)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nn[n-]n1 Show InChI InChI=1S/C22H22ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3/q-1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation |
J Med Chem 35: 4027-38 (1992)
BindingDB Entry DOI: 10.7270/Q2G44P8Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-V
(Homo sapiens (Human)) | BDBM50296635
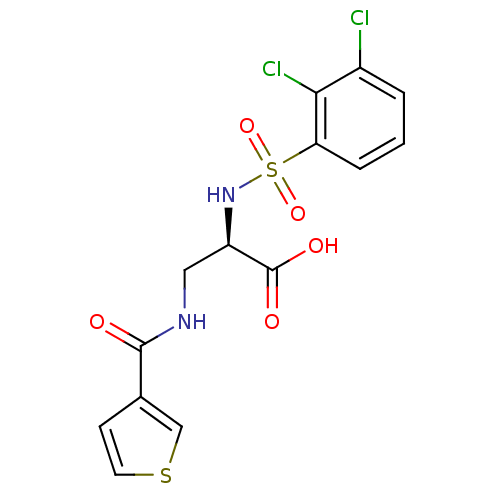
((R)-2-(2,3-dichlorophenylsulfonamido)-3-(thiophene...)Show SMILES OC(=O)[C@@H](CNC(=O)c1ccsc1)NS(=O)(=O)c1cccc(Cl)c1Cl |r| Show InChI InChI=1S/C14H12Cl2N2O5S2/c15-9-2-1-3-11(12(9)16)25(22,23)18-10(14(20)21)6-17-13(19)8-4-5-24-7-8/h1-5,7,10,18H,6H2,(H,17,19)(H,20,21)/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of fibrinogen binding to alphavbeta3 integrin |
Bioorg Med Chem Lett 19: 4832-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.041
BindingDB Entry DOI: 10.7270/Q2CJ8DJQ |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50414635
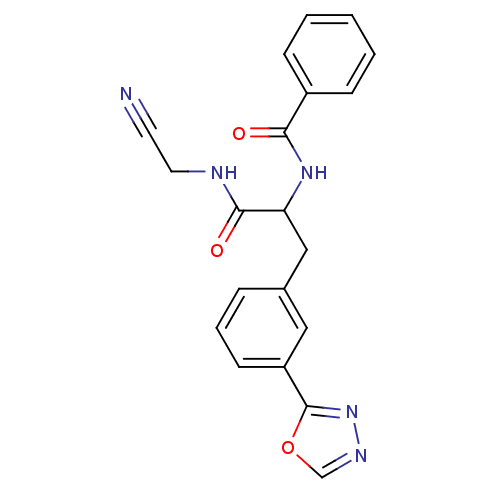
(CHEMBL564626)Show SMILES O=C(NCC#N)C(Cc1cccc(c1)-c1nnco1)NC(=O)c1ccccc1 Show InChI InChI=1S/C20H17N5O3/c21-9-10-22-19(27)17(24-18(26)15-6-2-1-3-7-15)12-14-5-4-8-16(11-14)20-25-23-13-28-20/h1-8,11,13,17H,10,12H2,(H,22,27)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM50414637

(CHEMBL562915)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nnco1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C22H25N7O3/c1-22(2,3)18-12-17(29(4)28-18)20(31)26-16(19(30)24-9-8-23)11-14-6-5-7-15(10-14)21-27-25-13-32-21/h5-7,10,12-13,16H,9,11H2,1-4H3,(H,24,30)(H,26,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L2 assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Procathepsin L [114-333,T223A]
(Homo sapiens (Human)) | BDBM31984

(Dipeptidyl nitrile inhibitor, 17)Show SMILES Cc1ccc2cc(C)cc(C(=O)N[C@@H](Cc3cccc(Cl)c3)C(=O)NCC#N)c2c1 |r| Show InChI InChI=1S/C24H22ClN3O2/c1-15-6-7-18-10-16(2)12-21(20(18)11-15)23(29)28-22(24(30)27-9-8-26)14-17-4-3-5-19(25)13-17/h3-7,10-13,22H,9,14H2,1-2H3,(H,27,30)(H,28,29)/t22-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca
