

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50238182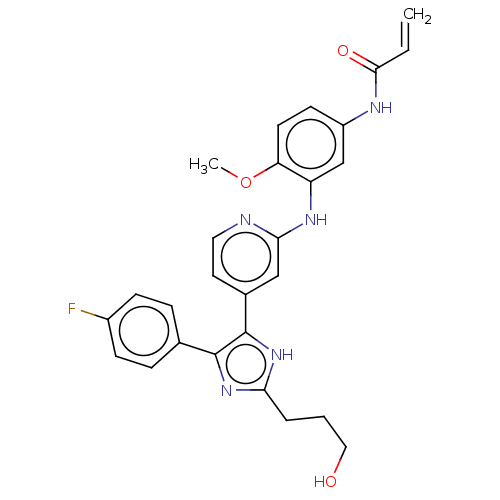 (CHEMBL4100860) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University T£bingen Curated by ChEMBL | Assay Description Inhibition of binding of [3H][D-Ala2,D-Leu5]enkephalin to Opioid receptor delta 1 in the rat brain homogenate | J Med Chem 60: 5613-5637 (2017) Article DOI: 10.1021/acs.jmedchem.7b00316 BindingDB Entry DOI: 10.7270/Q2V98BCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50238177 (CHEMBL4098072) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University T£bingen Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-fused EGFR cytoplasmic domain (669 to 1210 residues) expressed in baculovirus using TK-substrate-biotin incubated ... | J Med Chem 60: 5613-5637 (2017) Article DOI: 10.1021/acs.jmedchem.7b00316 BindingDB Entry DOI: 10.7270/Q2V98BCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM431867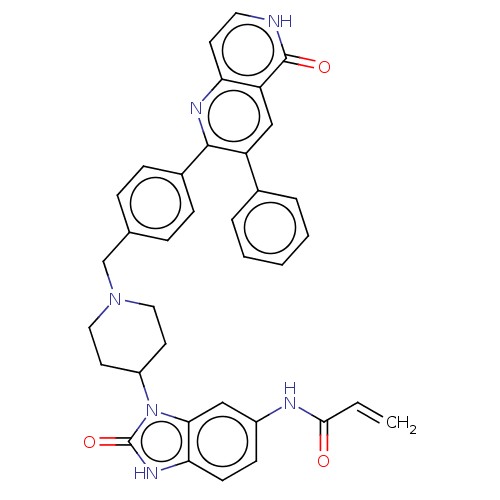 (US10550114, Compound 1a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TECHNISCHE UNIVERSITÄT DORTMUND US Patent | Assay Description iFLiK and HTRF studies were carried out as described in Z. Fang, J. R. Simard, D. Plenker, H. D. Nguyen, T. Phan, P. Wolle, S. Baumeister, D. Rauh, A... | US Patent US10550114 (2020) BindingDB Entry DOI: 10.7270/Q2DV1N97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50238183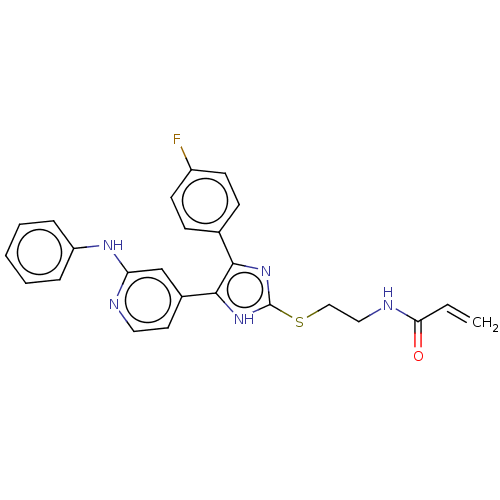 (CHEMBL4071012) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University T£bingen Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-fused EGFR cytoplasmic domain (669 to 1210 residues) expressed in baculovirus using TK-substrate-biotin incubated ... | J Med Chem 60: 5613-5637 (2017) Article DOI: 10.1021/acs.jmedchem.7b00316 BindingDB Entry DOI: 10.7270/Q2V98BCK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM431879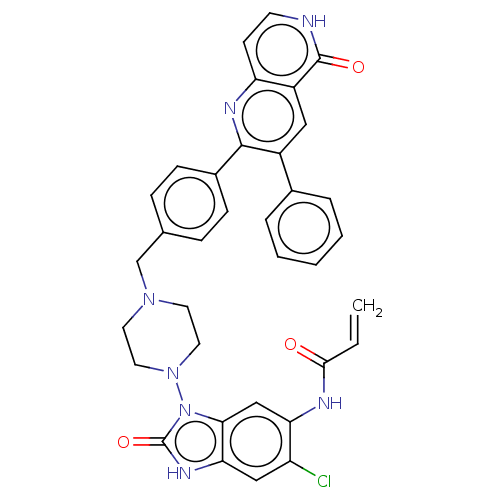 (US10550114, Compound 33) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TECHNISCHE UNIVERSITÄT DORTMUND US Patent | Assay Description iFLiK and HTRF studies were carried out as described in Z. Fang, J. R. Simard, D. Plenker, H. D. Nguyen, T. Phan, P. Wolle, S. Baumeister, D. Rauh, A... | US Patent US10550114 (2020) BindingDB Entry