

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50454329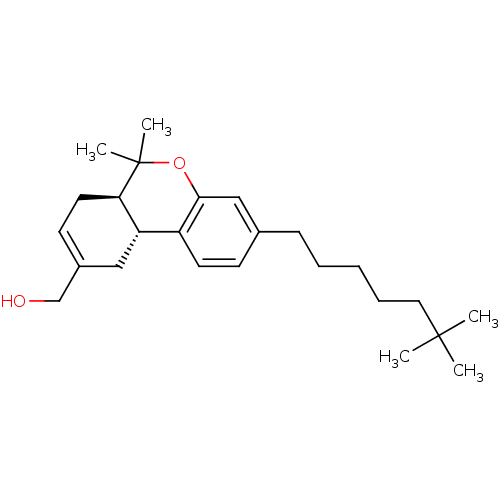 (CHEMBL2112647) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clemson University Curated by ChEMBL | Assay Description Ability to bind with Cannabinoid receptor 2 using [H]CP 55,940 as radioligand from cloned human receptor preparation | J Med Chem 39: 3875-7 (1996) Article DOI: 10.1021/jm960394y BindingDB Entry DOI: 10.7270/Q2R49PVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233601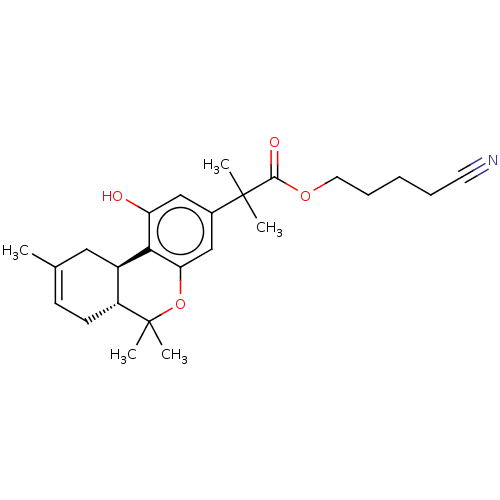 (CHEMBL4062745) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029978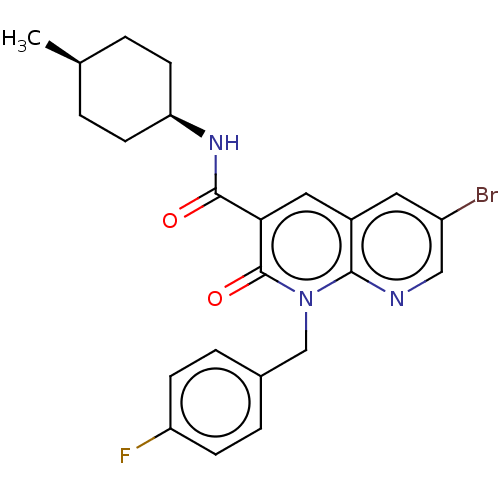 (CHEMBL3353441) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029963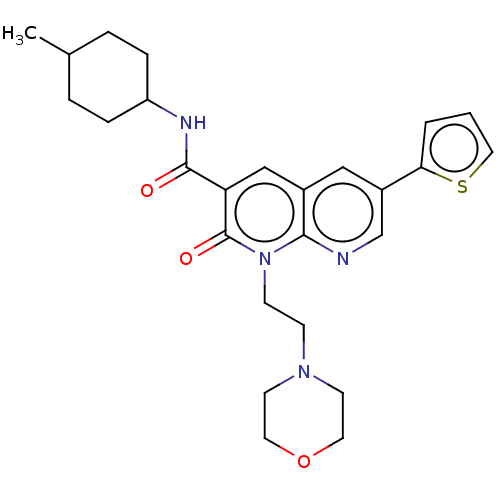 (CHEMBL3353452) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029958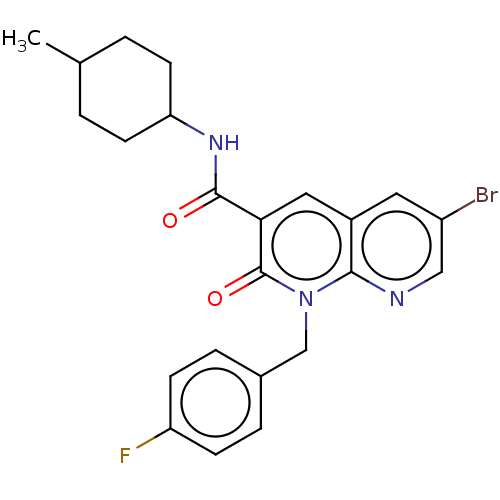 (CHEMBL3353439) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029962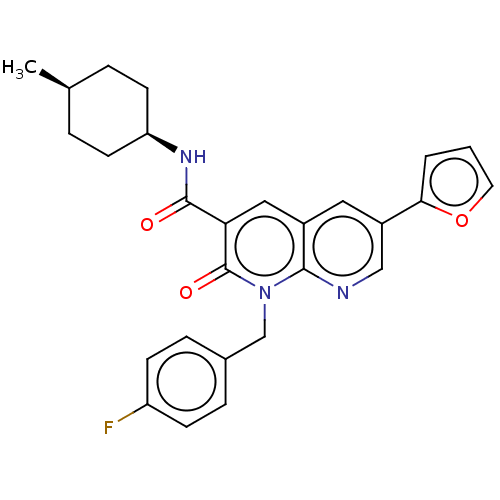 (CHEMBL3353450) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kennesaw State University Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Cannabinoid receptor 2 in Guinea pig ileum (GPI) using [3H]CP-55940 ligand | J Med Chem 41: 5177-87 (1999) Article