| Assay Description
IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. |
Bioorg Med Chem Lett 19: 4280-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.071
BindingDB Entry DOI: 10.7270/Q2TT4PBM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Cavia porcellus) | BDBM50003400
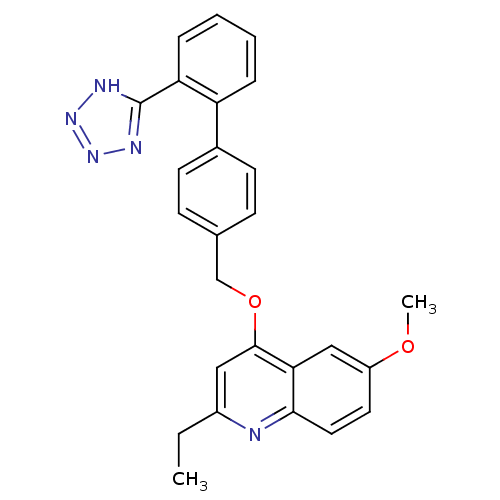
(2-Ethyl-6-methoxy-4-[2'-(1H-tetrazol-5-yl)-bipheny...)Show SMILES CCc1cc(OCc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c2cc(OC)ccc2n1 Show InChI InChI=1S/C26H23N5O2/c1-3-19-14-25(23-15-20(32-2)12-13-24(23)27-19)33-16-17-8-10-18(11-9-17)21-6-4-5-7-22(21)26-28-30-31-29-26/h4-15H,3,16H2,1-2H3,(H,28,29,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation |
J Med Chem 35: 4027-38 (1992)
BindingDB Entry DOI: 10.7270/Q2G44P8Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-V
(Homo sapiens (Human)) | BDBM50296597
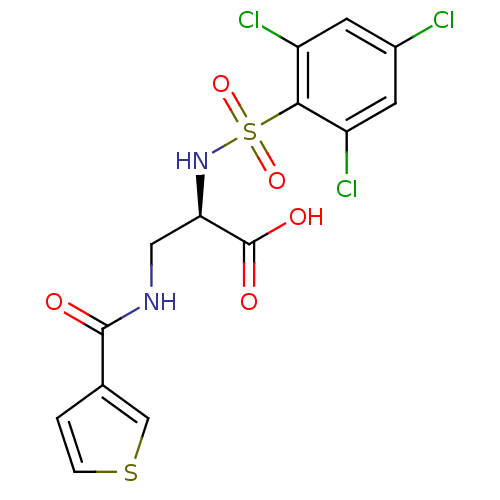
((R)-3-(thiophene-3-carboxamido)-2-(2,4,6-trichloro...)Show SMILES OC(=O)[C@@H](CNC(=O)c1ccsc1)NS(=O)(=O)c1c(Cl)cc(Cl)cc1Cl |r| Show InChI InChI=1S/C14H11Cl3N2O5S2/c15-8-3-9(16)12(10(17)4-8)26(23,24)19-11(14(21)22)5-18-13(20)7-1-2-25-6-7/h1-4,6,11,19H,5H2,(H,18,20)(H,21,22)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of fibrinogen binding to alphavbeta3 integrin |
Bioorg Med Chem Lett 19: 4832-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.041
BindingDB Entry DOI: 10.7270/Q2CJ8DJQ |
More data for this
Ligand-Target Pair | |
Integrin alpha-V
(Homo sapiens (Human)) | BDBM50296603
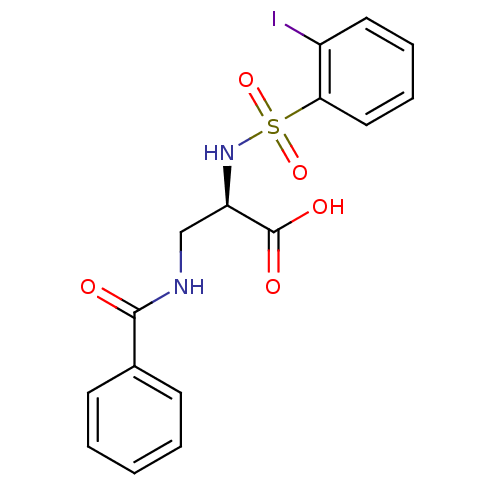
((R)-3-benzamido-2-(2-iodophenylsulfonamido)propano...)Show SMILES OC(=O)[C@@H](CNC(=O)c1ccccc1)NS(=O)(=O)c1ccccc1I |r| Show InChI InChI=1S/C16H15IN2O5S/c17-12-8-4-5-9-14(12)25(23,24)19-13(16(21)22)10-18-15(20)11-6-2-1-3-7-11/h1-9,13,19H,10H2,(H,18,20)(H,21,22)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of fibrinogen binding to alphavbeta3 integrin |