DOI: 10.7270/Q2DV1N97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50323701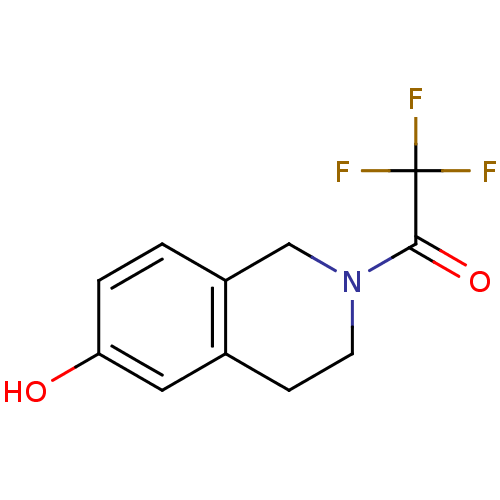 (2-(Trifluoroacetyl)-1,2,3,4-tetrahydro-6-isoquinol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut für Molekulare Physiologie Curated by ChEMBL | Assay Description Displacement of [3H]estradiol from human ERalpha ligand binding domain expressed in Escherichia coli BL21 (DE3) | Nat Chem Biol 5: 585-92 (2009) Article DOI: 10.1038/nchembio.188 BindingDB Entry DOI: 10.7270/Q2MP53F2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50131550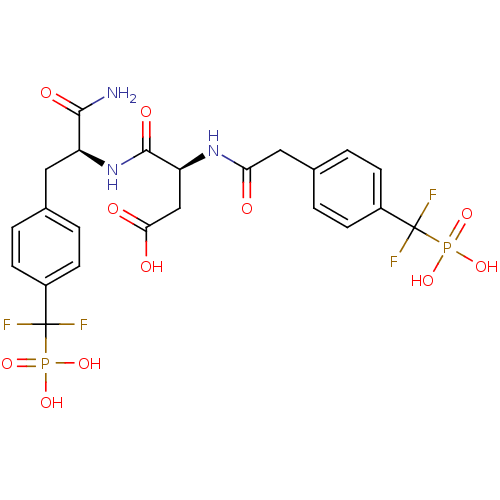 ((3S)-3-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology Curated by ChEMBL | Assay Description Inhibition of PTP1B | Bioorg Med Chem 19: 2145-55 (2011) Article DOI: 10.1016/j.bmc.2011.02.047 BindingDB Entry DOI: 10.7270/Q2BR8SHK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM431877 (US10550114, Compound 32) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TECHNISCHE UNIVERSITÄT DORTMUND US Patent | Assay Description iFLiK and HTRF studies were carried out as described in Z. Fang, J. R. Simard, D. Plenker, H. D. Nguyen, T. Phan, P. Wolle, S. Baumeister, D. Rauh, A... | US Patent US10550114 (2020) BindingDB Entry DOI: 10.7270/Q2DV1N97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50237140 (CHEMBL4068763) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University Curated by ChEMBL | Assay Description Reversible inhibition of wild-type human N-terminal GST-tagged EGFR cytoplasmic domain (669 to 1210 end amino acid residues) expressed in baculovirus... | J Med Chem 60: 2361-2372 (2017) Article DOI: 10.1021/acs.jmedchem.6b01626 BindingDB Entry DOI: 10.7270/Q2FB556R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50102904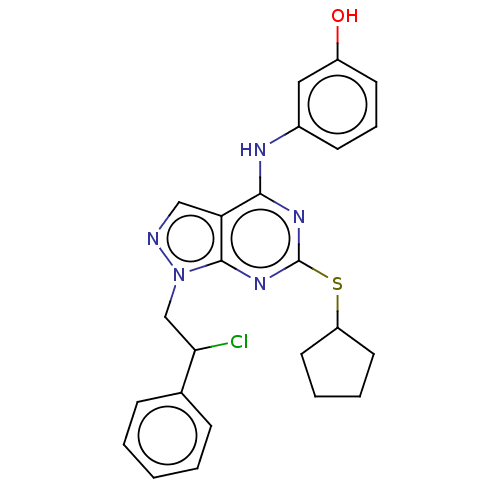 (CHEMBL3394091) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant src kinase using KVEKIGEGTYGVVYK as substrate by filter binding assay in presence of [gamma-32P]ATP | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50102908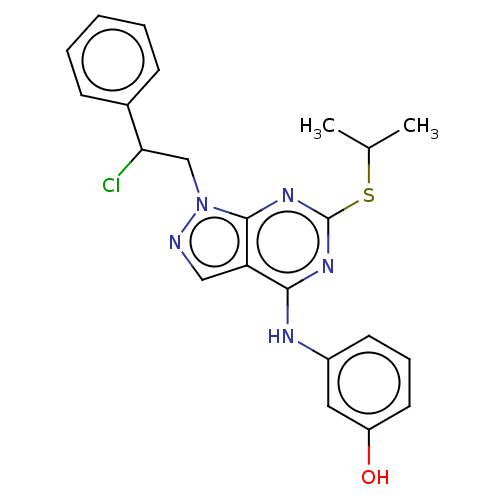 (CHEMBL3393071) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant src kinase using KVEKIGEGTYGVVYK as substrate by filter binding assay in presence of [gamma-32P]ATP | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM431882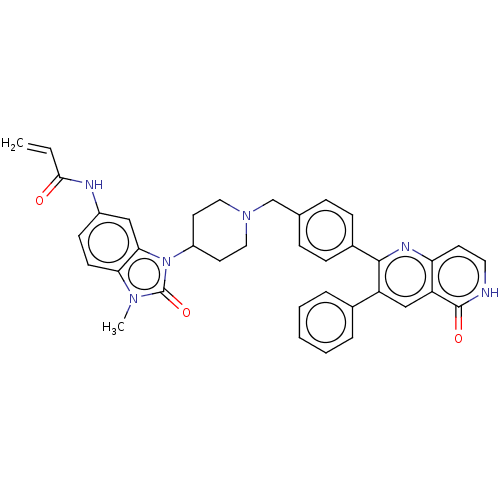 (US10550114, Compound 46) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TECHNISCHE UNIVERSITÄT DORTMUND US Patent | Assay Description iFLiK and HTRF studies were carried out as described in Z. Fang, J. R. Simard, D. Plenker, H. D. Nguyen, T. Phan, P. Wolle, S. Baumeister, D. Rauh, A... | US Patent US10550114 (2020) BindingDB Entry DOI: 10.7270/Q2DV1N97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50029668 (AZD-9291 | Osimertinib | US10085983, Compound AZD-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University T£bingen Curated by ChEMBL | Assay Description Inhibition of human N-terminal GST-fused EGFR cytoplasmic domain (669 to 1210 residues) expressed in baculovirus using TK-substrate-biotin incubated ... | J Med Chem 60: 5613-5637 (2017) Article DOI: 10.1021/acs.jmedchem.7b00316 BindingDB Entry DOI: 10.7270/Q2V98BCK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50029668 (AZD-9291 | Osimertinib | US10085983, Compound AZD-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University Curated by ChEMBL | Assay Description Reversible inhibition of wild-type human N-terminal GST-tagged EGFR cytoplasmic domain (669 to 1210 end amino acid residues) expressed in baculovirus... | J Med Chem 60: 2361-2372 (2017) Article DOI: 10.1021/acs.jmedchem.6b01626 BindingDB Entry DOI: 10.7270/Q2FB556R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM431875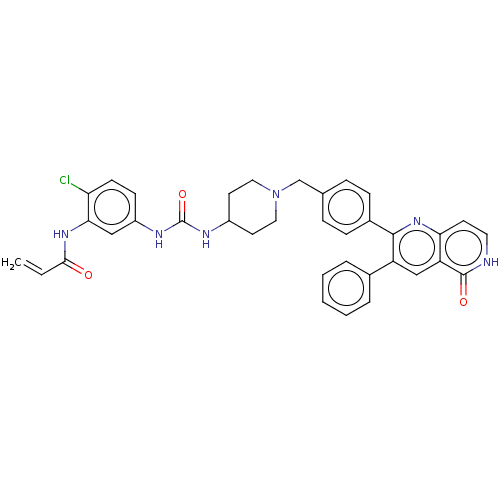 (US10550114, Compound 30) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 18.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TECHNISCHE UNIVERSITÄT DORTMUND US Patent | Assay Description iFLiK and HTRF studies were carried out as described in Z. Fang, J. R. Simard, D. Plenker, H. D. Nguyen, T. Phan, P. Wolle, S. Baumeister, D. Rauh, A... | US Patent US10550114 (2020) BindingDB Entry DOI: 10.7270/Q2DV1N97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myosin light chain kinase, smooth muscle (Homo sapiens (Human)) | BDBM6760 ((+)-K-252a | CHEMBL281948 | K-252a | methyl (15S,1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chemical Genomics Centre of the Max Planck Society Curated by ChEMBL | Assay Description Inhibition of MLCK by HTRF assay | Bioorg Med Chem 19: 429-39 (2011) Article DOI: 10.1016/j.bmc.2010.11.007 BindingDB Entry DOI: 10.7270/Q22F7NQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM431881 (US10550114, Compound 36) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 20.