 Found 179 hits with Last Name = 'rea' and Initial = 'ma'
Found 179 hits with Last Name = 'rea' and Initial = 'ma' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM82517
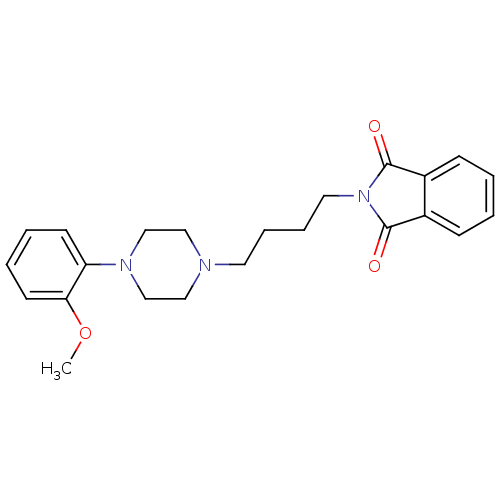
(2-{4-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-butyl}-...)Show SMILES COc1ccccc1N1CCN(CCCCN2C(=O)c3ccccc3C2=O)CC1 Show InChI InChI=1S/C23H27N3O3/c1-29-21-11-5-4-10-20(21)25-16-14-24(15-17-25)12-6-7-13-26-22(27)18-8-2-3-9-19(18)23(26)28/h2-5,8-11H,6-7,12-17H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM21392

(3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...)Show InChI InChI=1S/C11H13N3O/c12-4-3-8-6-14-10-2-1-7(11(13)15)5-9(8)10/h1-2,5-6,14H,3-4,12H2,(H2,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM78940

(METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...)Show InChI InChI=1S/C20H24N2S2/c1-21-9-11-22(12-10-21)18-13-15-5-3-4-6-19(15)24-20-8-7-16(23-2)14-17(18)20/h3-8,14,18H,9-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50027065
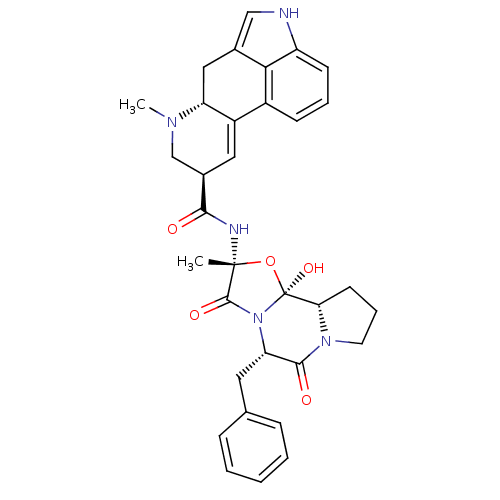
((5'alpha)-12'-hydroxy-2'-methyl-5'-(phenylmethyl)e...)Show SMILES CN1C[C@@H](C=C2[C@H]1Cc1c[nH]c3cccc2c13)C(=O)N[C@]1(C)O[C@@]2(O)[C@@H]3CCCN3C(=O)[C@H](Cc3ccccc3)N2C1=O |r,c:4| Show InChI InChI=1S/C33H35N5O5/c1-32(35-29(39)21-15-23-22-10-6-11-24-28(22)20(17-34-24)16-25(23)36(2)18-21)31(41)38-26(14-19-8-4-3-5-9-19)30(40)37-13-7-12-27(37)33(38,42)43-32/h3-6,8-11,15,17,21,25-27,34,42H,7,12-14,16,18H2,1-2H3,(H,35,39)/t21-,25-,26+,27+,32-,33+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM78940

(METHIOTHEPIN | MLS000859918 | Methiothepin mesylat...)Show InChI InChI=1S/C20H24N2S2/c1-21-9-11-22(12-10-21)18-13-15-5-3-4-6-19(15)24-20-8-7-16(23-2)14-17(18)20/h3-8,14,18H,9-13H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50024204

(1H-imidazo[4,5-c]pyridine derivative | 2N-[4,7-dim...)Show SMILES CN(C)S(=O)(=O)N[C@H]1C[C@H]2[C@@H](Cc3cn(C)c4cccc2c34)N(C)C1 Show InChI InChI=1S/C18H26N4O2S/c1-20(2)25(23,24)19-13-9-15-14-6-5-7-16-18(14)12(10-21(16)3)8-17(15)22(4)11-13/h5-7,10,13,15,17,19H,8-9,11H2,1-4H3/t13-,15+,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM30704
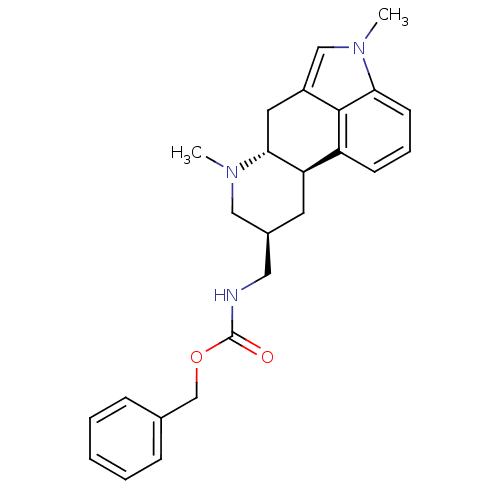
((phenylmethyl) N-[[(6aR,9S,10aR)-4,7-dimethyl-6,6a...)Show SMILES CN1C[C@H](CNC(=O)OCc2ccccc2)C[C@H]2[C@H]1Cc1cn(C)c3cccc2c13 Show InChI InChI=1S/C25H29N3O2/c1-27-14-18(13-26-25(29)30-16-17-7-4-3-5-8-17)11-21-20-9-6-10-22-24(20)19(12-23(21)27)15-28(22)2/h3-10,15,18,21,23H,11-14,16H2,1-2H3,(H,26,29)/t18-,21+,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50027065

