

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082842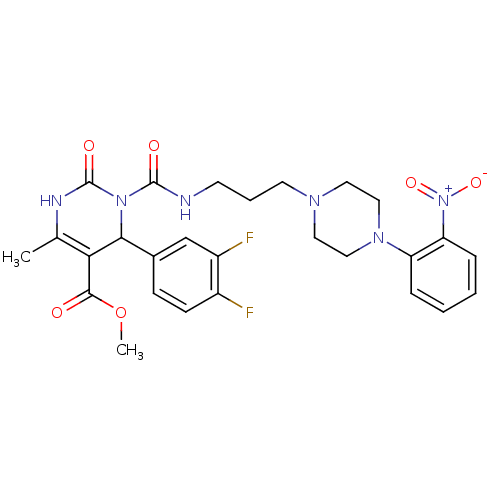 (4-(3,4-Difluoro-phenyl)-6-methyl-3-{3-[4-(2-nitro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082839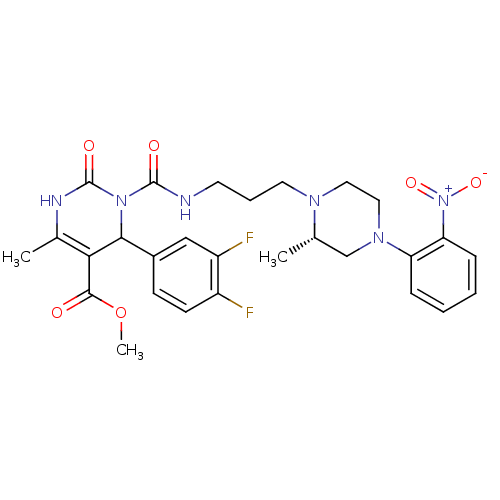 (4-(3,4-Difluoro-phenyl)-6-methyl-3-{3-[(S)-2-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (OK) | BDBM81811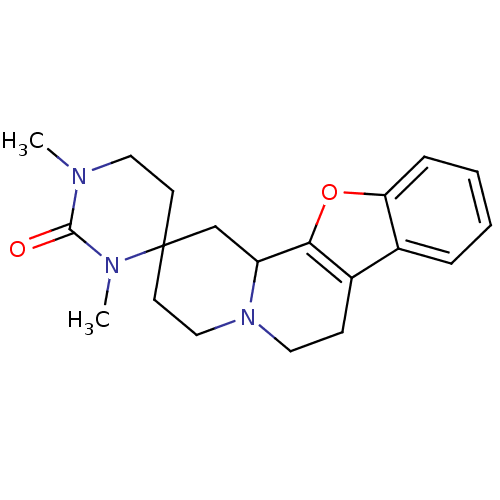 (CAS_123679 | L-657,743 | MK-912 | NSC_123679) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Curated by PDSP Ki Database | J Pharmacol Exp Ther 259: 323-9 (1991) BindingDB Entry DOI: 10.7270/Q2Z899WV | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 receptor (Homo sapiens (Human)) | BDBM50356880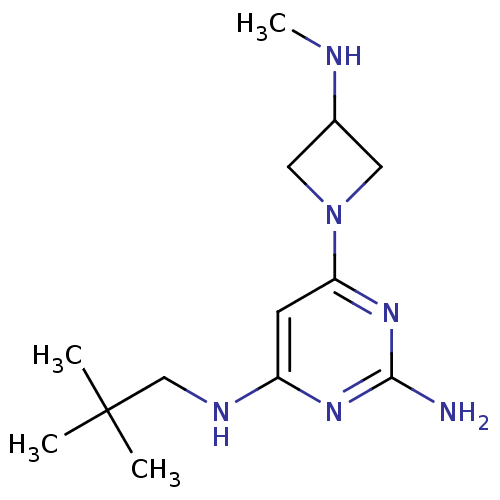 (CHEMBL1915536) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at full length human H4R expressed in HEK293 cells assessed as reversal of forskolin-induced cAMP production by CRE-beta-lactamas... | Bioorg Med Chem Lett 21: 6596-602 (2011) Article DOI: 10.1016/j.bmcl.2011.07.125 BindingDB Entry DOI: 10.7270/Q20C4W6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (OK) | BDBM50013515 ((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Curated by PDSP Ki Database | J Pharmacol Exp Ther 259: 323-9 (1991) BindingDB Entry DOI: 10.7270/Q2Z899WV | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545960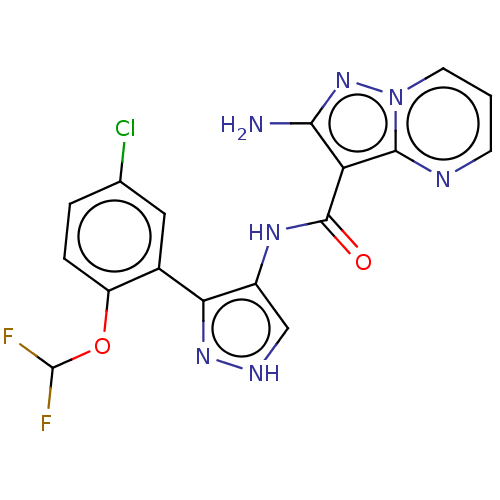 (CHEMBL4740778 | US11649241, Example 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545980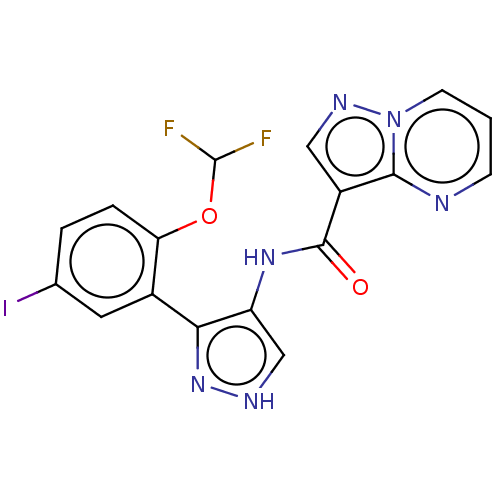 (CHEMBL4764019) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545979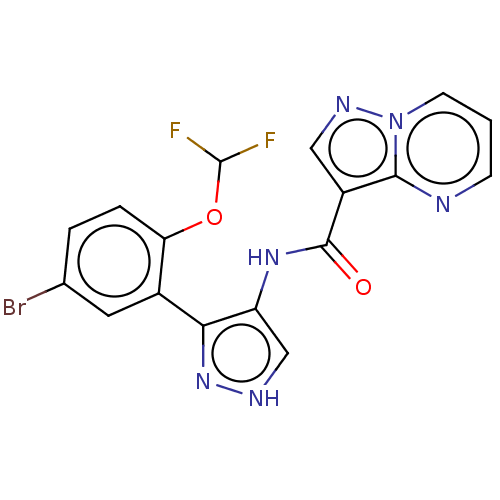 (CHEMBL4742159) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (OK) | BDBM81806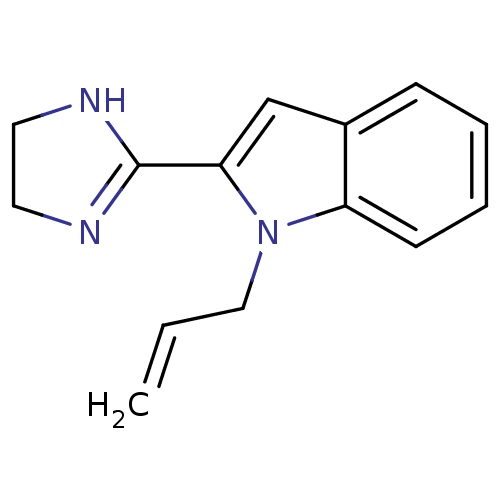 (2-(4,5-dihydro-1h-imidazol-2-yl)-2,3-dihydro-1-(2-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Curated by PDSP Ki Database | J Pharmacol Exp Ther 259: 323-9 (1991) BindingDB Entry DOI: 10.7270/Q2Z899WV | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM81806 (2-(4,5-dihydro-1h-imidazol-2-yl)-2,3-dihydro-1-(2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Curated by PDSP Ki Database | J Pharmacol Exp Ther 259: 323-9 (1991) BindingDB Entry DOI: 10.7270/Q2Z899WV | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082851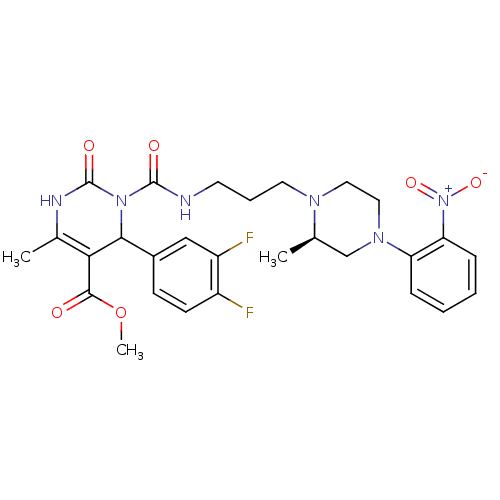 (4-(3,4-Difluoro-phenyl)-6-methyl-3-{3-[(R)-2-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545987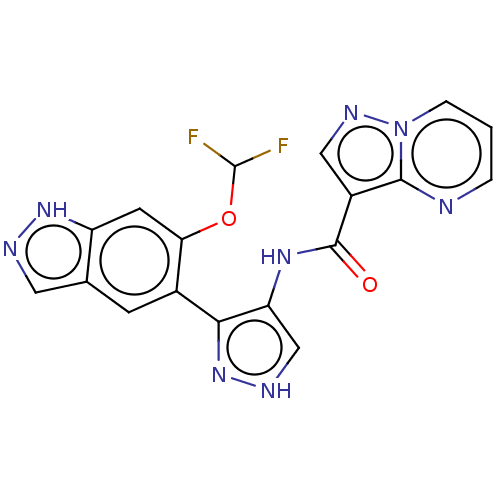 (CHEMBL4746726) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545986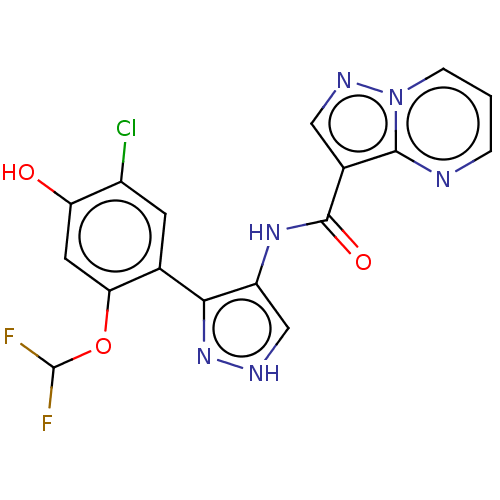 (CHEMBL4789075) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens (Human)) | BDBM81804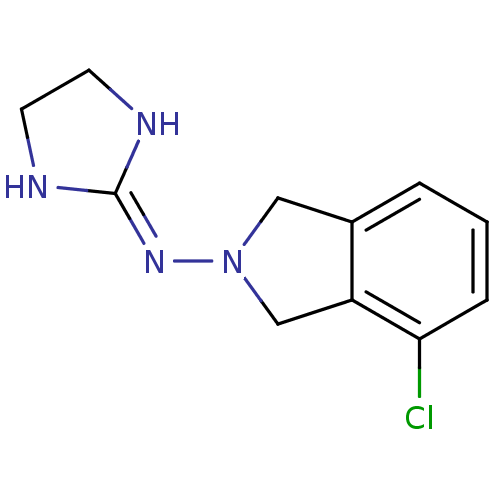 (4-chloro-2-(2-imidazolin-2-ylamino)isoindoline | B...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Curated by PDSP Ki Database | J Pharmacol Exp Ther 259: 323-9 (1991) BindingDB Entry DOI: 10.7270/Q2Z899WV | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM232618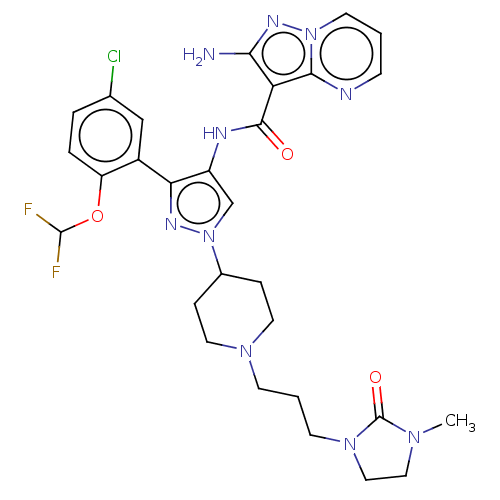 (US9346815, 164 | US9604984, Example 164) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | -57.