Bioorg Med Chem Lett 19: 4832-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.041
BindingDB Entry DOI: 10.7270/Q2CJ8DJQ |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM50414636

(CHEMBL559880)Show SMILES O=C(NCC#N)C(Cc1cccc(c1)-c1nncs1)NC(=O)c1ccccc1 Show InChI InChI=1S/C20H17N5O2S/c21-9-10-22-19(27)17(24-18(26)15-6-2-1-3-7-15)12-14-5-4-8-16(11-14)20-25-23-13-28-20/h1-8,11,13,17H,10,12H2,(H,22,27)(H,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L2 assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Cavia porcellus) | BDBM50003377
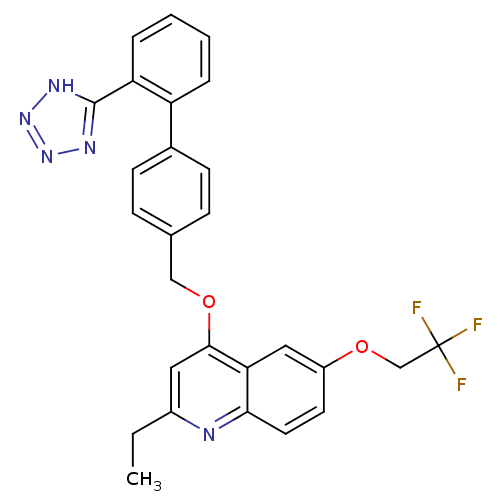
(2-Ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCc1cc(OCc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c2cc(OCC(F)(F)F)ccc2n1 Show InChI InChI=1S/C27H22F3N5O2/c1-2-19-13-25(23-14-20(11-12-24(23)31-19)37-16-27(28,29)30)36-15-17-7-9-18(10-8-17)21-5-3-4-6-22(21)26-32-34-35-33-26/h3-14H,2,15-16H2,1H3,(H,32,33,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation |
J Med Chem 35: 4027-38 (1992)
BindingDB Entry DOI: 10.7270/Q2G44P8Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Cavia porcellus) | BDBM50003403
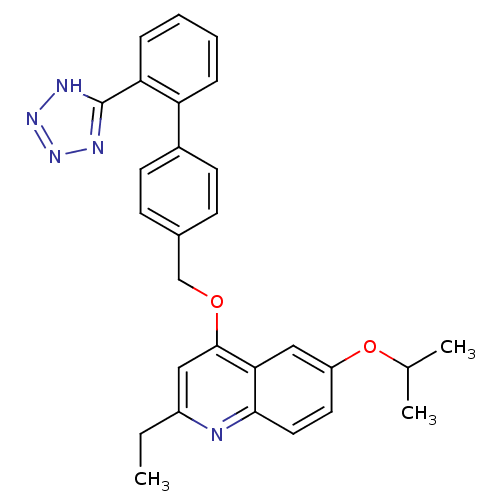
(2-Ethyl-6-isopropoxy-4-[2'-(1H-tetrazol-5-yl)-biph...)Show SMILES CCc1cc(OCc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c2cc(OC(C)C)ccc2n1 Show InChI InChI=1S/C28H27N5O2/c1-4-21-15-27(25-16-22(35-18(2)3)13-14-26(25)29-21)34-17-19-9-11-20(12-10-19)23-7-5-6-8-24(23)28-30-32-33-31-28/h5-16,18H,4,17H2,1-3H3,(H,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation |
J Med Chem 35: 4027-38 (1992)
BindingDB Entry DOI: 10.7270/Q2G44P8Q |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Cavia porcellus) | BDBM50003387
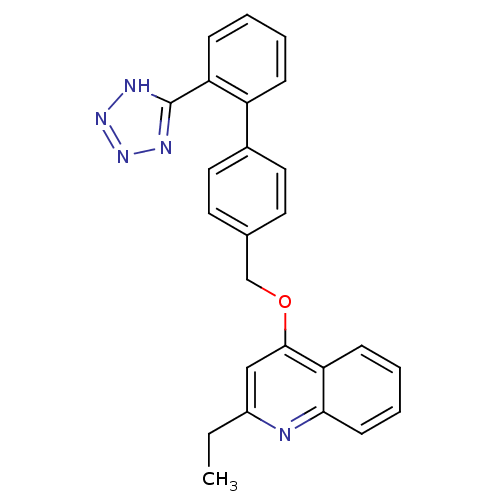