DOI: 10.1021/jm9801197 BindingDB Entry DOI: 10.7270/Q2ST7QJJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233599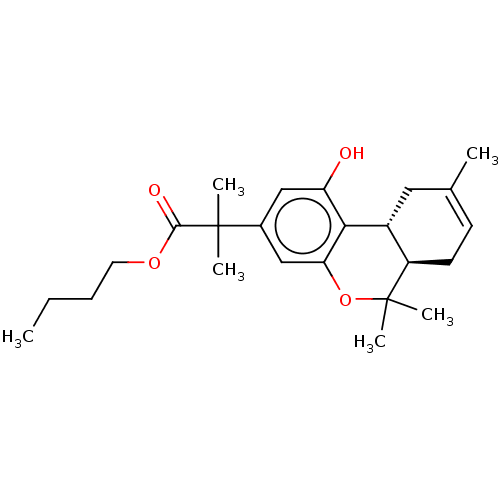 (CHEMBL3104360) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233619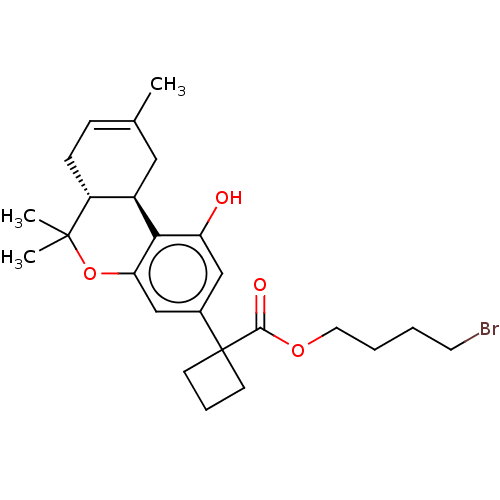 (CHEMBL4085404 | US10882838, Example 1.18) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50200170 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor transfected in HEK293EBNA cell membranes after 90 mins by liquid scintillation counting analysis | Eur J Med Chem 101: 651-67 (2015) Article DOI: 10.1016/j.ejmech.2015.06.057 BindingDB Entry DOI: 10.7270/Q2KP83ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029965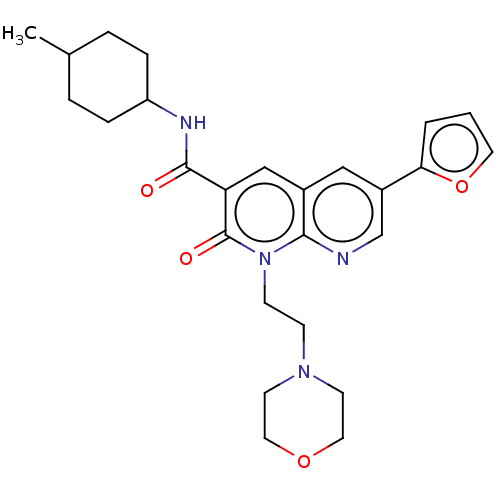 (CHEMBL3353454) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233606 (CHEMBL4068675) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233602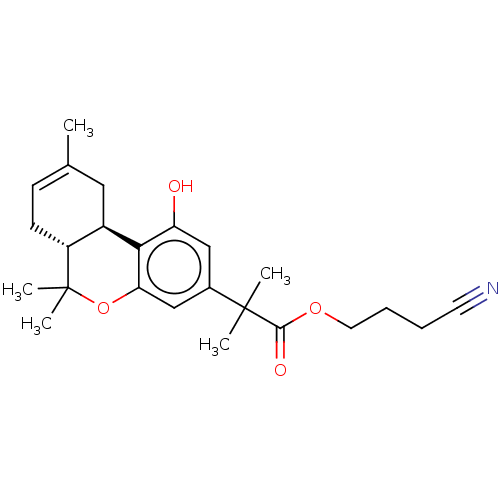 (CHEMBL4071389 | US10882838, Example 1.8) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233613 (CHEMBL4092136 | US10882838, Example 1.33) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233622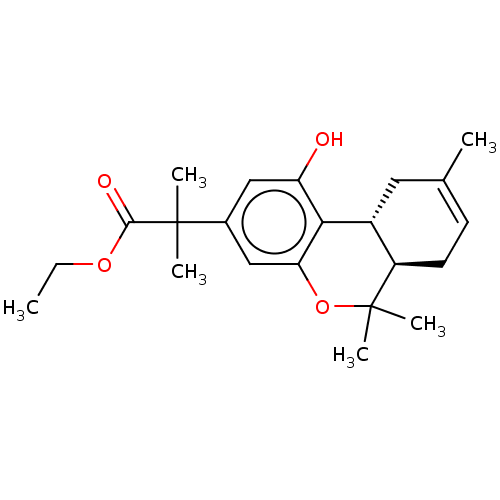 (CHEMBL4073011 | US10882838, Example 