((5'alpha)-12'-hydroxy-2'-methyl-5'-(phenylmethyl)e...)Show SMILES CN1C[C@@H](C=C2[C@H]1Cc1c[nH]c3cccc2c13)C(=O)N[C@]1(C)O[C@@]2(O)[C@@H]3CCCN3C(=O)[C@H](Cc3ccccc3)N2C1=O |r,c:4| Show InChI InChI=1S/C33H35N5O5/c1-32(35-29(39)21-15-23-22-10-6-11-24-28(22)20(17-34-24)16-25(23)36(2)18-21)31(41)38-26(14-19-8-4-3-5-9-19)30(40)37-13-7-12-27(37)33(38,42)43-32/h3-6,8-11,15,17,21,25-27,34,42H,7,12-14,16,18H2,1-2H3,(H,35,39)/t21-,25-,26+,27+,32-,33+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50031942
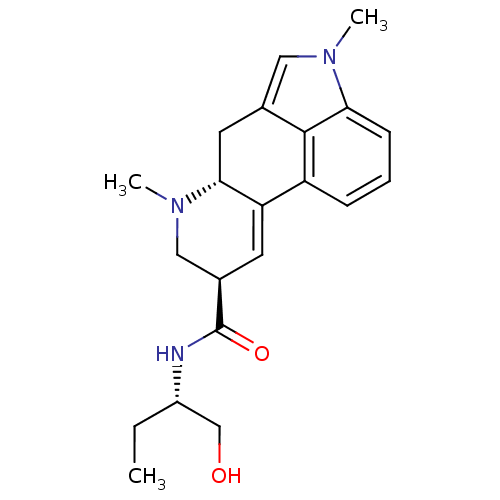
((6aR,9R)-4,6a,7-Trimethyl-4,6,6a,7,8,9-hexahydro-i...)Show SMILES CC[C@@H](CO)NC(=O)[C@H]1CN(C)[C@@H]2Cc3cn(C)c4cccc(C2=C1)c34 |r,c:24| Show InChI InChI=1S/C21H27N3O2/c1-4-15(12-25)22-21(26)14-8-17-16-6-5-7-18-20(16)13(10-23(18)2)9-19(17)24(3)11-14/h5-8,10,14-15,19,25H,4,9,11-12H2,1-3H3,(H,22,26)/t14-,15+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM21393

(7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...)Show InChI InChI=1S/C16H25NO/c1-3-10-17(11-4-2)14-9-8-13-6-5-7-16(18)15(13)12-14/h5-7,14,18H,3-4,8-12H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50019443
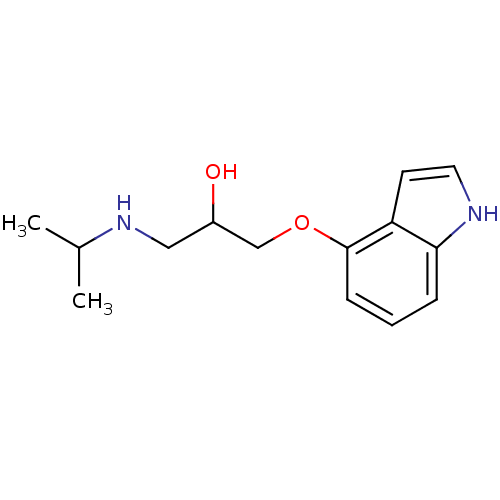
(1-(1H-Indol-4-yloxy)-3-isopropylamino-propan-2-ol ...)Show InChI InChI=1S/C14H20N2O2/c1-10(2)16-8-11(17)9-18-14-5-3-4-13-12(14)6-7-15-13/h3-7,10-11,15-17H,8-9H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50001775