(2-Ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCc1cc(OCc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c2ccccc2n1 Show InChI InChI=1S/C25H21N5O/c1-2-19-15-24(22-9-5-6-10-23(22)26-19)31-16-17-11-13-18(14-12-17)20-7-3-4-8-21(20)25-27-29-30-28-25/h3-15H,2,16H2,1H3,(H,27,28,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation |
J Med Chem 35: 4027-38 (1992)
BindingDB Entry DOI: 10.7270/Q2G44P8Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-V
(Homo sapiens (Human)) | BDBM50296611
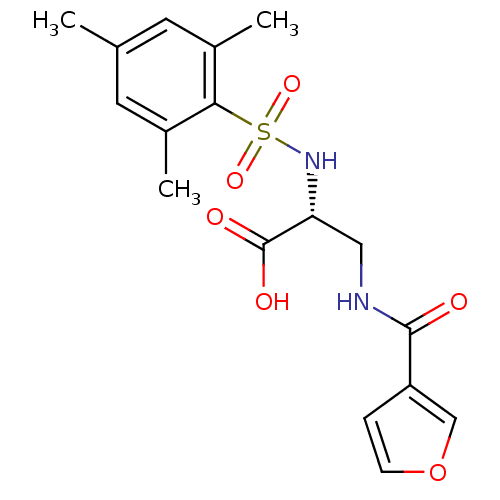
((R)-3-(furan-3-carboxamido)-2-(2,4,6-trimethylphen...)Show SMILES Cc1cc(C)c(c(C)c1)S(=O)(=O)N[C@H](CNC(=O)c1ccoc1)C(O)=O |r| Show InChI InChI=1S/C17H20N2O6S/c1-10-6-11(2)15(12(3)7-10)26(23,24)19-14(17(21)22)8-18-16(20)13-4-5-25-9-13/h4-7,9,14,19H,8H2,1-3H3,(H,18,20)(H,21,22)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of fibrinogen binding to alphavbeta3 integrin |
Bioorg Med Chem Lett 19: 4832-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.041
BindingDB Entry DOI: 10.7270/Q2CJ8DJQ |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM50414643

(CHEMBL557455)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nnc(CN)o1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C23H28N8O3/c1-23(2,3)18-12-17(31(4)30-18)21(33)27-16(20(32)26-9-8-24)11-14-6-5-7-15(10-14)22-29-28-19(13-25)34-22/h5-7,10,12,16H,9,11,13,25H2,1-4H3,(H,26,32)(H,27,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L2 assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Cavia porcellus) | BDBM50003394
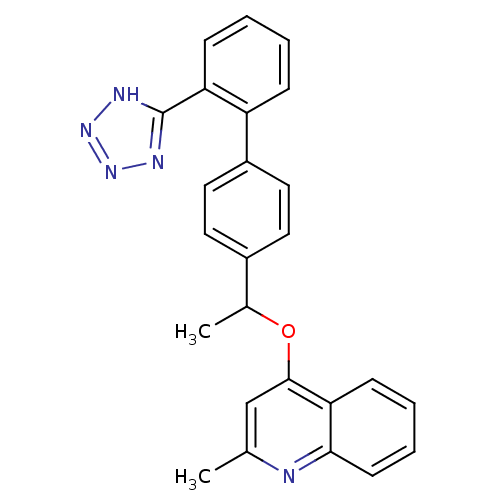
(2-Methyl-4-{1-[2'-(1H-tetrazol-5-yl)-biphenyl-4-yl...)Show SMILES CC(Oc1cc(C)nc2ccccc12)c1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C25H21N5O/c1-16-15-24(22-9-5-6-10-23(22)26-16)31-17(2)18-11-13-19(14-12-18)20-7-3-4-8-21(20)25-27-29-30-28-25/h3-15,17H,1-2H3,(H,27,28,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation |
J Med Chem 35: 4027-38 (1992)
BindingDB Entry DOI: 10.7270/Q2G44P8Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-V
(Homo sapiens (Human)) | BDBM50296609