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TECHNISCHE UNIVERSITÄT DORTMUND US Patent | Assay Description iFLiK and HTRF studies were carried out as described in Z. Fang, J. R. Simard, D. Plenker, H. D. Nguyen, T. Phan, P. Wolle, S. Baumeister, D. Rauh, A... | US Patent US10550114 (2020) BindingDB Entry DOI: 10.7270/Q2DV1N97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50341987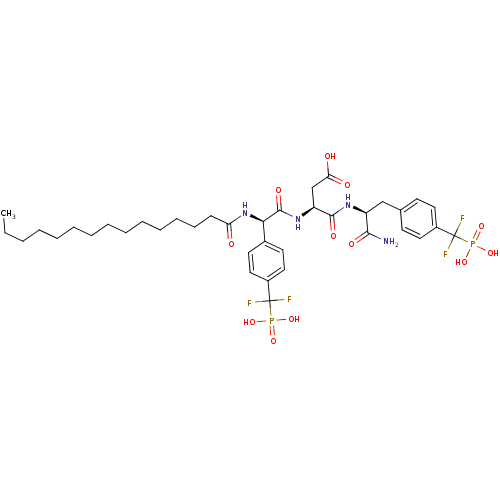 ((S)-4-((S)-1-amino-3-(4-(difluoro(phosphono)methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology Curated by ChEMBL | Assay Description Inhibition of PTP1B | Bioorg Med Chem 19: 2145-55 (2011) Article DOI: 10.1016/j.bmc.2011.02.047 BindingDB Entry DOI: 10.7270/Q2BR8SHK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50106497 (6-Chloro-7-(2-morpholin-4-yl-ethylamino)-quinoline...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology Curated by ChEMBL | Assay Description Inhibition of human Cdc25A | Bioorg Med Chem 19: 2145-55 (2011) Article DOI: 10.1016/j.bmc.2011.02.047 BindingDB Entry DOI: 10.7270/Q2BR8SHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50102872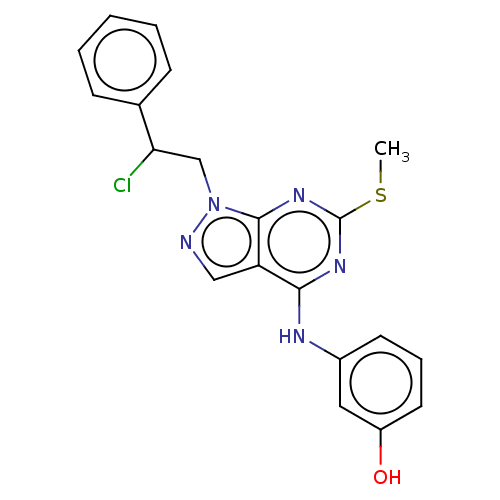 (CHEMBL3394083) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant src kinase using KVEKIGEGTYGVVYK as substrate by filter binding assay in presence of [gamma-32P]ATP | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50101585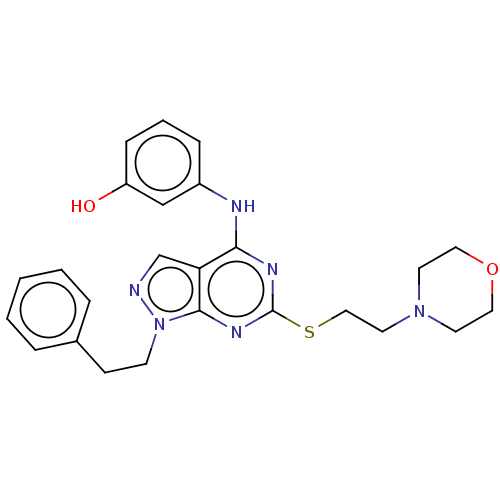 (CHEMBL3393986) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant src kinase using KVEKIGEGTYGVVYK as substrate by filter binding assay in presence of [gamma-32P]ATP | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM431880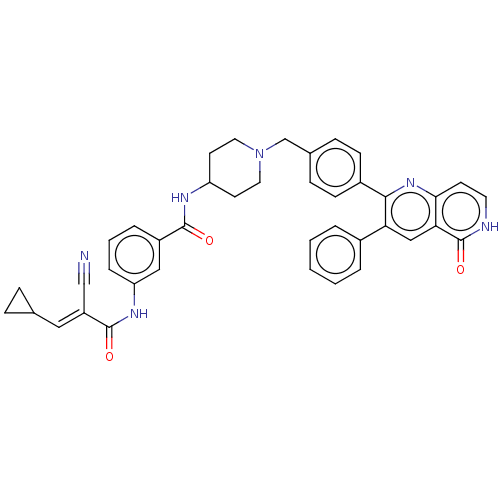 (US10550114, Compound 45) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 45.