1.35) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50053357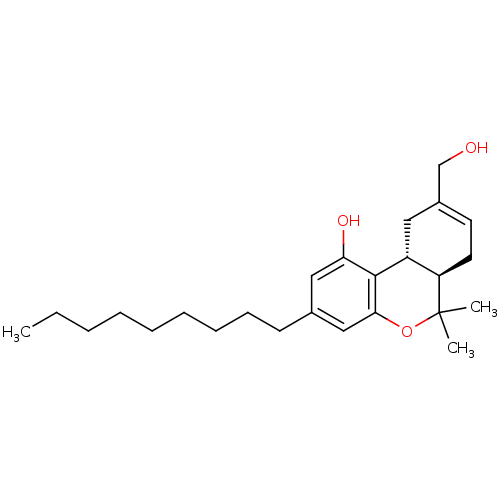 ((6aR,10aR)-9-Hydroxymethyl-6,6-dimethyl-3-nonyl-6a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clemson University Curated by ChEMBL | Assay Description Ability to bind with Cannabinoid receptor 2 using [H]CP 55,940 as radioligand from cloned human receptor preparation | J Med Chem 39: 3875-7 (1996) Article DOI: 10.1021/jm960394y BindingDB Entry DOI: 10.7270/Q2R49PVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029989 (CHEMBL3353437) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50200170 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Sassari Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor transfected in CHO cell membranes by liquid scintillation counting analysis | Eur J Med Chem 101: 651-67 (2015) Article DOI: 10.1016/j.ejmech.2015.06.057 BindingDB Entry DOI: 10.7270/Q2KP83ZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50180022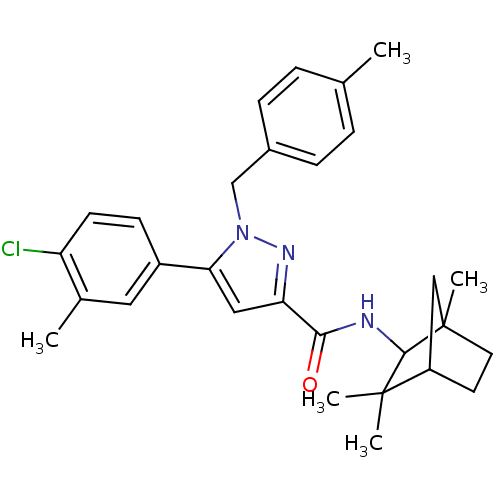 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50200170 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas-Pan American Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor expressed in CHO cells | J Med Chem 56: 6593-612 (2013) Article DOI: 10.1021/jm400070u