((R)-3-(2-methyloxazole-4-carboxamido)-2-(2,4,6-tri...)Show SMILES Cc1nc(co1)C(=O)NC[C@@H](NS(=O)(=O)c1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C17H21N3O6S/c1-9-5-10(2)15(11(3)6-9)27(24,25)20-13(17(22)23)7-18-16(21)14-8-26-12(4)19-14/h5-6,8,13,20H,7H2,1-4H3,(H,18,21)(H,22,23)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of fibrinogen binding to alphavbeta3 integrin |
Bioorg Med Chem Lett 19: 4832-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.041
BindingDB Entry DOI: 10.7270/Q2CJ8DJQ |
More data for this
Ligand-Target Pair | |
Integrin alpha-V
(Homo sapiens (Human)) | BDBM50296612
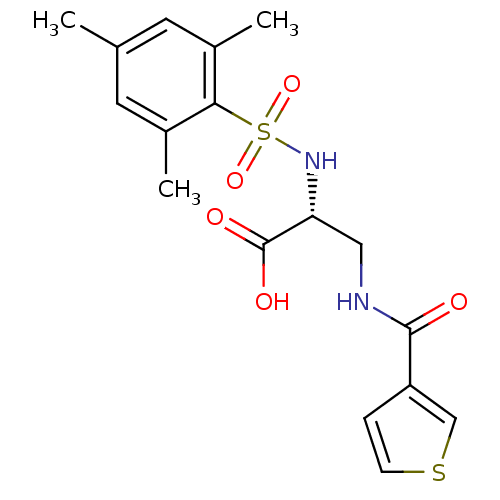
((R)-3-(thiophene-3-carboxamido)-2-(2,4,6-trimethyl...)Show SMILES Cc1cc(C)c(c(C)c1)S(=O)(=O)N[C@H](CNC(=O)c1ccsc1)C(O)=O |r| Show InChI InChI=1S/C17H20N2O5S2/c1-10-6-11(2)15(12(3)7-10)26(23,24)19-14(17(21)22)8-18-16(20)13-4-5-25-9-13/h4-7,9,14,19H,8H2,1-3H3,(H,18,20)(H,21,22)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of fibrinogen binding to alphavbeta3 integrin |
Bioorg Med Chem Lett 19: 4832-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.041
BindingDB Entry DOI: 10.7270/Q2CJ8DJQ |
More data for this
Ligand-Target Pair | |
Procathepsin L [114-333,T223A]
(Homo sapiens (Human)) | BDBM31980

(Dipeptidyl nitrile inhibitor, 13)Show SMILES Cc1cc(C)cc(c1)C(=O)N[C@@H](Cc1cccc(Cl)c1)C(=O)NCC#N |r| Show InChI InChI=1S/C20H20ClN3O2/c1-13-8-14(2)10-16(9-13)19(25)24-18(20(26)23-7-6-22)12-15-4-3-5-17(21)11-15/h3-5,8-11,18H,7,12H2,1-2H3,(H,23,26)(H,24,25)/t18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca
| Assay Description
IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. |
Bioorg Med Chem Lett 19: 4280-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.071
BindingDB Entry DOI: 10.7270/Q2TT4PBM |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Cavia porcellus) | BDBM50003376