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TECHNISCHE UNIVERSITÄT DORTMUND US Patent | Assay Description iFLiK and HTRF studies were carried out as described in Z. Fang, J. R. Simard, D. Plenker, H. D. Nguyen, T. Phan, P. Wolle, S. Baumeister, D. Rauh, A... | US Patent US10550114 (2020) BindingDB Entry DOI: 10.7270/Q2DV1N97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM431876 (US10550114, Compound 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 67.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TECHNISCHE UNIVERSITÄT DORTMUND US Patent | Assay Description iFLiK and HTRF studies were carried out as described in Z. Fang, J. R. Simard, D. Plenker, H. D. Nguyen, T. Phan, P. Wolle, S. Baumeister, D. Rauh, A... | US Patent US10550114 (2020) BindingDB Entry DOI: 10.7270/Q2DV1N97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50102878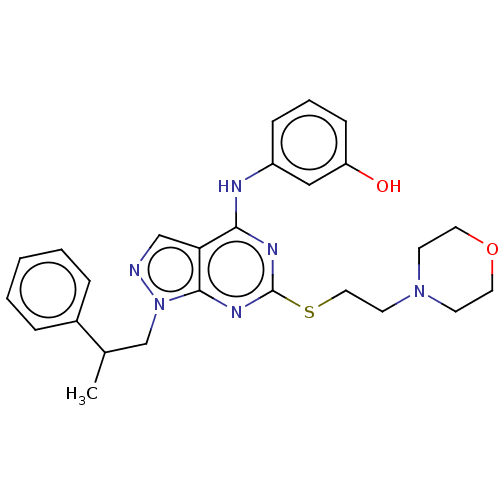 (CHEMBL3394082) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant src kinase using KVEKIGEGTYGVVYK as substrate by filter binding assay in presence of [gamma-32P]ATP | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM149404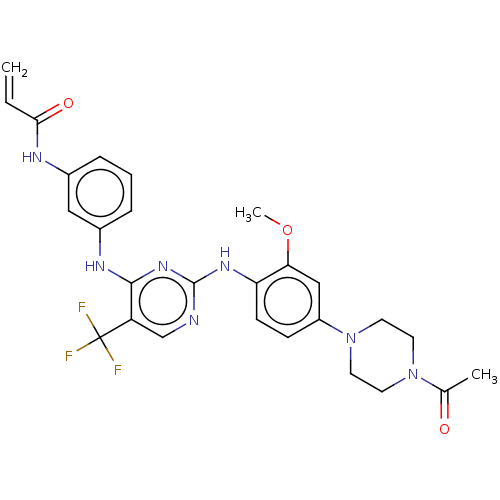 (AVL-301 | CHEMBL3545308 | CNX-419 | CO-1686 | Roci...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University Curated by ChEMBL | Assay Description Reversible inhibition of wild-type human N-terminal GST-tagged EGFR cytoplasmic domain (669 to 1210 end amino acid residues) expressed in baculovirus... | J Med Chem 60: 2361-2372 (2017) Article DOI: 10.1021/acs.jmedchem.6b01626 BindingDB Entry DOI: 10.7270/Q2FB556R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50106497 (6-Chloro-7-(2-morpholin-4-yl-ethylamino)-quinoline...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology Curated by ChEMBL | Assay Description Inhibition of human Cdc25C | Bioorg Med Chem 19: 2145-55 (2011) Article DOI: 10.1016/j.bmc.2011.02.047 BindingDB Entry DOI: 10.7270/Q2BR8SHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [E17K] (Homo sapiens (Human)) | BDBM431867 (US10550114, Compound 1a) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TECHNISCHE UNIVERSITÄT DORTMUND US Patent | Assay Description iFLiK and HTRF studies were carried out as described in Z. Fang, J. R. Simard, D. Plenker, H. D. Nguyen, T. Phan, P. Wolle, S. Baumeister, D. Rauh, A... | US Patent US10550114 (2020) BindingDB Entry DOI: 10.7270/Q2DV1N97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50106497 (6-Chloro-7-(2-morpholin-4-yl-ethylamino)-quinoline...