 Found 1364 hits with Last Name = 'rankovic' and Initial = 'z'
Found 1364 hits with Last Name = 'rankovic' and Initial = 'z' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50412114
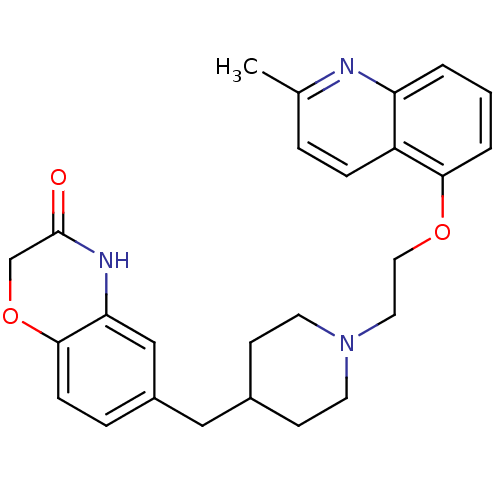
(CHEMBL183460 | SB-649915)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4ccc5OCC(=O)Nc5c4)CC3)cccc2n1 Show InChI InChI=1S/C26H29N3O3/c1-18-5-7-21-22(27-18)3-2-4-24(21)31-14-13-29-11-9-19(10-12-29)15-20-6-8-25-23(16-20)28-26(30)17-32-25/h2-8,16,19H,9-15,17H2,1H3,(H,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm causing 50% receptor occupancy against 5-hydroxytryptamine 1A receptor |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50150105
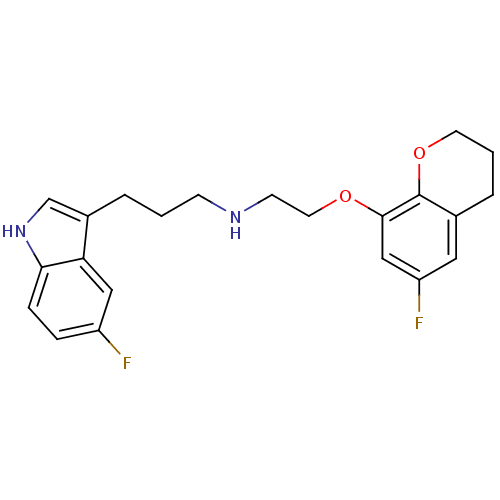
(CHEMBL124069 | [2-(6-Fluoro-chroman-8-yloxy)-ethyl...)Show SMILES Fc1cc2CCCOc2c(OCCNCCCc2c[nH]c3ccc(F)cc23)c1 Show InChI InChI=1S/C22H24F2N2O2/c23-17-5-6-20-19(12-17)16(14-26-20)3-1-7-25-8-10-27-21-13-18(24)11-15-4-2-9-28-22(15)21/h5-6,11-14,25-26H,1-4,7-10H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against serotonin transporter |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm causing 50% receptor occupancy against beta-2 adrenergic receptor |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027185

(CHEMBL183921)Show SMILES CC(COc1ccc2OCC(=O)Nc2c1)NCC(O)COc1cccc2[nH]ccc12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm causing 50% receptor occupancy against 5-hydroxytryptamine 1A receptor |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50175523
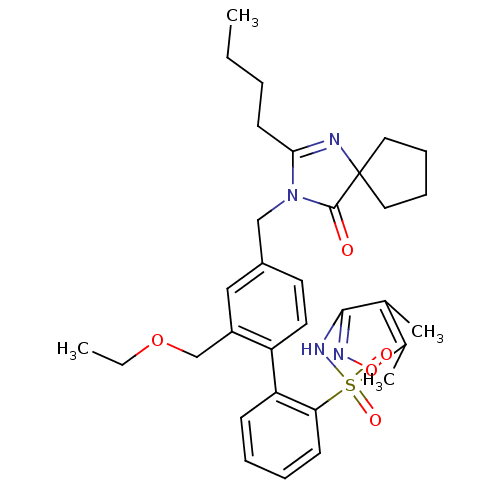
(4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(c(COCC)c1)-c1ccccc1S(=O)(=O)Nc1noc(C)c1C |t:4| Show InChI InChI=1S/C32H40N4O5S/c1-5-7-14-29-33-32(17-10-11-18-32)31(37)36(29)20-24-15-16-26(25(19-24)21-40-6-2)27-12-8-9-13-28(27)42(38,39)35-30-22(3)23(4)41-34-30/h8-9,12-13,15-16,19H,5-7,10-11,14,17-18,20-21H2,1-4H3,(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against angiotensin II receptor, type 1 |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50042235

(2-butyl-3-{[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 |t:4| Show InChI InChI=1S/C25H28N6O/c1-2-3-10-22-26-25(15-6-7-16-25)24(32)31(22)17-18-11-13-19(14-12-18)20-8-4-5-9-21(20)23-27-29-30-28-23/h4-5,8-9,11-14H,2-3,6-7,10,15-17H2,1H3,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against angiotensin II receptor, type 1 |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50109061
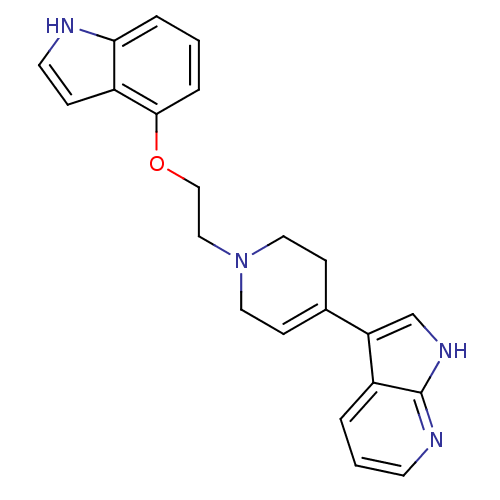
(3-{1-[2-(1H-Indol-4-yloxy)-ethyl]-1,2,3,6-tetrahyd...)Show SMILES C(CN1CCC(=CC1)c1c[nH]c2ncccc12)Oc1cccc2[nH]ccc12 |c:5| Show InChI InChI=1S/C22H22N4O/c1-4-20-18(6-10-23-20)21(5-1)27-14-13-26-11-7-16(8-12-26)19-15-25-22-17(19)3-2-9-24-22/h1-7,9-10,15,23H,8,11-14H2,(H,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against alpha adrenergic receptor |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416116