(2-Ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...)Show SMILES CCc1cc(OCc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c2c(cccc2n1)C#N Show InChI InChI=1S/C26H20N6O/c1-2-20-14-24(25-19(15-27)6-5-9-23(25)28-20)33-16-17-10-12-18(13-11-17)21-7-3-4-8-22(21)26-29-31-32-30-26/h3-14H,2,16H2,1H3,(H,29,30,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of the specific binding of [125I]-angiotensin II to a guinea pig adrenal membrane preparation |
J Med Chem 35: 4027-38 (1992)
BindingDB Entry DOI: 10.7270/Q2G44P8Q |
More data for this
Ligand-Target Pair | |
Integrin alpha-V
(Homo sapiens (Human)) | BDBM50296600
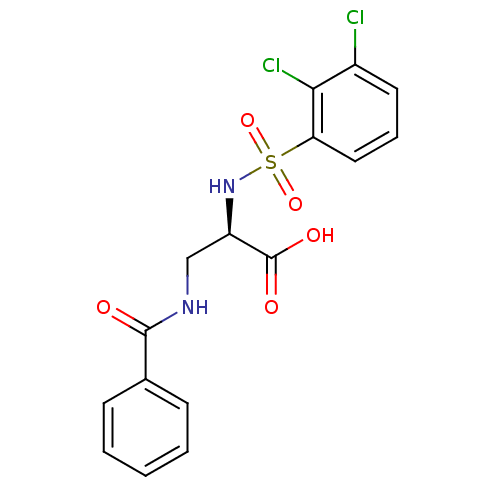
((R)-3-benzamido-2-(2,3-dichlorophenylsulfonamido)p...)Show SMILES OC(=O)[C@@H](CNC(=O)c1ccccc1)NS(=O)(=O)c1cccc(Cl)c1Cl |r| Show InChI InChI=1S/C16H14Cl2N2O5S/c17-11-7-4-8-13(14(11)18)26(24,25)20-12(16(22)23)9-19-15(21)10-5-2-1-3-6-10/h1-8,12,20H,9H2,(H,19,21)(H,22,23)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of fibrinogen binding to alphavbeta3 integrin |
Bioorg Med Chem Lett 19: 4832-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.041
BindingDB Entry DOI: 10.7270/Q2CJ8DJQ |
More data for this
Ligand-Target Pair | |
Procathepsin L [114-333,T223A]
(Homo sapiens (Human)) | BDBM31990
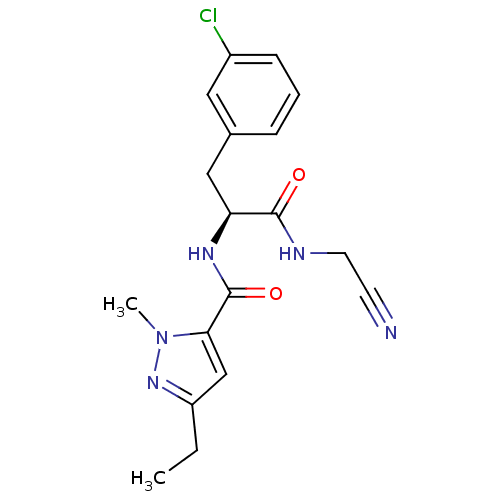
(Dipeptidyl nitrile inhibitor, 23)Show SMILES CCc1cc(C(=O)N[C@@H](Cc2cccc(Cl)c2)C(=O)NCC#N)n(C)n1 |r| Show InChI InChI=1S/C18H20ClN5O2/c1-3-14-11-16(24(2)23-14)18(26)22-15(17(25)21-8-7-20)10-12-5-4-6-13(19)9-12/h4-6,9,11,15H,3,8,10H2,1-2H3,(H,21,25)(H,22,26)/t15-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | 7.0 | 37 |
AstraZeneca
| Assay Description
IC50 values for inhibition of Cathepsins were determined from dose dependent inhibition of cleavage of fluorogenic, AMC-tagged, peptide substrates. |
Bioorg Med Chem Lett 19: 4280-3 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.071
BindingDB Entry DOI: 10.7270/Q2TT4PBM |
More data for this
Ligand-Target Pair | |
Integrin alpha-V
(Homo sapiens (Human)) | BDBM50296607
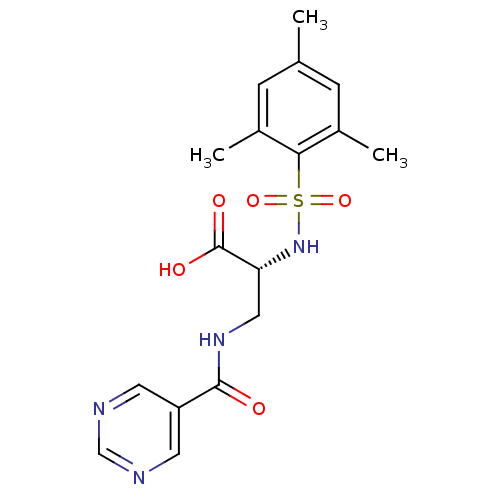