((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM21393

(7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...)Show InChI InChI=1S/C16H25NO/c1-3-10-17(11-4-2)14-9-8-13-6-5-7-16(18)15(13)12-14/h5-7,14,18H,3-4,8-12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50001859
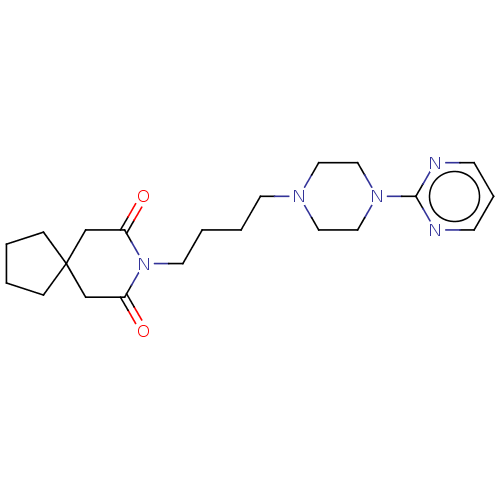
((buspirone) 8-[4-(4-Pyrimidin-2-yl-piperazin-1-yl)...)Show SMILES O=C1CC2(CCCC2)CC(=O)N1CCCCN1CCN(CC1)c1ncccn1 Show InChI InChI=1S/C21H31N5O2/c27-18-16-21(6-1-2-7-21)17-19(28)26(18)11-4-3-10-24-12-14-25(15-13-24)20-22-8-5-9-23-20/h5,8-9H,1-4,6-7,10-17H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 68.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM82517

(2-{4-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-butyl}-...)Show SMILES COc1ccccc1N1CCN(CCCCN2C(=O)c3ccccc3C2=O)CC1 Show InChI InChI=1S/C23H27N3O3/c1-29-21-11-5-4-10-20(21)25-16-14-24(15-17-25)12-6-7-13-26-22(27)18-8-2-3-9-19(18)23(26)28/h2-5,8-11H,6-7,12-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM10755

(14C-5-hydroxy tryptamine creatinine disulfate | 2-...)Show InChI InChI=1S/C10H12N2O/c11-4-3-7-6-12-10-2-1-8(13)5-9(7)10/h1-2,5-6,12-13H,3-4,11H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM30704

((phenylmethyl) N-[[(6aR,9S,10aR)-4,7-dimethyl-6,6a...)Show SMILES CN1C[C@H](CNC(=O)OCc2ccccc2)C[C@H]2[C@H]1Cc1cn(C)c3cccc2c13 Show InChI InChI=1S/C25H29N3O2/c1-27-14-18(13-26-25(29)30-16-17-7-4-3-5-8-17)11-21-20-9-6-10-22-24(20)19(12-23(21)27)15-28(22)2/h3-10,15,18,21,23H,11-14,16H2,1-2H3,(H,26,29)/t18-,21+,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 273 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50001859

((buspirone) 8-[4-(4-Pyrimidin-2-yl-piperazin-1-yl)...)Show SMILES O=C1CC2(CCCC2)CC(=O)N1CCCCN1CCN(CC1)c1ncccn1 Show InChI InChI=1S/C21H31N5O2/c27-18-16-21(6-1-2-7-21)17-19(28)26(18)11-4-3-10-24-12-14-25(15-13-24)20-22-8-5-9-23-20/h5,8-9H,1-4,6-7,10-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 375 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM55121

(3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...)Show InChI InChI=1S/C8H11NO2/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,10-11H,3-4,9H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 545 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50024204

(1H-imidazo[4,5-c]pyridine derivative | 2N-[4,7-dim...)Show SMILES CN(C)S(=O)(=O)N[C@H]1C[C@H]2[C@@H](Cc3cn(C)c4cccc2c34)N(C)C1 Show InChI InChI=1S/C18H26N4O2S/c1-20(2)25(23,24)19-13-9-15-14-6-5-7-16-18(14)12(10-21(16)3)8-17(15)22(4)11-13/h5-7,10,13,15,17,19H,8-9,11H2,1-4H3/t13-,15+,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 545 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50031942

((6aR,9R)-4,6a,7-Trimethyl-4,6,6a,7,8,9-hexahydro-i...)Show SMILES CC[C@@H](CO)NC(=O)[C@H]1CN(C)[C@@H]2Cc3cn(C)c4cccc(C2=C1)c34 |r,c:24| Show InChI InChI=1S/C21H27N3O2/c1-4-15(12-25)22-21(26)14-8-17-16-6-5-7-18-20(16)13(10-23(18)2)9-19(17)24(3)11-14/h5-8,10,14-15,19,25H,4,9,11-12H2,1-3H3,(H,22,26)/t14-,15+,19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 545 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM35234

(DL-[7-3H]norepinephrine | NOREPINEPHRINE | Noradre...)Show InChI InChI=1S/C8H11NO3/c9-4-8(12)5-1-2-6(10)7(11)3-5/h1-3,8,10-12H,4,9H2 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 545 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM55121

(3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...)Show InChI InChI=1S/C8H11NO2/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,10-11H,3-4,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 545 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Rattus norvegicus (rat)) | BDBM50001775

((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...)Show SMILES [#6]-c1nc2sccn2c(=O)c1-[#6]-[#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6](\c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H25F2N3OS/c1-18-24(26(33)32-16-17-34-27(32)30-18)12-15-31-13-10-21(11-14-31)25(19-2-6-22(28)7-3-19)20-4-8-23(29)9-5-20/h2-9,16-17H,10-15H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 545 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50000492