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Genentech, Inc. US Patent | Assay Description The activity of the isolated recombinant JAK1 and JAK2 kinase domain was measured by monitoring phosphorylation of a peptide derived from JAK3 (Val-A... | US Patent US9346815 (2016) BindingDB Entry DOI: 10.7270/Q2NS0SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082827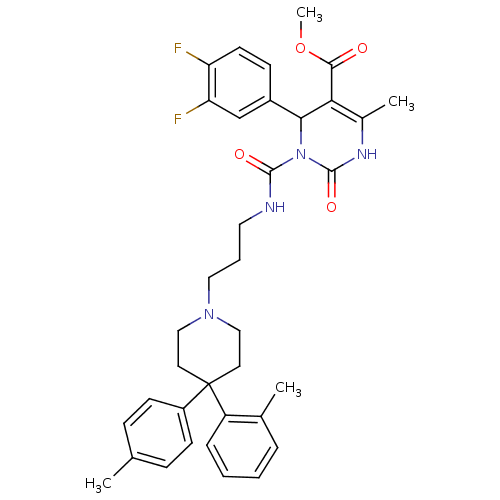 (4-(3,4-Difluoro-phenyl)-6-methyl-2-oxo-3-[3-(4-o-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM254931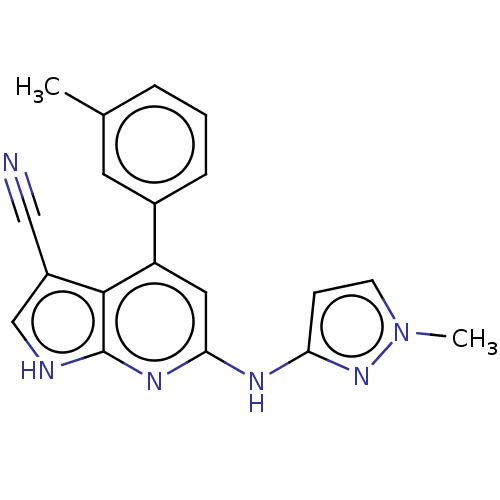 (US9499542, 14 | US9675594, 14) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd. Curated by ChEMBL | Assay Description Inhibition of wild type recombinant human GST-tagged LRRK2 (970 to 2527 residues) expressed in baculovirus using fluorescein-LRRKtide as substrate af... | J Med Chem 60: 8945-8962 (2017) Article DOI: 10.1021/acs.jmedchem.7b01186 BindingDB Entry DOI: 10.7270/Q2BR8VMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082811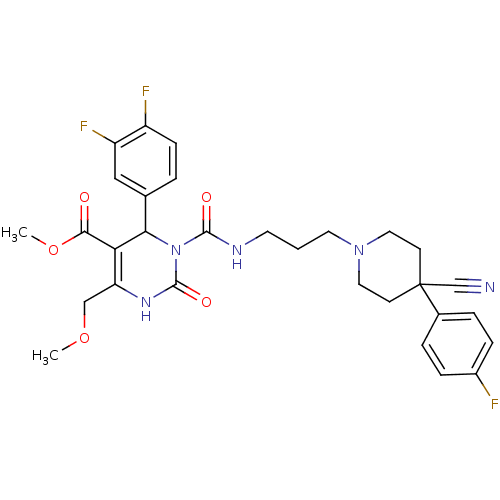 (3-{3-[4-Cyano-4-(4-fluoro-phenyl)-piperidin-1-yl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM232618 (US9346815, 164 | US9604984, Example 164) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description The activity of the isolated recombinant JAK1 and JAK2 kinase domain was measured by monitoring phosphorylation of a peptide derived from JAK3 (Val-A... | US Patent US9604984 (2017) BindingDB Entry DOI: 10.7270/Q26D5W25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090035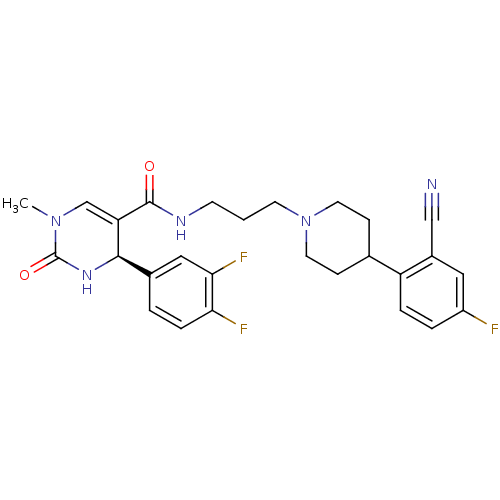 (4-(3,4-Difluoro-phenyl)-1-methyl-2-oxo-1,2,3,4-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM232751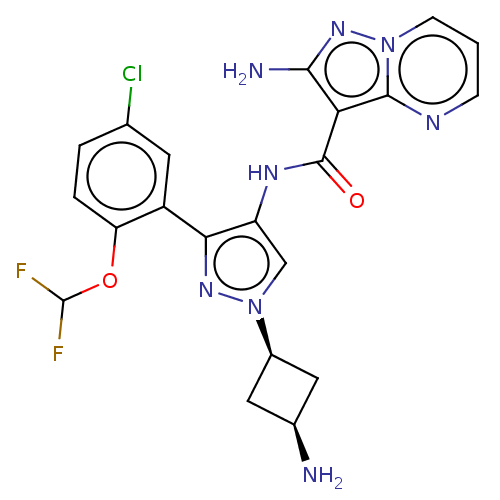 (US9346815, 297 | US9604984, Example 297) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0700 | -57.4 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Genentech, Inc. US Patent | Assay Description The activity of the isolated recombinant JAK1 and JAK2 kinase domain was measured by monitoring phosphorylation of a peptide derived from JAK3 (Val-A... | US Patent US9346815 (2016) BindingDB Entry DOI: 10.7270/Q2NS0SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50090023 (4-(3,4-Difluoro-phenyl)-2-oxo-1,2,3,4-tetrahydro-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluated for the ability to displace [125I]- HEAT from human cloned Alpha-1A adrenergic receptor stably expressed in CHO cells. | J Med Chem 43: 2703-18 (2000) BindingDB Entry DOI: 10.7270/Q2XK8DTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM232751 (US9346815, 297 | US9604984, Example 297) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description The activity of the isolated recombinant JAK1 and JAK2 kinase domain was measured by monitoring phosphorylation of a peptide derived from JAK3 (Val-A... | US Patent US9604984 (2017) BindingDB Entry DOI: 10.7270/Q26D5W25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545944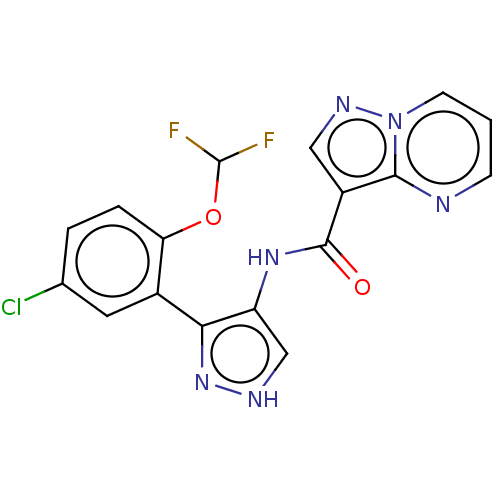 (CHEMBL4744172) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545961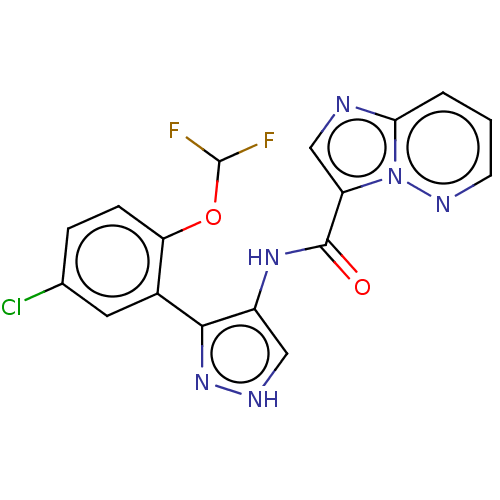 (CHEMBL4793262) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50545987 (CHEMBL4746726) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK1 (854 to 1154 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545964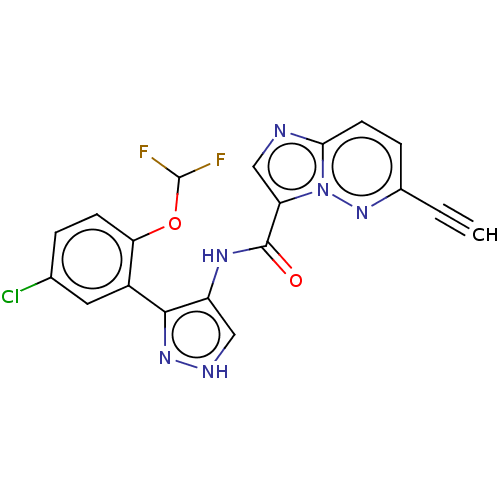 (CHEMBL4746416) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (OPOSSUM) | BDBM50013515 ((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Curated by PDSP Ki Database | J Pharmacol Exp Ther 259: 323-9 (1991) BindingDB Entry DOI: 10.7270/Q2Z899WV | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM366982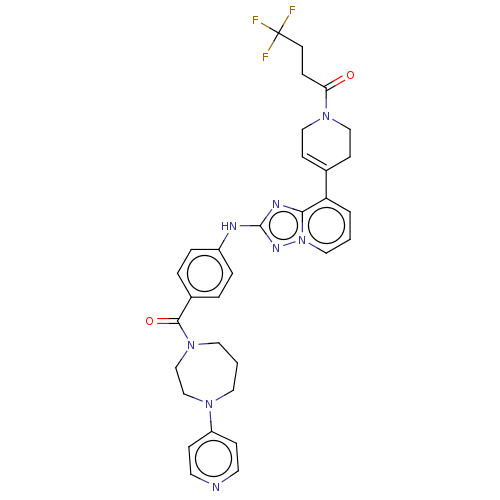 (4,4,4-trifluoro-1-[4-[2- [4-[4-(4-pyridyl)-1,4- di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University | Assay Description The activity of the isolated recombinant JAK1 and JAK2 kinase domain was measured by monitoring phosphorylation of a peptide derived from JAK3 (Val-A... | J Med Chem 51: 68-76 (2008) BindingDB Entry DOI: 10.7270/Q2CC130F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (OK) | BDBM81804 (4-chloro-2-(2-imidazolin-2-ylamino)isoindoline | B...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska Curated by PDSP Ki Database | J Pharmacol Exp Ther 259: 323-9 (1991) BindingDB Entry DOI: 10.7270/Q2Z899WV | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545965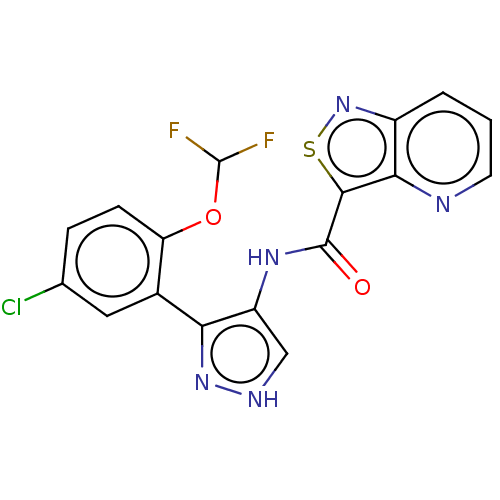 (CHEMBL4788860 | US11649241, Example 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545945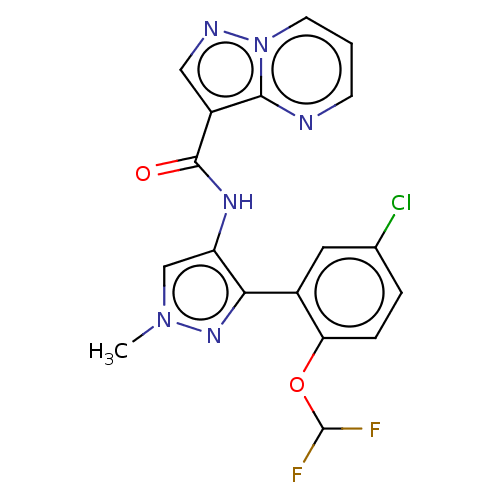 (CHEMBL4777342) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.0880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM232628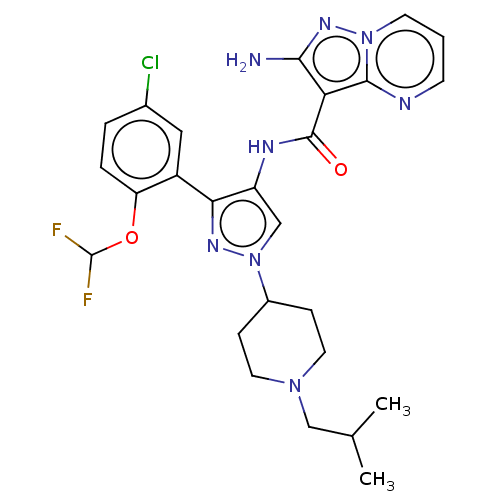 (US9346815, 174 | US9604984, Example 174) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0900 | -56.