BindingDB Entry DOI: 10.7270/Q2BG2RXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233608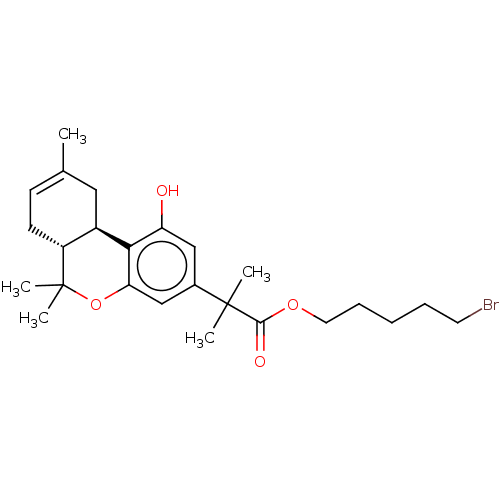 (CHEMBL4094603) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029972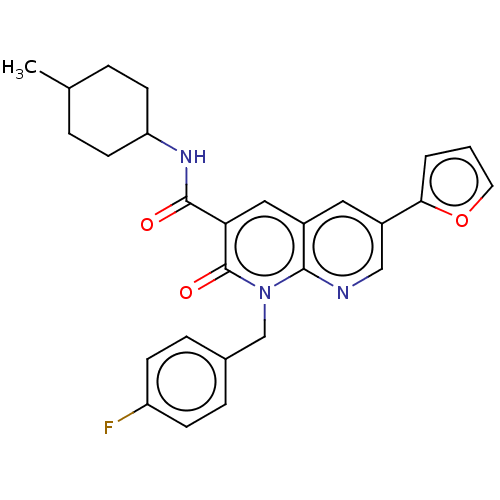 (CHEMBL3353448) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029964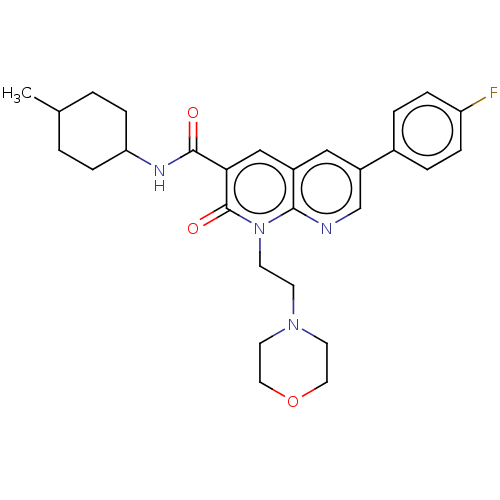 (CHEMBL3353453) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233606 (CHEMBL4068675) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233612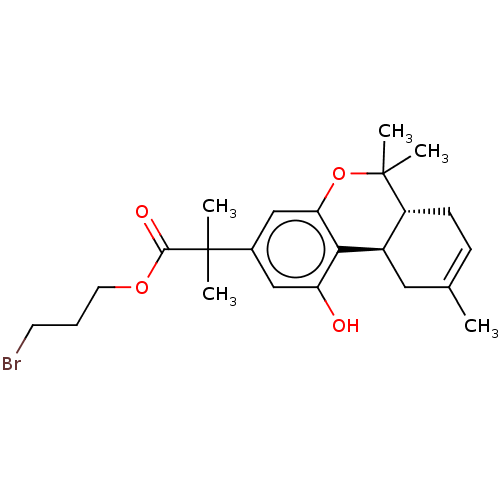 (CHEMBL4102348 | US10882838, Example 1.7) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233619 (CHEMBL4085404 | US10882838, Example 1.18) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233605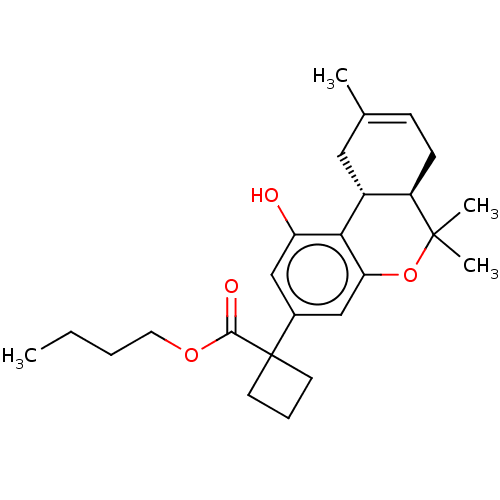 (CHEMBL3104362 | US10882838, Example 1.20) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233601 (CHEMBL4062745) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50053357 ((6aR,10aR)-9-Hydroxymethyl-6,6-dimethyl-3-nonyl-6a...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clemson University Curated by ChEMBL | Assay Description Compound was evaluated for Pharmacological response in the Cannabinoid receptor 1 | J Med Chem 39: 3875-7 (1996) Article