((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CN2CCC1CC2 |(27.19,-33.96,;28.52,-34.73,;28.53,-36.27,;27.2,-37.04,;27.2,-38.58,;25.86,-39.35,;28.53,-39.36,;28.53,-40.89,;29.87,-38.58,;29.86,-37.03,;31.19,-36.26,;31.19,-34.72,;32.53,-37.02,;33.86,-36.25,;35.2,-37.02,;36.52,-36.25,;36.52,-34.71,;35.19,-33.94,;33.85,-34.71,;34.61,-36.04,;35.74,-34.91,)| Show InChI InChI=1S/C15H20ClN3O2/c1-21-14-7-12(17)11(16)6-10(14)15(20)18-13-8-19-4-2-9(13)3-5-19/h6-7,9,13H,2-5,8,17H2,1H3,(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 545 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50005835

((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...)Show InChI InChI=1S/C14H21N3O2S/c1-15-20(18,19)10-11-4-5-14-13(8-11)12(9-16-14)6-7-17(2)3/h4-5,8-9,15-16H,6-7,10H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 545 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50019443

(1-(1H-Indol-4-yloxy)-3-isopropylamino-propan-2-ol ...)Show InChI InChI=1S/C14H20N2O2/c1-10(2)16-8-11(17)9-18-14-5-3-4-13-12(14)6-7-15-13/h3-7,10-11,15-17H,8-9H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 545 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM35234

(DL-[7-3H]norepinephrine | NOREPINEPHRINE | Noradre...)Show InChI InChI=1S/C8H11NO3/c9-4-8(12)5-1-2-6(10)7(11)3-5/h1-3,8,10-12H,4,9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 545 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by PDSP Ki Database
| |
Neuron 11: 449-58 (1993)
Article DOI: 10.1016/0896-6273(93)90149-l
BindingDB Entry DOI: 10.7270/Q269723D |
More data for this
Ligand-Target Pair | |
Smoothened homolog
(Homo sapiens (Human)) | BDBM50293788
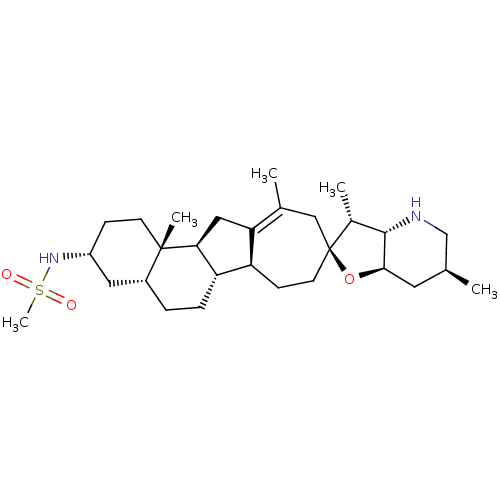
(CHEMBL538867 | N-((2S,3R,3aS,3'R,4a'R,6S,6a'R,6b'S...)Show SMILES C[C@@H]1[C@@H]2NC[C@@H](C)C[C@H]2O[C@]11CC[C@H]2[C@@H]3CC[C@@H]4C[C@@H](CC[C@]4(C)[C@H]3CC2=C(C)C1)NS(C)(=O)=O |r,t:31| Show InChI InChI=1S/C29H48N2O3S/c1-17-12-26-27(30-16-17)19(3)29(34-26)11-9-22-23-7-6-20-13-21(31-35(5,32)33)8-10-28(20,4)25(23)14-24(22)18(2)15-29/h17,19-23,25-27,30-31H,6-16H2,1-5H3/t17-,19+,20+,21+,22-,23-,25-,26+,27-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant SMO expressed in mouse C3H10T1/2 cells assessed as inhibition of association of BODIPY-cyclopamine |
J Med Chem 52: 4400-18 (2009)
Article DOI: 10.1021/jm900305z
BindingDB Entry DOI: 10.7270/Q2CC10QM |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068324
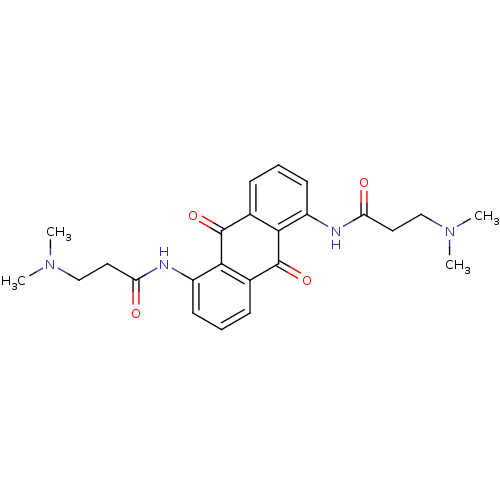
(3-Dimethylamino-N-[5-(3-dimethylamino-propionylami...)Show SMILES CN(C)CCC(=O)Nc1cccc2C(=O)c3c(NC(=O)CCN(C)C)cccc3C(=O)c12 Show InChI InChI=1S/C24H28N4O4/c1-27(2)13-11-19(29)25-17-9-5-7-15-21(17)23(31)16-8-6-10-18(22(16)24(15)32)26-20(30)12-14-28(3)4/h5-10H,11-14H2,1-4H3,(H,25,29)(H,26,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068304
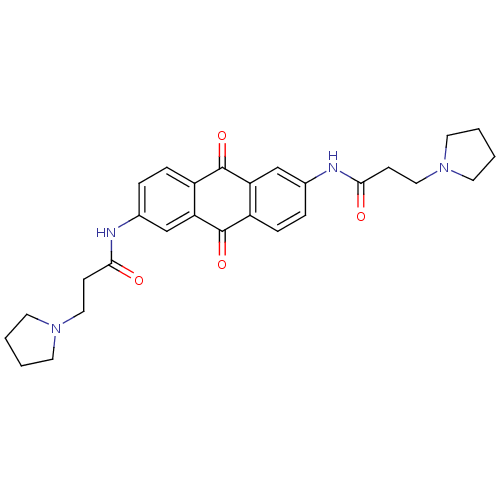
(CHEMBL343445 | N-[9,10-Dioxo-6-(3-pyrrolidin-1-yl-...)Show SMILES O=C(CCN1CCCC1)Nc1ccc2C(=O)c3cc(NC(=O)CCN4CCCC4)ccc3C(=O)c2c1 Show InChI InChI=1S/C28H32N4O4/c33-25(9-15-31-11-1-2-12-31)29-19-5-7-21-23(17-19)27(35)22-8-6-20(18-24(22)28(21)36)30-26(34)10-16-32-13-3-4-14-32/h5-8,17-18H,1-4,9-16H2,(H,29,33)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50082524
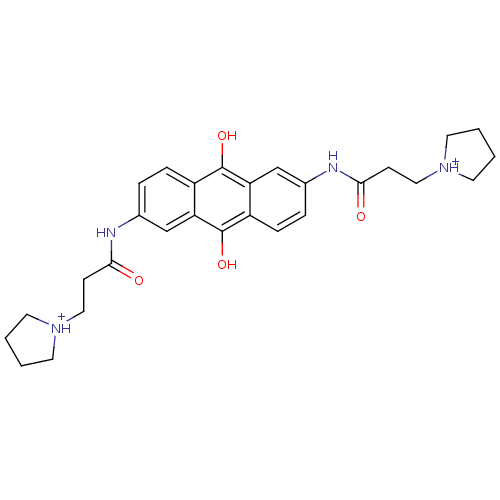
(2,6-Bis(3-pyrrolidinopropionamido)anthracene-9,10-...)Show SMILES Oc1c2ccc(NC(=O)CC[NH+]3CCCC3)cc2c(O)c2ccc(NC(=O)CC[NH+]3CCCC3)cc12 Show InChI InChI=1S/C28H34N4O4/c33-25(9-15-31-11-1-2-12-31)29-19-5-7-21-23(17-19)27(35)22-8-6-20(18-24(22)28(21)36)30-26(34)10-16-32-13-3-4-14-32/h5-8,17-18,35-36H,1-4,9-16H2,(H,29,33)(H,30,34)/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Telomerase evaluated by TRAP Assay studies. |
J Med Chem 42: 4538-46 (1999)
BindingDB Entry DOI: 10.7270/Q2J103VC |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068320