(CHEMBL1084315)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)N2CCCNCC2)c2ccccc12 Show InChI InChI=1S/C25H27N3O4S/c1-32-23-11-12-24(20-9-3-2-8-19(20)23)33(30,31)28-17-21(18-7-4-5-10-22(18)28)25(29)27-15-6-13-26-14-16-27/h2-5,7-12,21,26H,6,13-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50115848
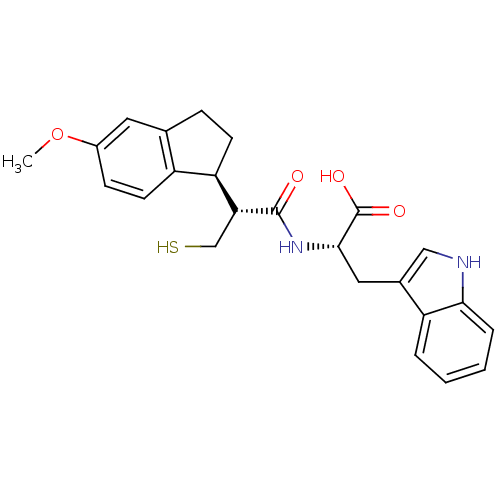
((S)-3-(1H-Indol-3-yl)-2-[(R)-3-mercapto-2-((S)-5-m...)Show SMILES COc1ccc2[C@@H](CCc2c1)[C@@H](CS)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C24H26N2O4S/c1-30-16-7-9-17-14(10-16)6-8-19(17)20(13-31)23(27)26-22(24(28)29)11-15-12-25-21-5-3-2-4-18(15)21/h2-5,7,9-10,12,19-20,22,25,31H,6,8,11,13H2,1H3,(H,26,27)(H,28,29)/t19-,20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against angiotensin I converting enzyme |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50109061

(3-{1-[2-(1H-Indol-4-yloxy)-ethyl]-1,2,3,6-tetrahyd...)Show SMILES C(CN1CCC(=CC1)c1c[nH]c2ncccc12)Oc1cccc2[nH]ccc12 |c:5| Show InChI InChI=1S/C22H22N4O/c1-4-20-18(6-10-23-20)21(5-1)27-14-13-26-11-7-16(8-12-26)19-15-25-22-17(19)3-2-9-24-22/h1-7,9-10,15,23H,8,11-14H2,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against serotonin transporter |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027184

(CHEMBL180700)Show InChI InChI=1S/C21H23N3O4/c25-21-14-28-20-6-5-15(13-18(20)24-21)26-12-10-22-8-2-11-27-19-4-1-3-17-16(19)7-9-23-17/h1,3-7,9,13,22-23H,2,8,10-12,14H2,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm causing 50% receptor occupancy against 5-hydroxytryptamine 1A receptor |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50117910
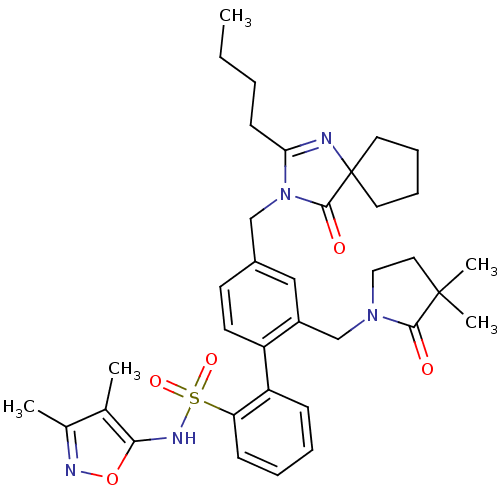
(4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(c(CN2CCC(C)(C)C2=O)c1)-c1ccccc1S(=O)(=O)Nc1onc(C)c1C |t:4| Show InChI InChI=1S/C36H45N5O5S/c1-6-7-14-31-37-36(17-10-11-18-36)34(43)41(31)22-26-15-16-28(27(21-26)23-40-20-19-35(4,5)33(40)42)29-12-8-9-13-30(29)47(44,45)39-32-24(2)25(3)38-46-32/h8-9,12-13,15-16,21,39H,6-7,10-11,14,17-20,22-23H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against endothelin receptor type A |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50057128