DOI: 10.1021/jm960394y BindingDB Entry DOI: 10.7270/Q2R49PVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50053359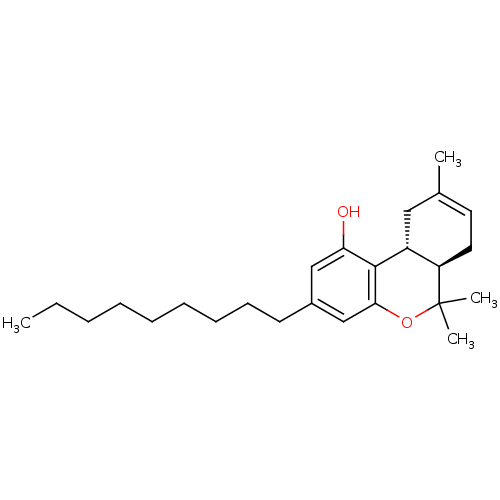 ((6aR,10aR)-6,6,9-Trimethyl-3-nonyl-6a,7,10,10a-tet...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clemson University Curated by ChEMBL | Assay Description Compound was evaluated for Pharmacological response in the Cannabinoid receptor 1 | J Med Chem 39: 3875-7 (1996) Article DOI: 10.1021/jm960394y BindingDB Entry DOI: 10.7270/Q2R49PVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233602 (CHEMBL4071389 | US10882838, Example 1.8) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233609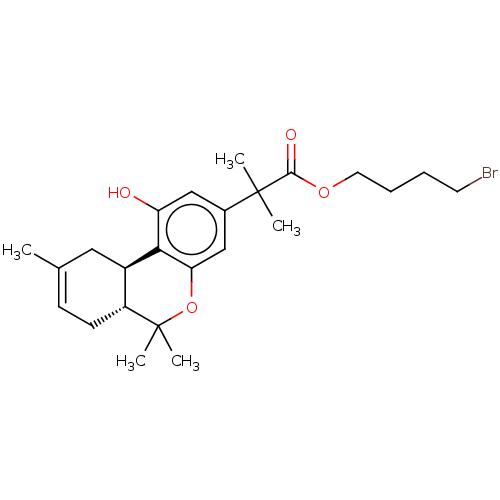 (CHEMBL4074070) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233606 (CHEMBL4068675) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233612 (CHEMBL4102348 | US10882838, Example 1.7) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233609 (CHEMBL4074070) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233601 (CHEMBL4062745) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233604 (CHEMBL4063822 | US10882838, Example 1.19) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50258652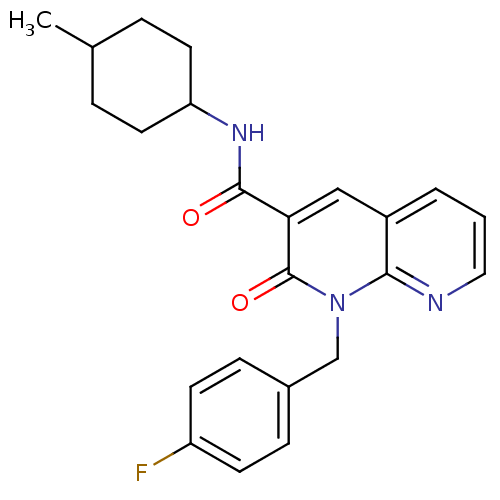 (CHEMBL466651 | N-(4-Methylcyclohexyl)-1-(p-fluorob...