(2,7-Bis[3-(pyrrolidino)propionamido]anthraquinone ...)Show SMILES O=C(CCN1CCCC1)Nc1ccc2C(=O)c3ccc(NC(=O)CCN4CCCC4)cc3C(=O)c2c1 Show InChI InChI=1S/C28H32N4O4/c33-25(9-15-31-11-1-2-12-31)29-19-5-7-21-23(17-19)28(36)24-18-20(6-8-22(24)27(21)35)30-26(34)10-16-32-13-3-4-14-32/h5-8,17-18H,1-4,9-16H2,(H,29,33)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068330

(CHEMBL144848 | N-[9,10-Dioxo-5-(3-piperidin-1-yl-p...)Show SMILES O=C(CCN1CCCCC1)Nc1cccc2C(=O)c3c(NC(=O)CCN4CCCCC4)cccc3C(=O)c12 Show InChI InChI=1S/C30H36N4O4/c35-25(13-19-33-15-3-1-4-16-33)31-23-11-7-9-21-27(23)29(37)22-10-8-12-24(28(22)30(21)38)32-26(36)14-20-34-17-5-2-6-18-34/h7-12H,1-6,13-20H2,(H,31,35)(H,32,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068303
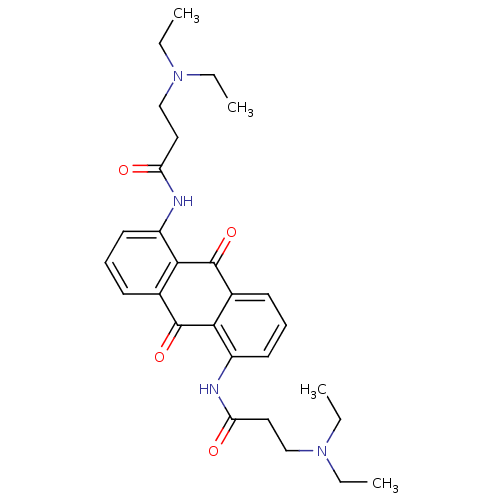
(1,5-BIS[3-(DIETHYLAMINO)PROPIONAMIDO]ANTHRACENE-9,...)Show SMILES CCN(CC)CCC(=O)Nc1cccc2C(=O)c3c(NC(=O)CCN(CC)CC)cccc3C(=O)c12 Show InChI InChI=1S/C28H36N4O4/c1-5-31(6-2)17-15-23(33)29-21-13-9-11-19-25(21)27(35)20-12-10-14-22(26(20)28(19)36)30-24(34)16-18-32(7-3)8-4/h9-14H,5-8,15-18H2,1-4H3,(H,29,33)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50080847