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Genentech, Inc. US Patent | Assay Description The activity of the isolated recombinant JAK1 and JAK2 kinase domain was measured by monitoring phosphorylation of a peptide derived from JAK3 (Val-A... | US Patent US9346815 (2016) BindingDB Entry DOI: 10.7270/Q2NS0SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM232631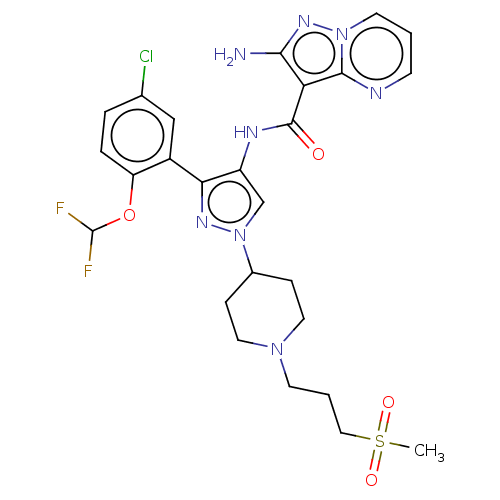 (US9346815, 177 | US9604984, Example 177) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0900 | -56.8 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Genentech, Inc. US Patent | Assay Description The activity of the isolated recombinant JAK1 and JAK2 kinase domain was measured by monitoring phosphorylation of a peptide derived from JAK3 (Val-A... | US Patent US9346815 (2016) BindingDB Entry DOI: 10.7270/Q2NS0SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM232631 (US9346815, 177 | US9604984, Example 177) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description The activity of the isolated recombinant JAK1 and JAK2 kinase domain was measured by monitoring phosphorylation of a peptide derived from JAK3 (Val-A... | US Patent US9604984 (2017) BindingDB Entry DOI: 10.7270/Q26D5W25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM232628 (US9346815, 174 | US9604984, Example 174) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description The activity of the isolated recombinant JAK1 and JAK2 kinase domain was measured by monitoring phosphorylation of a peptide derived from JAK3 (Val-A... | US Patent US9604984 (2017) BindingDB Entry DOI: 10.7270/Q26D5W25 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22000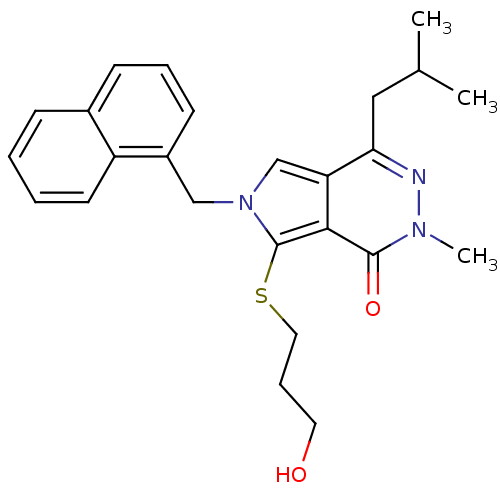 (7-[(3-hydroxypropyl)sulfanyl]-2-methyl-4-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0955 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22000 (7-[(3-hydroxypropyl)sulfanyl]-2-methyl-4-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | -56.