((R)-3-(pyrimidine-5-carboxamido)-2-(2,4,6-trimethy...)Show SMILES Cc1cc(C)c(c(C)c1)S(=O)(=O)N[C@H](CNC(=O)c1cncnc1)C(O)=O |r| Show InChI InChI=1S/C17H20N4O5S/c1-10-4-11(2)15(12(3)5-10)27(25,26)21-14(17(23)24)8-20-16(22)13-6-18-9-19-7-13/h4-7,9,14,21H,8H2,1-3H3,(H,20,22)(H,23,24)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of fibrinogen binding to alphavbeta3 integrin |
Bioorg Med Chem Lett 19: 4832-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.041
BindingDB Entry DOI: 10.7270/Q2CJ8DJQ |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50414642

(CHEMBL549791)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nnc(CO)o1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C23H27N7O4/c1-23(2,3)18-12-17(30(4)29-18)21(33)26-16(20(32)25-9-8-24)11-14-6-5-7-15(10-14)22-28-27-19(13-31)34-22/h5-7,10,12,16,31H,9,11,13H2,1-4H3,(H,25,32)(H,26,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50414644

(CHEMBL555122)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nnc(CCN)o1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C24H30N8O3/c1-24(2,3)19-14-18(32(4)31-19)22(34)28-17(21(33)27-11-10-26)13-15-6-5-7-16(12-15)23-30-29-20(35-23)8-9-25/h5-7,12,14,17H,8-9,11,13,25H2,1-4H3,(H,27,33)(H,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM50414633

(CHEMBL556436)Show SMILES O=C(NCC#N)C(Cc1cccc(c1)-c1cccnn1)NC(=O)c1ccccc1 Show InChI InChI=1S/C22H19N5O2/c23-11-13-24-22(29)20(26-21(28)17-7-2-1-3-8-17)15-16-6-4-9-18(14-16)19-10-5-12-25-27-19/h1-10,12,14,20H,13,15H2,(H,24,29)(H,26,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L2 assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50414639

(CHEMBL550872)Show SMILES Cc1nnc(o1)-c1cccc(CC(NC(=O)c2cc(nn2C)C(C)(C)C)C(=O)NCC#N)c1 Show InChI InChI=1S/C23H27N7O3/c1-14-27-28-22(33-14)16-8-6-7-15(11-16)12-17(20(31)25-10-9-24)26-21(32)18-13-19(23(2,3)4)29-30(18)5/h6-8,11,13,17H,10,12H2,1-5H3,(H,25,31)(H,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data