((2-Benzofuran-6-yl-ethyl)-((R)-5-methoxy-1,2,3,4-t...)Show InChI InChI=1S/C23H27NO2/c1-24(13-11-17-9-10-18-12-14-26-23(18)15-17)16-19-5-3-7-21-20(19)6-4-8-22(21)25-2/h4,6,8-10,12,14-15,19H,3,5,7,11,13,16H2,1-2H3/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against 5-hydroxytryptamine 1A receptor |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50175525
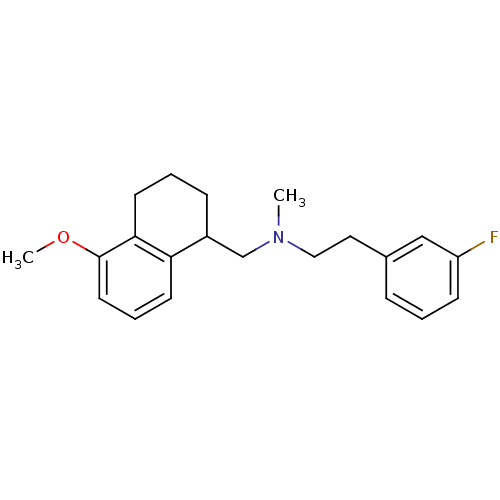
(CHEMBL198045 | [2-(3-Fluoro-phenyl)-ethyl]-(5-meth...)Show InChI InChI=1S/C21H26FNO/c1-23(13-12-16-6-3-8-18(22)14-16)15-17-7-4-10-20-19(17)9-5-11-21(20)24-2/h3,5-6,8-9,11,14,17H,4,7,10,12-13,15H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against 5-hydroxytryptamine 1A receptor |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4A
(Homo sapiens (Human)) | BDBM50513343
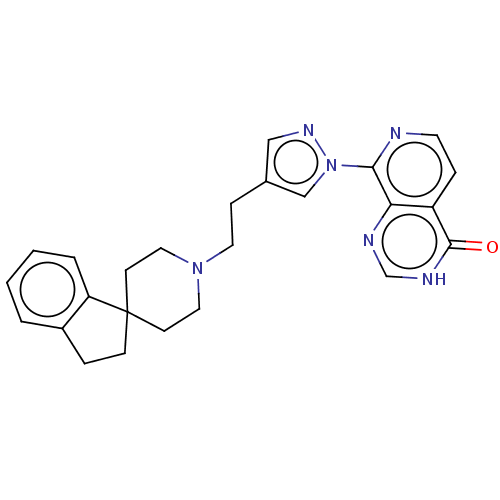
(CHEMBL4449500)Show SMILES O=c1[nH]cnc2c(nccc12)-n1cc(CCN2CCC3(CCc4ccccc34)CC2)cn1 Show InChI InChI=1S/C25H26N6O/c32-24-20-6-11-26-23(22(20)27-17-28-24)31-16-18(15-29-31)7-12-30-13-9-25(10-14-30)8-5-19-3-1-2-4-21(19)25/h1-4,6,11,15-17H,5,7-10,12-14H2,(H,27,28,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00680
BindingDB Entry DOI: 10.7270/Q2W95F76 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM84745

(CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...)Show InChI InChI=1S/C18H19NOS/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16/h2-10,13,17,19H,11-12H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against serotonin transporter |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50145347
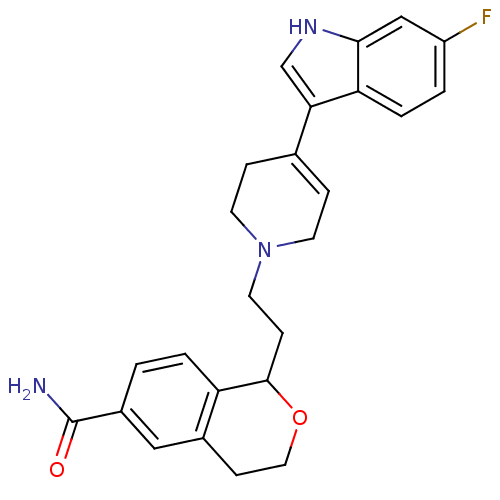
(1-(2-(4-(6-fluoro-1H-indol-3-yl)-5,6-dihydropyridi...)Show SMILES NC(=O)c1ccc2C(CCN3CCC(=CC3)c3c[nH]c4cc(F)ccc34)OCCc2c1 |c:13| Show InChI InChI=1S/C25H26FN3O2/c26-19-2-4-21-22(15-28-23(21)14-19)16-5-9-29(10-6-16)11-7-24-20-3-1-18(25(27)30)13-17(20)8-12-31-24/h1-5,13-15,24,28H,6-12H2,(H2,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against dopamine receptor D2 |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416120
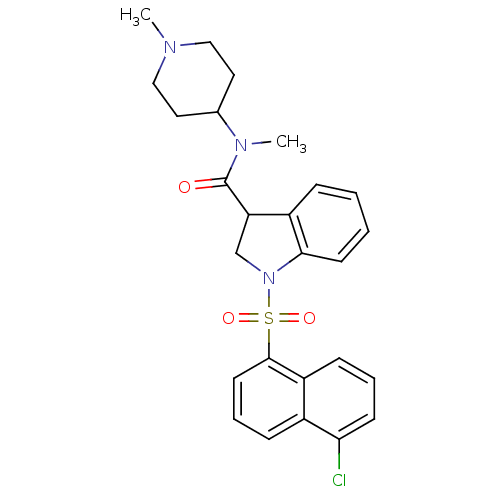
(CHEMBL1082484)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1cccc2c(Cl)cccc12 Show InChI InChI=1S/C26H28ClN3O3S/c1-28-15-13-18(14-16-28)29(2)26(31)22-17-30(24-11-4-3-7-20(22)24)34(32,33)25-12-6-8-19-21(25)9-5-10-23(19)27/h3-12,18,22H,13-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50108392

((3-ENDO)-8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL 1H-I...)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)c1c[nH]c2ccccc12 |r,THB:9:7:4.3:1| Show InChI InChI=1S/C17H20N2O2/c1-19-11-6-7-12(19)9-13(8-11)21-17(20)15-10-18-16-5-3-2-4-14(15)16/h2-5,10-13,18H,6-9H2,1H3/t11-,12+,13+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against 5-hydroxytryptamine 1A receptor |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50412114