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology Curated by ChEMBL | Assay Description Inhibition of human Cdc25B | Bioorg Med Chem 19: 2145-55 (2011) Article DOI: 10.1016/j.bmc.2011.02.047 BindingDB Entry DOI: 10.7270/Q2BR8SHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50102909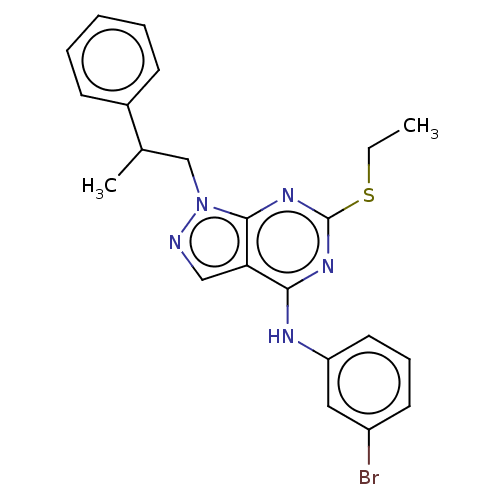 (CHEMBL3394077) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant src kinase using KVEKIGEGTYGVVYK as substrate by filter binding assay in presence of [gamma-32P]ATP | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50102906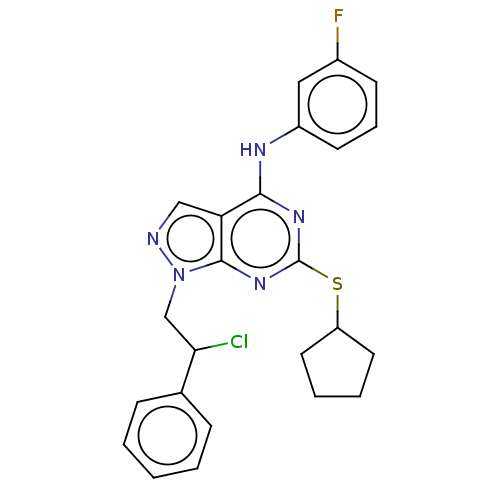 (CHEMBL3394089) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant src kinase using KVEKIGEGTYGVVYK as substrate by filter binding assay in presence of [gamma-32P]ATP | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50102875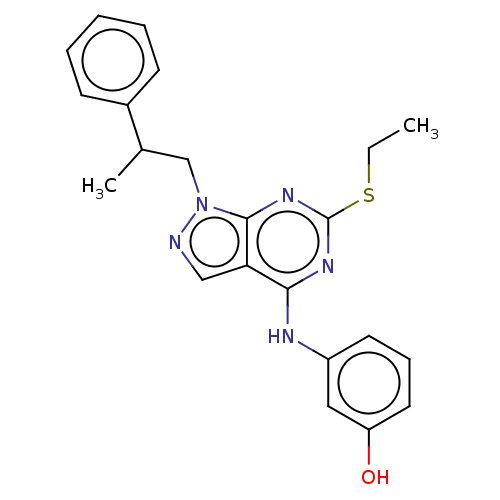 (CHEMBL3394078) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant src kinase using KVEKIGEGTYGVVYK as substrate by filter binding assay in presence of [gamma-32P]ATP | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50102907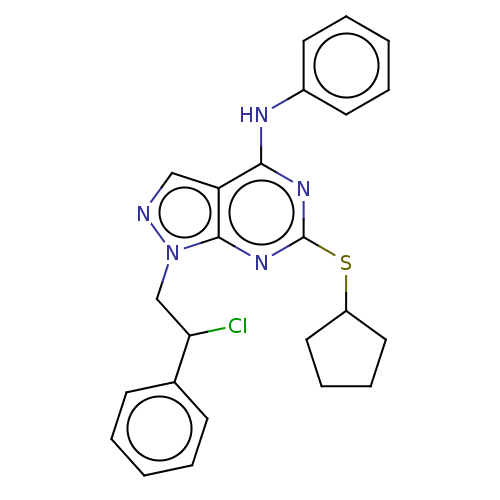 (CHEMBL3394088) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant src kinase using KVEKIGEGTYGVVYK as substrate by filter binding assay in presence of [gamma-32P]ATP | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50102908 (CHEMBL3393071) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant Abl kinase using [gamma-32P]ATP as substrate by filter binding assay | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50102903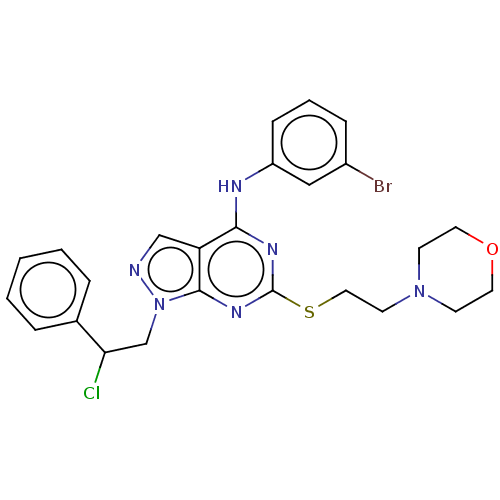 (CHEMBL3394092) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant Abl kinase using [gamma-32P]ATP as substrate by filter binding assay | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50101585 (CHEMBL3393986) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant Abl kinase using [gamma-32P]ATP as substrate by filter binding assay | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50102871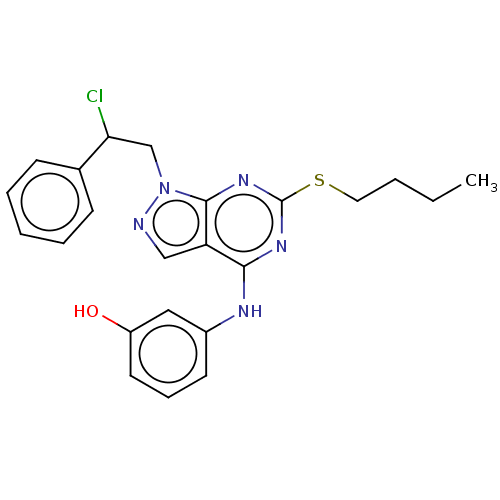 (CHEMBL3394084) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant src kinase using KVEKIGEGTYGVVYK as substrate by filter binding assay in presence of [gamma-32P]ATP | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50102903 (CHEMBL3394092) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant src kinase using KVEKIGEGTYGVVYK as substrate by filter binding assay in presence of [gamma-32P]ATP | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [E17K] (Homo sapiens (Human)) | BDBM431879 (US10550114, Compound 33) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TECHNISCHE UNIVERSITÄT DORTMUND US Patent | Assay Description iFLiK and HTRF studies were carried out as described in Z. Fang, J. R. Simard, D. Plenker, H. D. Nguyen, T. Phan, P. Wolle, S. Baumeister, D. Rauh, A... | US Patent US10550114 (2020) BindingDB Entry DOI: 10.7270/Q2DV1N97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50237139 (CHEMBL4089863) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University Curated by ChEMBL | Assay Description In vitro Binding affinity towards 5-hydroxytryptamine 3 receptor was determined | J Med Chem 60: 2361-2372 (2017) Article DOI: 10.1021/acs.jmedchem.6b01626 BindingDB Entry DOI: 10.7270/Q2FB556R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50102872 (CHEMBL3394083) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant Abl kinase using [gamma-32P]ATP as substrate by filter binding assay | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TYR_PHOSPHATASE_2 domain-containing protein (Mycobacterium tuberculosis) | BDBM50341982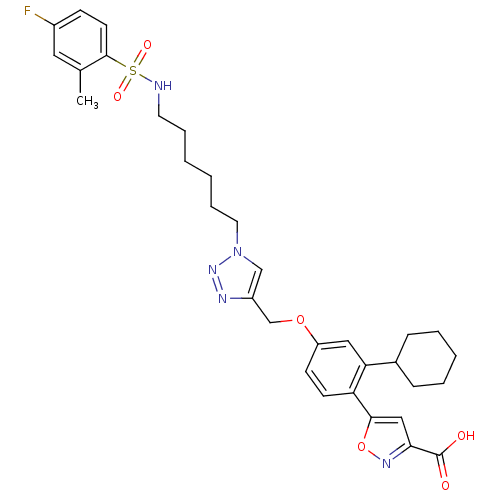 (5-(2-cyclohexyl-4-((1-(6-(4-fluoro-2-methylphenyls...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis ptpB | Bioorg Med Chem 19: 2145-55 (2011) Article DOI: 10.1016/j.bmc.2011.02.047 BindingDB Entry DOI: 10.7270/Q2BR8SHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50102904 (CHEMBL3394091) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant Abl kinase using [gamma-32P]ATP as substrate by filter binding assay | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50343430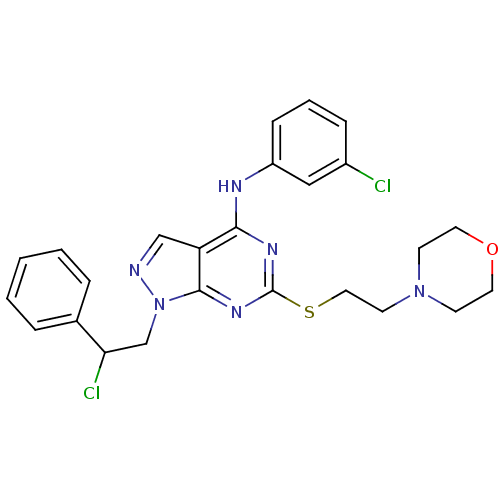 (CHEMBL1775036 | N-(3-Chlorophenyl)-1-(2-chloro-2-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant Abl kinase using [gamma-32P]ATP as substrate