(3,6-Bis(3-piperidinopropionamido)acridine | 3-Pipe...)Show SMILES O=C(CCN1CCCCC1)Nc1ccc2cc3ccc(NC(=O)CCN4CCCCC4)cc3nc2c1 Show InChI InChI=1S/C29H37N5O2/c35-28(11-17-33-13-3-1-4-14-33)30-24-9-7-22-19-23-8-10-25(21-27(23)32-26(22)20-24)31-29(36)12-18-34-15-5-2-6-16-34/h7-10,19-21H,1-6,11-18H2,(H,30,35)(H,31,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Telomerase evaluated by TRAP Assay studies. |
J Med Chem 42: 4538-46 (1999)
BindingDB Entry DOI: 10.7270/Q2J103VC |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068328
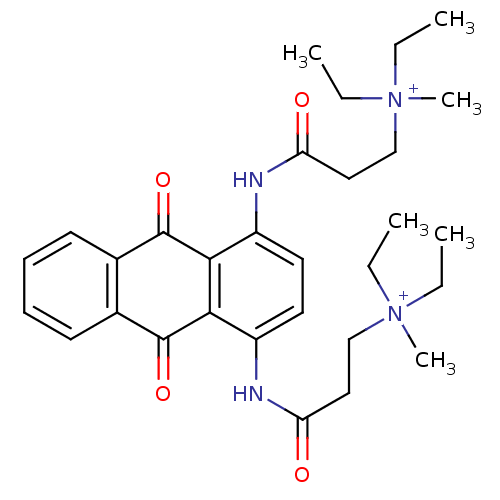
(CHEMBL144303 | N-[9,10-Dioxo-4-(3-(N,N-diethyl-N-m...)Show SMILES CC[N+](C)(CC)CCC(=O)Nc1ccc(NC(=O)CC[N+](C)(CC)CC)c2C(=O)c3ccccc3C(=O)c12 Show InChI InChI=1S/C30H40N4O4/c1-7-33(5,8-2)19-17-25(35)31-23-15-16-24(32-26(36)18-20-34(6,9-3)10-4)28-27(23)29(37)21-13-11-12-14-22(21)30(28)38/h11-16H,7-10,17-20H2,1-6H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50080840
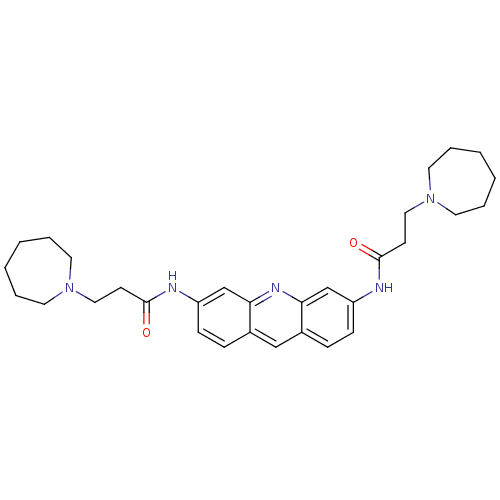
(3,6-Bis[3-(azepan-1-yl)propionamido]acridine | 3,6...)Show SMILES O=C(CCN1CCCCCC1)Nc1ccc2cc3ccc(NC(=O)CCN4CCCCCC4)cc3nc2c1 Show InChI InChI=1S/C31H41N5O2/c37-30(13-19-35-15-5-1-2-6-16-35)32-26-11-9-24-21-25-10-12-27(23-29(25)34-28(24)22-26)33-31(38)14-20-36-17-7-3-4-8-18-36/h9-12,21-23H,1-8,13-20H2,(H,32,37)(H,33,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Telomerase evaluated by TRAP Assay studies. |
J Med Chem 42: 4538-46 (1999)
BindingDB Entry DOI: 10.7270/Q2J103VC |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068321

(2,7-Bis[3-(piperidino)propionamido]anthraquinone |...)Show SMILES O=C(CCN1CCCCC1)Nc1ccc2C(=O)c3ccc(NC(=O)CCN4CCCCC4)cc3C(=O)c2c1 Show InChI InChI=1S/C30H36N4O4/c35-27(11-17-33-13-3-1-4-14-33)31-21-7-9-23-25(19-21)30(38)26-20-22(8-10-24(26)29(23)37)32-28(36)12-18-34-15-5-2-6-16-34/h7-10,19-20H,1-6,11-18H2,(H,31,35)(H,32,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against telomerase |
J Med Chem 42: 2679-84 (1999)
Article DOI: 10.1021/jm990084q
BindingDB Entry DOI: 10.7270/Q2DF6RX9 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068321