(CHEMBL183460 | SB-649915)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4ccc5OCC(=O)Nc5c4)CC3)cccc2n1 Show InChI InChI=1S/C26H29N3O3/c1-18-5-7-21-22(27-18)3-2-4-24(21)31-14-13-29-11-9-19(10-12-29)15-20-6-8-25-23(16-20)28-26(30)17-32-25/h2-8,16,19H,9-15,17H2,1H3,(H,28,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm causing 50% receptor occupancy against serotonin transporter |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50057093
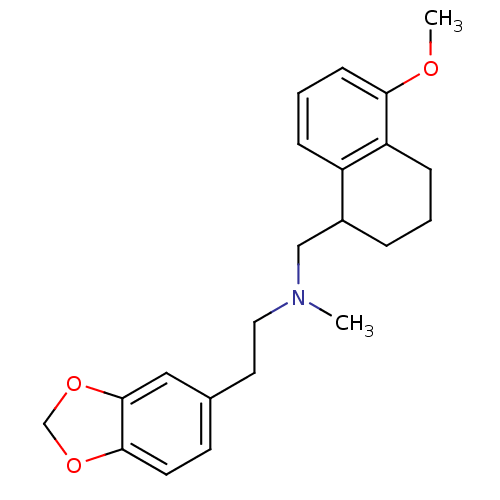
((2-Benzo[1,3]dioxol-5-yl-ethyl)-(5-methoxy-1,2,3,4...)Show InChI InChI=1S/C22H27NO3/c1-23(12-11-16-9-10-21-22(13-16)26-15-25-21)14-17-5-3-7-19-18(17)6-4-8-20(19)24-2/h4,6,8-10,13,17H,3,5,7,11-12,14-15H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against 5-hydroxytryptamine 1A receptor |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Homo sapiens (Human)) | BDBM50108392

((3-ENDO)-8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL 1H-I...)Show SMILES CN1[C@H]2CC[C@@H]1C[C@@H](C2)OC(=O)c1c[nH]c2ccccc12 |r,THB:9:7:4.3:1| Show InChI InChI=1S/C17H20N2O2/c1-19-11-6-7-12(19)9-13(8-11)21-17(20)15-10-18-16-5-3-2-4-14(15)16/h2-5,10-13,18H,6-9H2,1H3/t11-,12+,13+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against nicotinic acetylcholine receptor alpha7 |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 5B
(Homo sapiens (Human)) | BDBM50513343

(CHEMBL4449500)Show SMILES O=c1[nH]cnc2c(nccc12)-n1cc(CCN2CCC3(CCc4ccccc34)CC2)cn1 Show InChI InChI=1S/C25H26N6O/c32-24-20-6-11-26-23(22(20)27-17-28-24)31-16-18(15-29-31)7-12-30-13-9-25(10-14-30)8-5-19-3-1-2-4-21(19)25/h1-4,6,11,15-17H,5,7-10,12-14H2,(H,27,28,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00680
BindingDB Entry DOI: 10.7270/Q2W95F76 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50128875
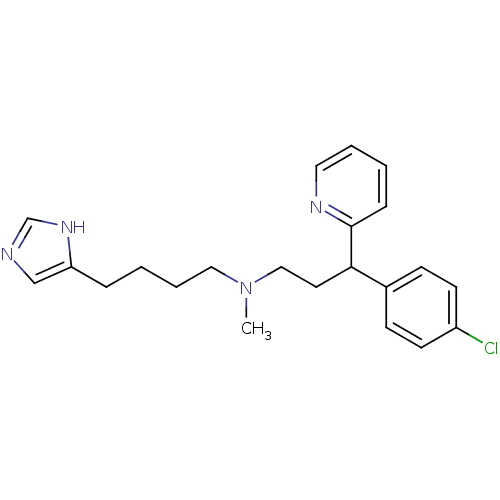
(CHEMBL292195 | [3-(4-Chloro-phenyl)-3-pyridin-2-yl...)Show SMILES CN(CCCCc1cnc[nH]1)CCC(c1ccc(Cl)cc1)c1ccccn1 Show InChI InChI=1S/C22H27ClN4/c1-27(14-5-3-6-20-16-24-17-26-20)15-12-21(22-7-2-4-13-25-22)18-8-10-19(23)11-9-18/h2,4,7-11,13,16-17,21H,3,5-6,12,14-15H2,1H3,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against histamine H1 receptor |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416108
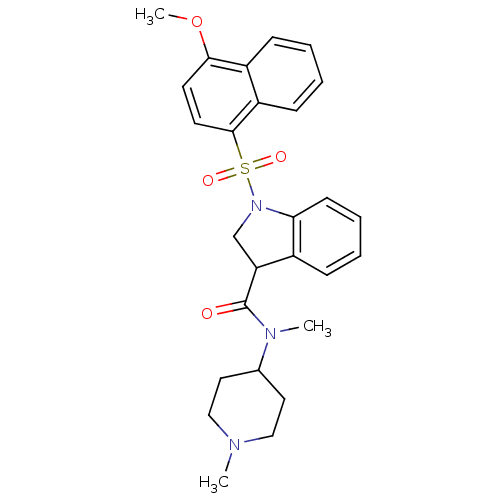
(CHEMBL1082818)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)N(C)C2CCN(C)CC2)c2ccccc12 Show InChI InChI=1S/C27H31N3O4S/c1-28-16-14-19(15-17-28)29(2)27(31)23-18-30(24-11-7-6-8-20(23)24)35(32,33)26-13-12-25(34-3)21-9-4-5-10-22(21)26/h4-13,19,23H,14-18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416114
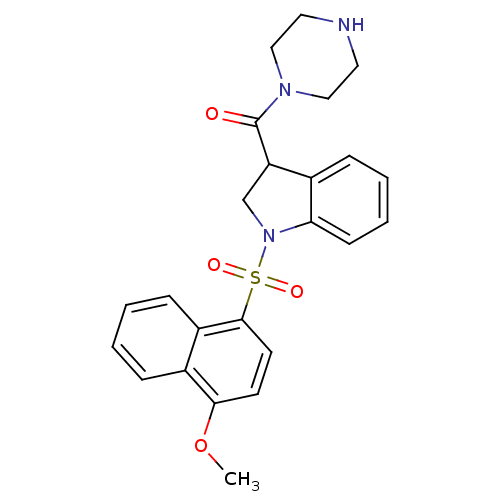
(CHEMBL1085608)Show SMILES COc1ccc(c2ccccc12)S(=O)(=O)N1CC(C(=O)N2CCNCC2)c2ccccc12 Show InChI InChI=1S/C24H25N3O4S/c1-31-22-10-11-23(19-8-3-2-7-18(19)22)32(29,30)27-16-20(17-6-4-5-9-21(17)27)24(28)26-14-12-25-13-15-26/h2-11,20,25H,12-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50082412
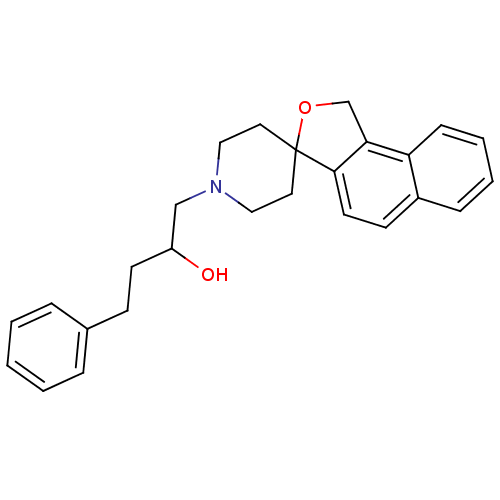
(1-phenyl-3-spiro[1,3-dihydrobenzo[e]isobenzofuran-...)Show SMILES OC(CCc1ccccc1)CN1CCC2(CC1)OCc1c2ccc2ccccc12 Show InChI InChI=1S/C26H29NO2/c28-22(12-10-20-6-2-1-3-7-20)18-27-16-14-26(15-17-27)25-13-11-21-8-4-5-9-23(21)24(25)19-29-26/h1-9,11,13,22,28H,10,12,14-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against 5-hydroxytryptamine 1A receptor |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50175523