by filter binding assay | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50102877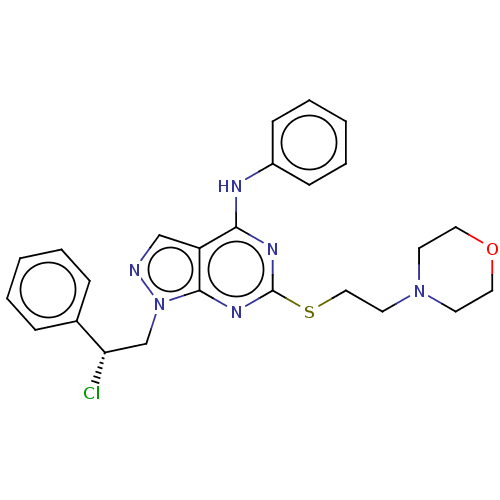 (CHEMBL3393979) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant src kinase using KVEKIGEGTYGVVYK as substrate by filter binding assay in presence of [gamma-32P]ATP | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50102887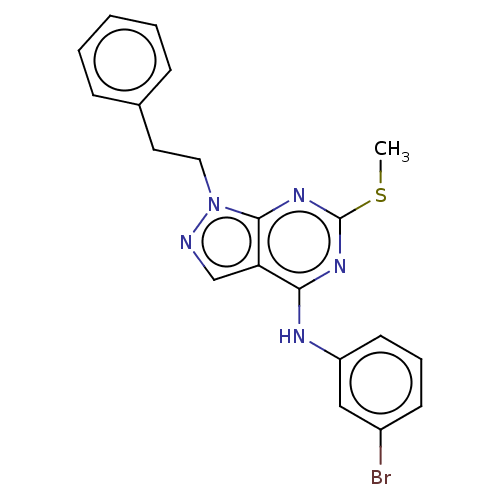 (CHEMBL3393983) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant src kinase using KVEKIGEGTYGVVYK as substrate by filter binding assay in presence of [gamma-32P]ATP | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50343431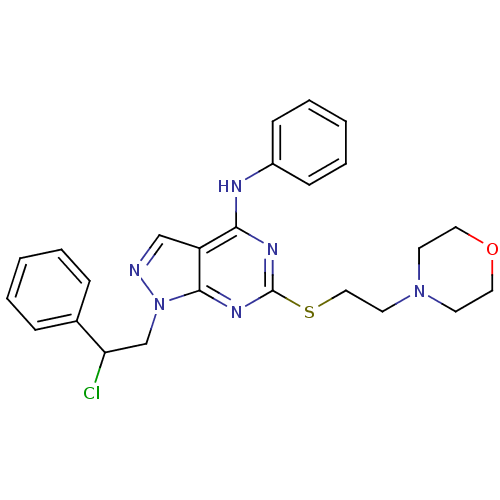 (1-(2-Chloro-2-phenylethyl)-6-[(2-morpholin-4-yleth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of c-src kinase (unknown origin) | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50343431 (1-(2-Chloro-2-phenylethyl)-6-[(2-morpholin-4-yleth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant Abl kinase using [gamma-32P]ATP as substrate by filter binding assay | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50102876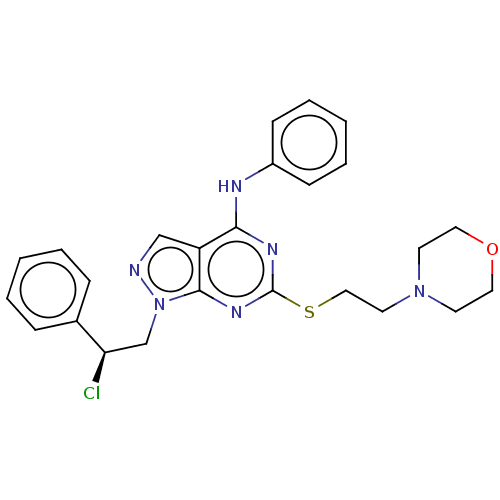 (CHEMBL3393980) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant src kinase using KVEKIGEGTYGVVYK as substrate by filter binding assay in presence of [gamma-32P]ATP | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50237153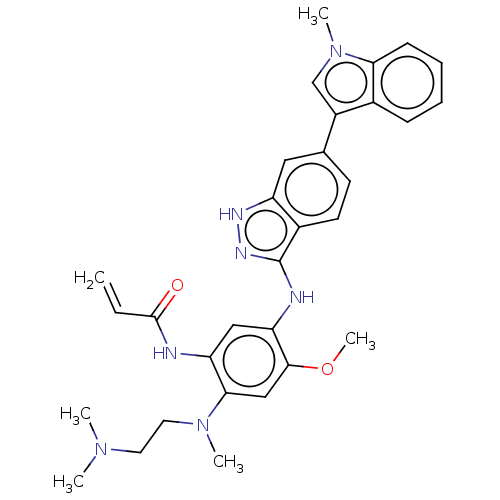 (CHEMBL4098444) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University Curated by ChEMBL | Assay Description Inhibition of cloned isozyme, human carbonic anhydrase II | J Med Chem 60: 2361-2372 (2017) Article DOI: 10.1021/acs.jmedchem.6b01626 BindingDB Entry DOI: 10.7270/Q2FB556R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50343431 (1-(2-Chloro-2-phenylethyl)-6-[(2-morpholin-4-yleth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Via Aldo Moro 2 Curated by ChEMBL | Assay Description Inhibition of human recombinant src kinase using KVEKIGEGTYGVVYK as substrate by filter binding assay in presence of [gamma-32P]ATP | J Med Chem 58: 347-61 (2015) Article DOI: 10.1021/jm5013159 BindingDB Entry DOI: 10.7270/Q2416ZTT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1710 total ) | Next | Last >> |