(2,7-Bis[3-(piperidino)propionamido]anthraquinone |...)Show SMILES O=C(CCN1CCCCC1)Nc1ccc2C(=O)c3ccc(NC(=O)CCN4CCCCC4)cc3C(=O)c2c1 Show InChI InChI=1S/C30H36N4O4/c35-27(11-17-33-13-3-1-4-14-33)31-21-7-9-23-25(19-21)30(38)26-20-22(8-10-24(26)29(23)37)32-28(36)12-18-34-15-5-2-6-16-34/h7-10,19-20H,1-6,11-18H2,(H,31,35)(H,32,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50005750
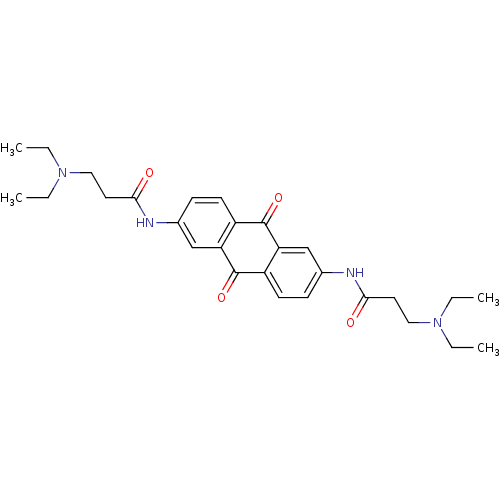
(3-Diethylamino-N-[6-(3-diethylamino-propionylamino...)Show SMILES CCN(CC)CCC(=O)Nc1ccc2C(=O)c3cc(NC(=O)CCN(CC)CC)ccc3C(=O)c2c1 Show InChI InChI=1S/C28H36N4O4/c1-5-31(6-2)15-13-25(33)29-19-9-11-21-23(17-19)27(35)22-12-10-20(18-24(22)28(21)36)30-26(34)14-16-32(7-3)8-4/h9-12,17-18H,5-8,13-16H2,1-4H3,(H,29,33)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068332
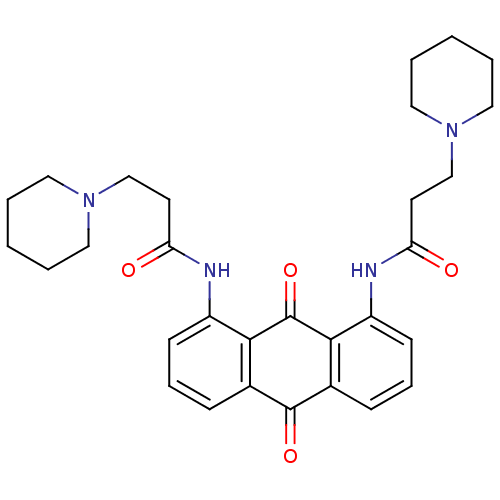
(CHEMBL144386 | N-[9,10-Dioxo-8-(3-piperidin-1-yl-p...)Show SMILES O=C(CCN1CCCCC1)Nc1cccc2C(=O)c3cccc(NC(=O)CCN4CCCCC4)c3C(=O)c12 Show InChI InChI=1S/C30H36N4O4/c35-25(13-19-33-15-3-1-4-16-33)31-23-11-7-9-21-27(23)30(38)28-22(29(21)37)10-8-12-24(28)32-26(36)14-20-34-17-5-2-6-18-34/h7-12H,1-6,13-20H2,(H,31,35)(H,32,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068311
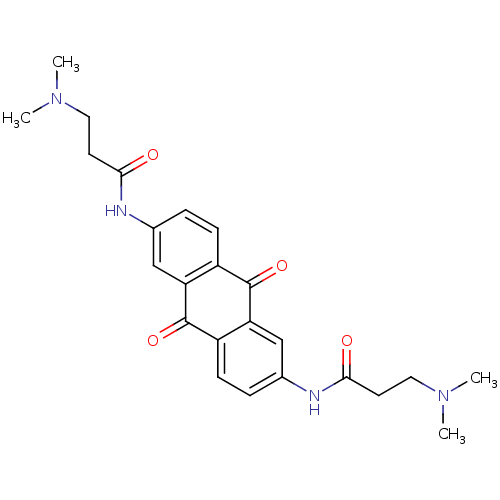
(3-Dimethylamino-N-[6-(3-dimethylamino-propionylami...)Show SMILES CN(C)CCC(=O)Nc1ccc2C(=O)c3cc(NC(=O)CCN(C)C)ccc3C(=O)c2c1 Show InChI InChI=1S/C24H28N4O4/c1-27(2)11-9-21(29)25-15-5-7-17-19(13-15)23(31)18-8-6-16(14-20(18)24(17)32)26-22(30)10-12-28(3)4/h5-8,13-14H,9-12H2,1-4H3,(H,25,29)(H,26,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068315

(3-Diethylamino-N-[8-(3-diethylamino-propionylamino...)Show SMILES CCN(CC)CCC(=O)Nc1cccc2C(=O)c3cccc(NC(=O)CCN(CC)CC)c3C(=O)c12 Show InChI InChI=1S/C28H36N4O4/c1-5-31(6-2)17-15-23(33)29-21-13-9-11-19-25(21)28(36)26-20(27(19)35)12-10-14-22(26)30-24(34)16-18-32(7-3)8-4/h9-14H,5-8,15-18H2,1-4H3,(H,29,33)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068312