(4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(c(COCC)c1)-c1ccccc1S(=O)(=O)Nc1noc(C)c1C |t:4| Show InChI InChI=1S/C32H40N4O5S/c1-5-7-14-29-33-32(17-10-11-18-32)31(37)36(29)20-24-15-16-26(25(19-24)21-40-6-2)27-12-8-9-13-28(27)42(38,39)35-30-22(3)23(4)41-34-30/h8-9,12-13,15-16,19H,5-7,10-11,14,17-18,20-21H2,1-4H3,(H,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against endothelin receptor type A |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50175502

(CHEMBL383581 | N-[2'-(4,5-Dimethyl-isoxazol-3-ylsu...)Show SMILES CN(Cc1cc(ccc1-c1ccccc1S(=O)(=O)Nc1noc(C)c1C)-c1ncco1)C(=O)CC(C)(C)C Show InChI InChI=1S/C28H32N4O5S/c1-18-19(2)37-30-26(18)31-38(34,35)24-10-8-7-9-23(24)22-12-11-20(27-29-13-14-36-27)15-21(22)17-32(6)25(33)16-28(3,4)5/h7-15H,16-17H2,1-6H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against endothelin receptor type A |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50117910

(4''-(2-Butyl-4-oxo-1,3-diaza-spiro[4.4]non-1-en-3-...)Show SMILES CCCCC1=NC2(CCCC2)C(=O)N1Cc1ccc(c(CN2CCC(C)(C)C2=O)c1)-c1ccccc1S(=O)(=O)Nc1onc(C)c1C |t:4| Show InChI InChI=1S/C36H45N5O5S/c1-6-7-14-31-37-36(17-10-11-18-36)34(43)41(31)22-26-15-16-28(27(21-26)23-40-20-19-35(4,5)33(40)42)29-12-8-9-13-30(29)47(44,45)39-32-24(2)25(3)38-46-32/h8-9,12-13,15-16,21,39H,6-7,10-11,14,17-20,22-23H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against angiotensin II receptor, type 1 |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50175502

(CHEMBL383581 | N-[2'-(4,5-Dimethyl-isoxazol-3-ylsu...)Show SMILES CN(Cc1cc(ccc1-c1ccccc1S(=O)(=O)Nc1noc(C)c1C)-c1ncco1)C(=O)CC(C)(C)C Show InChI InChI=1S/C28H32N4O5S/c1-18-19(2)37-30-26(18)31-38(34,35)24-10-8-7-9-23(24)22-12-11-20(27-29-13-14-36-27)15-21(22)17-32(6)25(33)16-28(3,4)5/h7-15H,16-17H2,1-6H3,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against angiotensin II receptor, type 1 |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Endothelin-converting enzyme 1
(Homo sapiens (Human)) | BDBM50115848

((S)-3-(1H-Indol-3-yl)-2-[(R)-3-mercapto-2-((S)-5-m...)Show SMILES COc1ccc2[C@@H](CCc2c1)[C@@H](CS)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C24H26N2O4S/c1-30-16-7-9-17-14(10-16)6-8-19(17)20(13-31)23(27)26-22(24(28)29)11-15-12-25-21-5-3-2-4-18(15)21/h2-5,7,9-10,12,19-20,22,25,31H,6,8,11,13H2,1H3,(H,26,27)(H,28,29)/t19-,20-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against endothelin converting enzyme 1 |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50082412