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
AstraZeneca | Assay Description Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ... | Bioorg Med Chem Lett 16: 2260-5 (2006) Article DOI: 10.1016/j.bmcl.2006.01.024 BindingDB Entry DOI: 10.7270/Q2C24TQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50582714 (CHEMBL5075978) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human LRRK2 WT incubated for 2 hrs by TR-FRET based Lanthascreen kinase activity assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00720 BindingDB Entry DOI: 10.7270/Q2MP5754 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM232602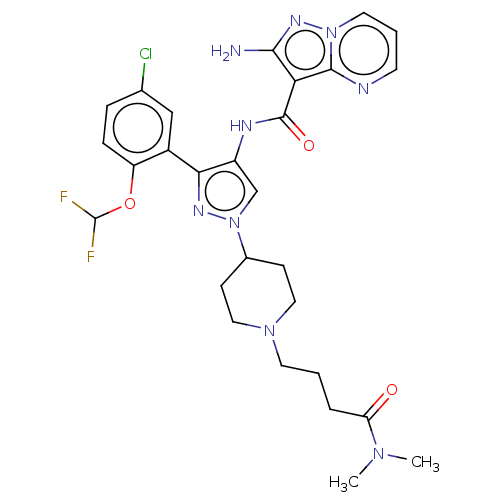 (US9346815, 148 | US9604984, Example 148) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | -56.5 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Genentech, Inc. US Patent | Assay Description The activity of the isolated recombinant JAK1 and JAK2 kinase domain was measured by monitoring phosphorylation of a peptide derived from JAK3 (Val-A... | US Patent US9346815 (2016) BindingDB Entry DOI: 10.7270/Q2NS0SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM232720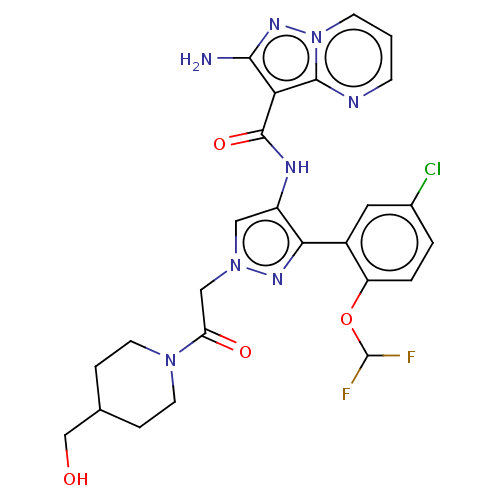 (US9346815, 266 | US9604984, Example 266) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | -56.5 | n/a | n/a | n/a | n/a | n/a | 7.2 | 22 |