(2,7-Bis[3-(diethylamino)propionamido]anthraquinone...)Show SMILES CCN(CC)CCC(=O)Nc1ccc2C(=O)c3ccc(NC(=O)CCN(CC)CC)cc3C(=O)c2c1 Show InChI InChI=1S/C28H36N4O4/c1-5-31(6-2)15-13-25(33)29-19-9-11-21-23(17-19)28(36)24-18-20(10-12-22(24)27(21)35)30-26(34)14-16-32(7-3)8-4/h9-12,17-18H,5-8,13-16H2,1-4H3,(H,29,33)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against telomerase |
J Med Chem 42: 2679-84 (1999)
Article DOI: 10.1021/jm990084q
BindingDB Entry DOI: 10.7270/Q2DF6RX9 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068312

(2,7-Bis[3-(diethylamino)propionamido]anthraquinone...)Show SMILES CCN(CC)CCC(=O)Nc1ccc2C(=O)c3ccc(NC(=O)CCN(CC)CC)cc3C(=O)c2c1 Show InChI InChI=1S/C28H36N4O4/c1-5-31(6-2)15-13-25(33)29-19-9-11-21-23(17-19)28(36)24-18-20(10-12-22(24)27(21)35)30-26(34)14-16-32(7-3)8-4/h9-12,17-18H,5-8,13-16H2,1-4H3,(H,29,33)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068335
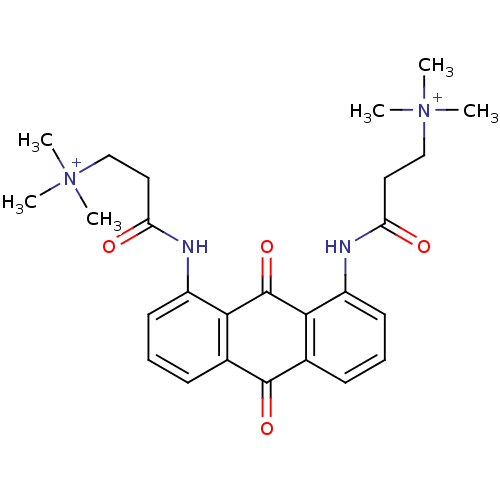
(CHEMBL422120 | N-[9,10-Dioxo-8-(3-(N,N,N-trimethyl...)Show SMILES C[N+](C)(C)CCC(=O)Nc1cccc2C(=O)c3cccc(NC(=O)CC[N+](C)(C)C)c3C(=O)c12 Show InChI InChI=1S/C26H32N4O4/c1-29(2,3)15-13-21(31)27-19-11-7-9-17-23(19)26(34)24-18(25(17)33)10-8-12-20(24)28-22(32)14-16-30(4,5)6/h7-12H,13-16H2,1-6H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50005746
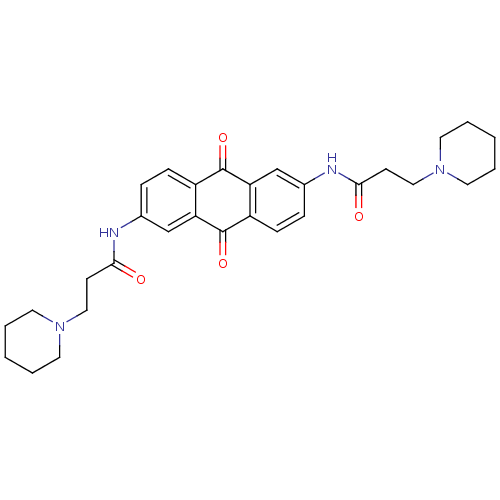
(CHEMBL109382 | CHEMBL33618 | N-[9,10-Dioxo-6-(3-pi...)Show SMILES O=C(CCN1CCCCC1)Nc1ccc2C(=O)c3cc(NC(=O)CCN4CCCCC4)ccc3C(=O)c2c1 Show InChI InChI=1S/C30H36N4O4/c35-27(11-17-33-13-3-1-4-14-33)31-21-7-9-23-25(19-21)29(37)24-10-8-22(20-26(24)30(23)38)32-28(36)12-18-34-15-5-2-6-16-34/h7-10,19-20H,1-6,11-18H2,(H,31,35)(H,32,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50082532

(2,6-Bis(3-piperidinopropionamido)anthracene-9,10-d...)Show SMILES Oc1c2ccc(NC(=O)CC[NH+]3CCCCC3)cc2c(O)c2ccc(NC(=O)CC[NH+]3CCCCC3)cc12 Show InChI InChI=1S/C30H38N4O4/c35-27(11-17-33-13-3-1-4-14-33)31-21-7-9-23-25(19-21)29(37)24-10-8-22(20-26(24)30(23)38)32-28(36)12-18-34-15-5-2-6-16-34/h7-10,19-20,37-38H,1-6,11-18H2,(H,31,35)(H,32,36)/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Telomerase evaluated by TRAP Assay studies. |
J Med Chem 42: 4538-46 (1999)
BindingDB Entry DOI: 10.7270/Q2J103VC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data