(1-phenyl-3-spiro[1,3-dihydrobenzo[e]isobenzofuran-...)Show SMILES OC(CCc1ccccc1)CN1CCC2(CC1)OCc1c2ccc2ccccc12 Show InChI InChI=1S/C26H29NO2/c28-22(12-10-20-6-2-1-3-7-20)18-27-16-14-26(15-17-27)25-13-11-21-8-4-5-9-23(21)24(25)19-29-26/h1-9,11,13,22,28H,10,12,14-19H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against serotonin transporter |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50109061

(3-{1-[2-(1H-Indol-4-yloxy)-ethyl]-1,2,3,6-tetrahyd...)Show SMILES C(CN1CCC(=CC1)c1c[nH]c2ncccc12)Oc1cccc2[nH]ccc12 |c:5| Show InChI InChI=1S/C22H22N4O/c1-4-20-18(6-10-23-20)21(5-1)27-14-13-26-11-7-16(8-12-26)19-15-25-22-17(19)3-2-9-24-22/h1-7,9-10,15,23H,8,11-14H2,(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against 5-hydroxytryptamine 1A receptor |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50002088
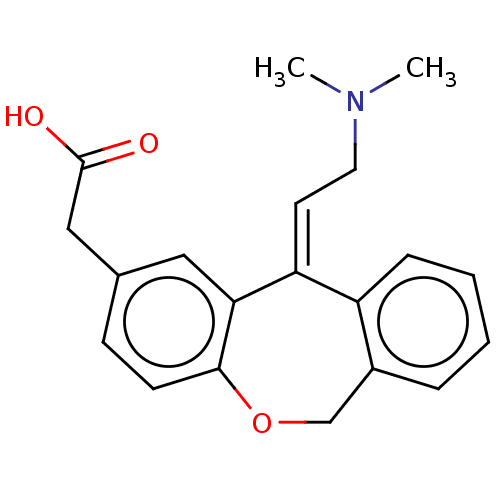
(CHEMBL302005 | [11-(2-Dimethylamino-ethylidene)-6,...)Show InChI InChI=1S/C20H21NO3/c1-21(2)10-9-17-16-6-4-3-5-15(16)13-24-19-8-7-14(11-18(17)19)12-20(22)23/h3-9,11H,10,12-13H2,1-2H3,(H,22,23)/b17-9+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against histamine H1 receptor |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22890

(2-(2-{4-[(4-chlorophenyl)(phenyl)methyl]piperazin-...)Show SMILES OC(=O)COCCN1CCN(CC1)C(c1ccccc1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H25ClN2O3/c22-19-8-6-18(7-9-19)21(17-4-2-1-3-5-17)24-12-10-23(11-13-24)14-15-27-16-20(25)26/h1-9,21H,10-16H2,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against histamine H1 receptor |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50175524
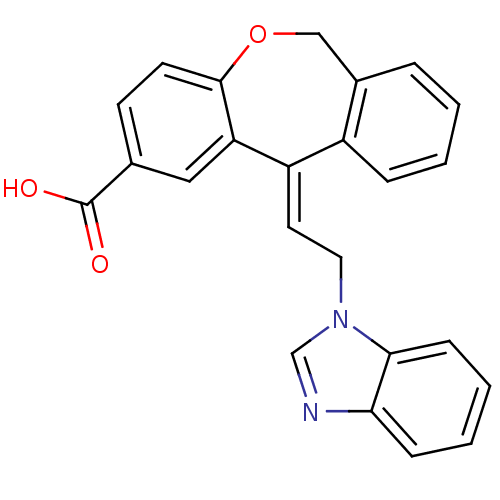
(11-[2-Benzoimidazol-1-yl-eth-(E)-ylidene]-6,11-dih...)Show SMILES OC(=O)c1ccc2OCc3ccccc3\C(=C/Cn3cnc4ccccc34)c2c1 Show InChI InChI=1S/C24H18N2O3/c27-24(28)16-9-10-23-20(13-16)19(18-6-2-1-5-17(18)14-29-23)11-12-26-15-25-21-7-3-4-8-22(21)26/h1-11,13,15H,12,14H2,(H,27,28)/b19-11+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against thromboxane A2 receptor to prostaglandin H2 receptor |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50128875

(CHEMBL292195 | [3-(4-Chloro-phenyl)-3-pyridin-2-yl...)Show SMILES CN(CCCCc1cnc[nH]1)CCC(c1ccc(Cl)cc1)c1ccccn1 Show InChI InChI=1S/C22H27ClN4/c1-27(14-5-3-6-20-16-24-17-26-20)15-12-21(22-7-2-4-13-25-22)18-8-10-19(23)11-9-18/h2,4,7-11,13,16-17,21H,3,5-6,12,14-15H2,1H3,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against histamine H3 receptor |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM84745

(CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...)Show InChI InChI=1S/C18H19NOS/c1-19-12-11-17(18-10-5-13-21-18)20-16-9-4-7-14-6-2-3-8-15(14)16/h2-10,13,17,19H,11-12H2,1H3/t17-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against norepinephrine transporter |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50145347