Genentech, Inc. US Patent | Assay Description The activity of the isolated recombinant JAK1 and JAK2 kinase domain was measured by monitoring phosphorylation of a peptide derived from JAK3 (Val-A... | US Patent US9346815 (2016) BindingDB Entry DOI: 10.7270/Q2NS0SRT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082837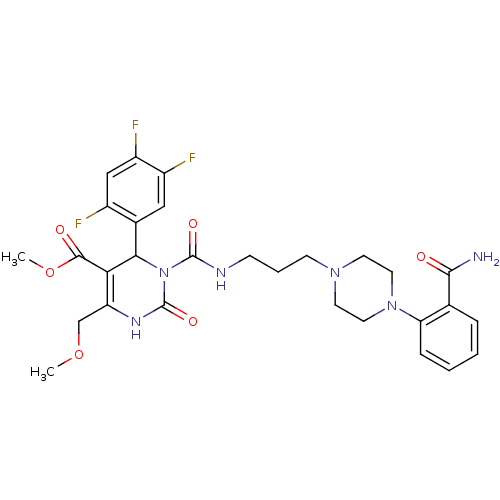 (3-{3-[4-(2-Carbamoyl-phenyl)-piperazin-1-yl]-propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082845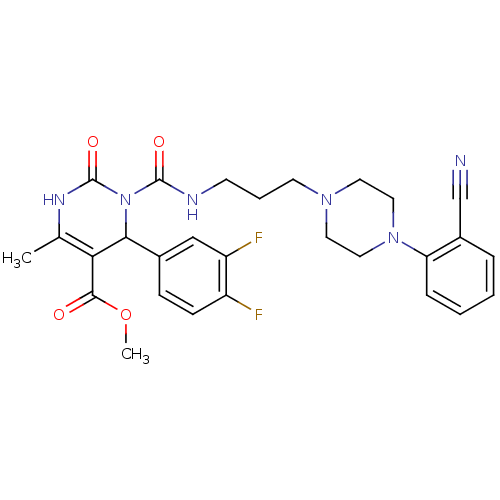 (3-{3-[4-(2-Cyano-phenyl)-piperazin-1-yl]-propylcar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082852 (4-(3,4-Difluoro-phenyl)-3-{3-[4-(2-methoxy-phenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082810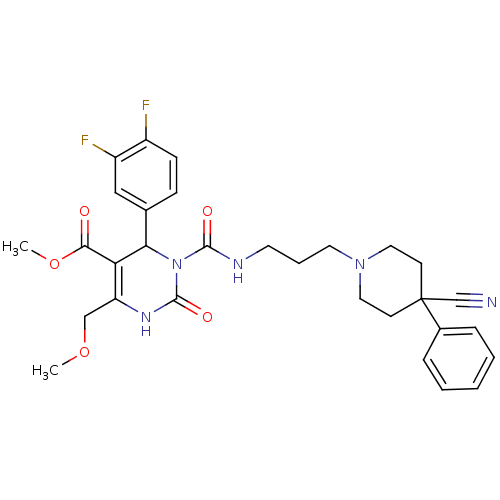 (3-[3-(4-Cyano-4-phenyl-piperidin-1-yl)-propylcarba...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082825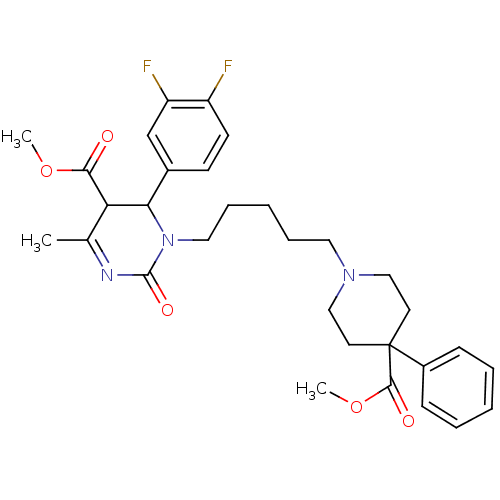 (4-(3,4-Difluoro-phenyl)-3-[5-(4-methoxycarbonyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082797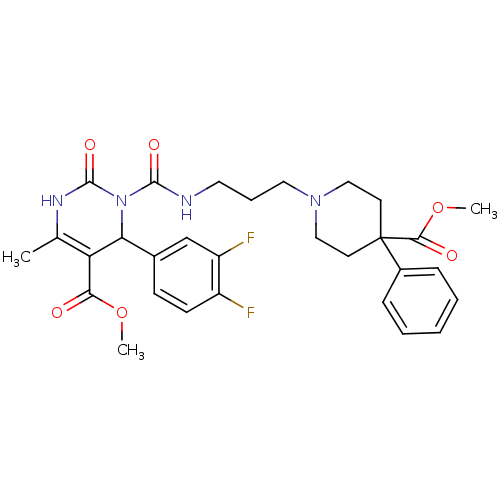 (4-(3,4-Difluoro-phenyl)-3-[3-(4-methoxycarbonyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082857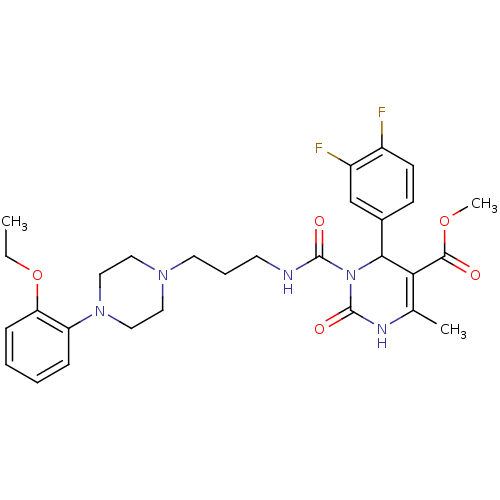 (4-(3,4-Difluoro-phenyl)-3-{3-[4-(2-ethoxy-phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082856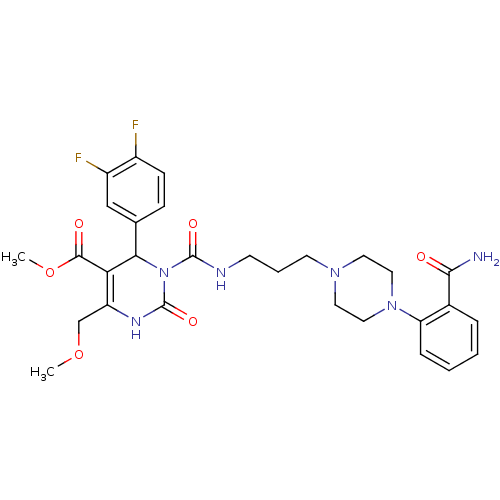 (3-{3-[4-(2-Carbamoyl-phenyl)-piperazin-1-yl]-propy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082845 (3-{3-[4-(2-Cyano-phenyl)-piperazin-1-yl]-propylcar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4794-803 (1999) BindingDB Entry DOI: 10.7270/Q25B01P0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 12378 total ) | Next | Last >> |