(1-(2-(4-(6-fluoro-1H-indol-3-yl)-5,6-dihydropyridi...)Show SMILES NC(=O)c1ccc2C(CCN3CCC(=CC3)c3c[nH]c4cc(F)ccc34)OCCc2c1 |c:13| Show InChI InChI=1S/C25H26FN3O2/c26-19-2-4-21-22(15-28-23(21)14-19)16-5-9-29(10-6-16)11-7-24-20-3-1-18(25(27)30)13-17(20)8-12-31-24/h1-5,13-15,24,28H,6-12H2,(H2,27,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against alpha adrenergic receptor |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416117
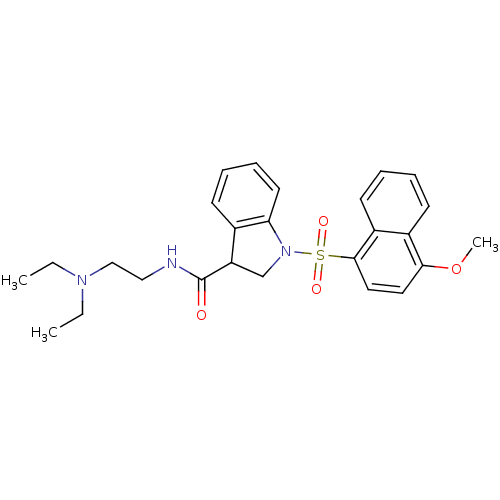
(CHEMBL1084316)Show SMILES CCN(CC)CCNC(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc(OC)c2ccccc12 Show InChI InChI=1S/C26H31N3O4S/c1-4-28(5-2)17-16-27-26(30)22-18-29(23-13-9-8-10-19(22)23)34(31,32)25-15-14-24(33-3)20-11-6-7-12-21(20)25/h6-15,22H,4-5,16-18H2,1-3H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416131
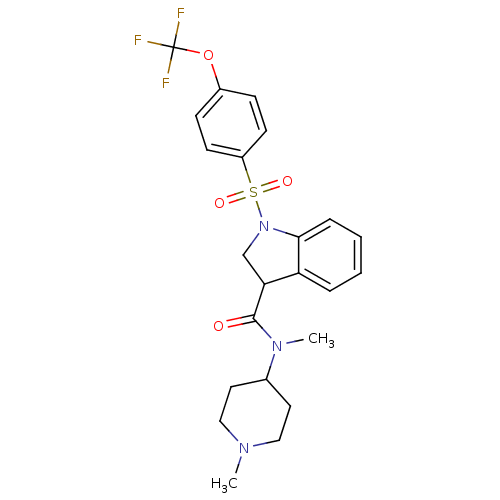
(CHEMBL1085965)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C23H26F3N3O4S/c1-27-13-11-16(12-14-27)28(2)22(30)20-15-29(21-6-4-3-5-19(20)21)34(31,32)18-9-7-17(8-10-18)33-23(24,25)26/h3-10,16,20H,11-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50175516
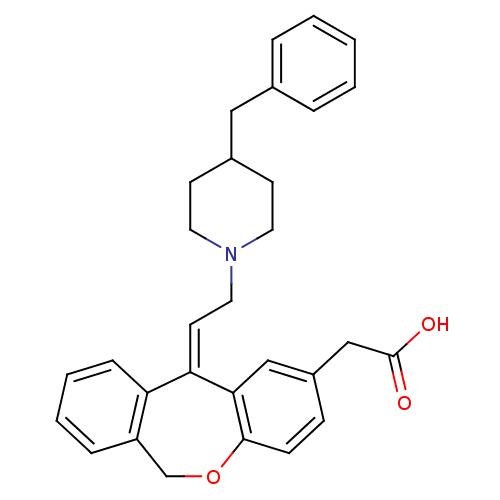
(CHEMBL372588 | {11-[2-(4-Benzyl-piperidin-1-yl)-et...)Show SMILES OC(=O)Cc1ccc2OCc3ccccc3\C(=C\CN3CCC(Cc4ccccc4)CC3)c2c1 Show InChI InChI=1S/C30H31NO3/c32-30(33)20-24-10-11-29-28(19-24)27(26-9-5-4-8-25(26)21-34-29)14-17-31-15-12-23(13-16-31)18-22-6-2-1-3-7-22/h1-11,14,19,23H,12-13,15-18,20-21H2,(H,32,33)/b27-14- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against histamine H1 receptor |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]naloxone from MOR (unknown origin) expressed in human 293T cells |
J Med Chem 58: 2584-608 (2015)
Article DOI: 10.1021/jm501535r
BindingDB Entry DOI: 10.7270/Q2JM2CBK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50027183
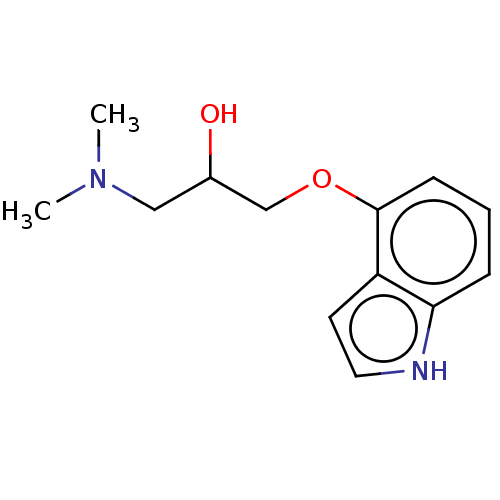
(CHEMBL326134) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against 5-hydroxytryptamine 1A receptor |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50115848

((S)-3-(1H-Indol-3-yl)-2-[(R)-3-mercapto-2-((S)-5-m...)Show SMILES COc1ccc2[C@@H](CCc2c1)[C@@H](CS)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C24H26N2O4S/c1-30-16-7-9-17-14(10-16)6-8-19(17)20(13-31)23(27)26-22(24(28)29)11-15-12-25-21-5-3-2-4-18(15)21/h2-5,7,9-10,12,19-20,22,25,31H,6,8,11,13H2,1H3,(H,26,27)(H,28,29)/t19-,20-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against neutral endopeptidase |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50403993
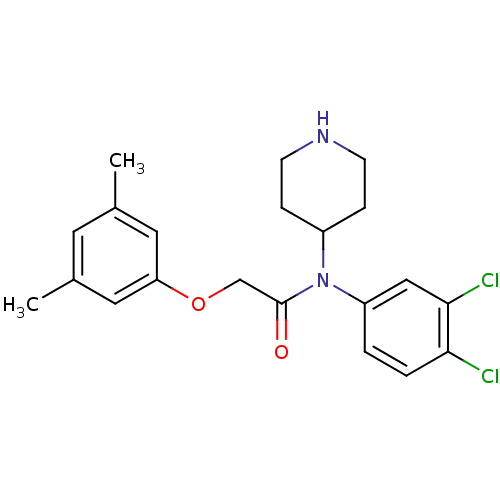
(CHEMBL91418)Show SMILES Cc1cc(C)cc(OCC(=O)N(C2CCNCC2)c2ccc(Cl)c(Cl)c2)c1 Show InChI InChI=1S/C21H24Cl2N2O2/c1-14-9-15(2)11-18(10-14)27-13-21(26)25(16-5-7-24-8-6-16)17-3-4-19(22)20(23)12-17/h3-4,9-12,16,24H,5-8,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Negative logarithm causing 50% receptor occupancy against tachykinin receptor 1 |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416132
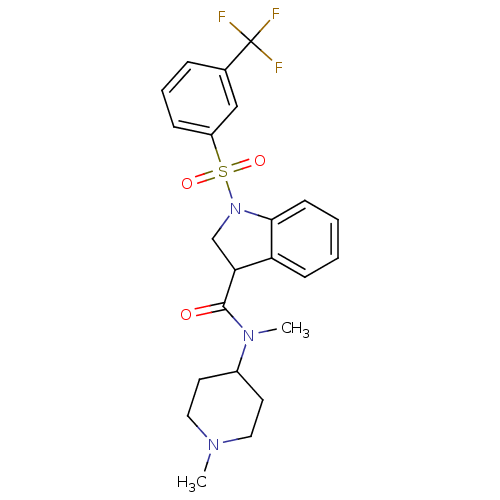
(CHEMBL1083297)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H26F3N3O3S/c1-27-12-10-17(11-13-27)28(2)22(30)20-15-29(21-9-4-3-8-19(20)21)33(31,32)18-7-5-6-16(14-18)23(24,25)26/h3-9,14,17,20H,10-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM14029
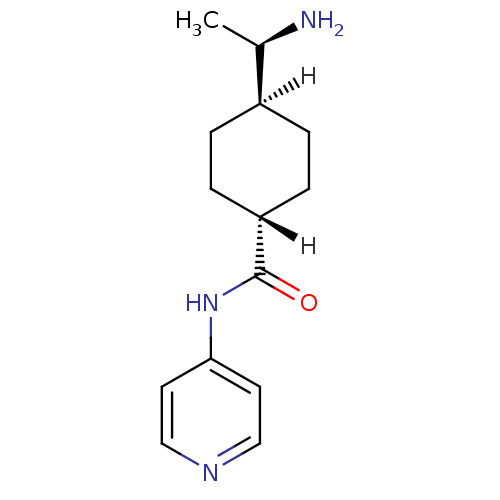
((R)-TRANS-4-(1-AMINOETHYL)-N-(4-PYRIDYL) CYCLOHEXA...)Show SMILES [H][C@@]1(CC[C@@]([H])(CC1)C(=O)Nc1ccncc1)[C@@H](C)N |r,wU:4.4,1.18,17.20,wD:4.8,1.0,(1.92,.41,;1.06,-.86,;-.27,-1.63,;-1.61,-.86,;-1.61,.68,;-1.61,2.22,;-.27,1.45,;1.06,.68,;-2.94,1.45,;-2.94,2.99,;-4.27,.68,;-5.61,1.45,;-5.61,2.99,;-6.94,3.76,;-8.28,2.99,;-8.28,1.45,;-6.94,.68,;2.6,-.86,;3.37,.47,;3.37,-2.2,)| Show InChI InChI=1S/C14H21N3O/c1-10(15)11-2-4-12(5-3-11)14(18)17-13-6-8-16-9-7-13/h6-12H,2-5,15H2,1H3,(H,16,17,18)/t10-,11-,12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD
Curated by ChEMBL
| Assay Description
Inhibition of ROCK-1 |
Bioorg Med Chem Lett 21: 97-101 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.060
BindingDB Entry DOI: 10.7270/Q29W0GQK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50416122
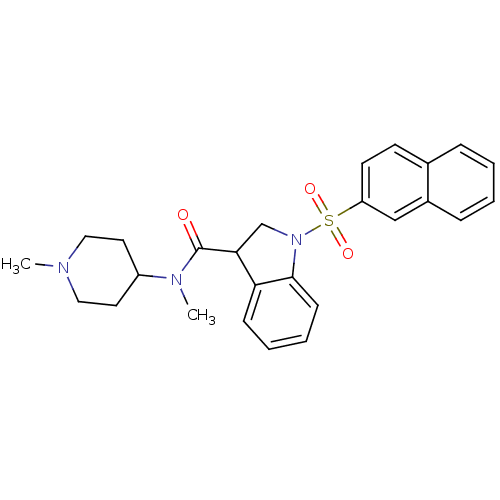
(CHEMBL1085351)Show SMILES CN(C1CCN(C)CC1)C(=O)C1CN(c2ccccc12)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C26H29N3O3S/c1-27-15-13-21(14-16-27)28(2)26(30)24-18-29(25-10-6-5-9-23(24)25)33(31,32)22-12-11-19-7-3-4-8-20(19)17-22/h3-12,17,21,24H,13-16,18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human 5HT6 expressed in CHO cells by FLIPR 384 assay |
Bioorg Med Chem Lett 20: 3713-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.085
BindingDB Entry DOI: 10.7270/Q2V69KV3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data