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029974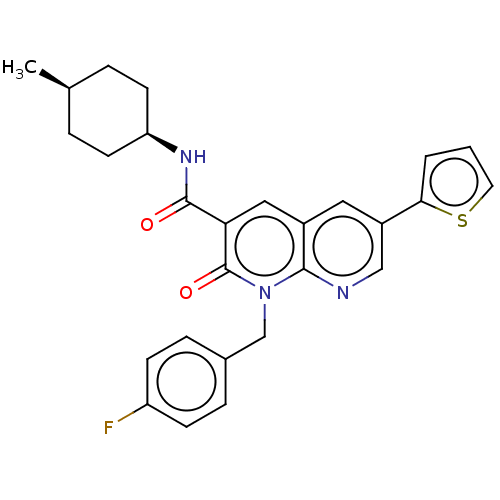 (CHEMBL3353446) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233613 (CHEMBL4092136 | US10882838, Example 1.33) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233608 (CHEMBL4094603) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029979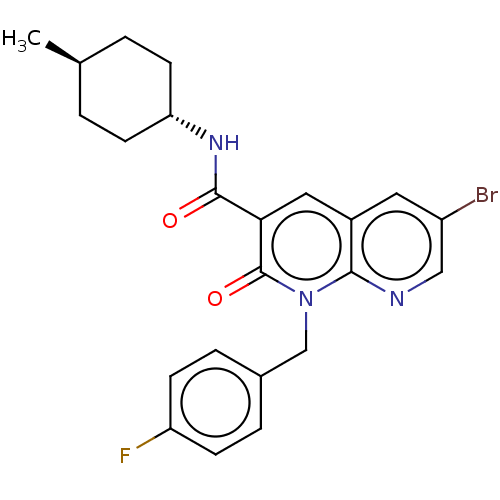 (CHEMBL3353440) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233612 (CHEMBL4102348 | US10882838, Example 1.7) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233609 (CHEMBL4074070) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50454329 (CHEMBL2112647) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clemson University Curated by ChEMBL | Assay Description Ability to bind with Cannabinoid receptor 1 using [H]CP-55940 as radioligand in rat brain membranes | J Med Chem 39: 3875-7 (1996) Article DOI: 10.1021/jm960394y BindingDB Entry DOI: 10.7270/Q2R49PVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50233608 (CHEMBL4094603) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from CB1 receptor in rat brain membranes by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233603 (CHEMBL4098489 | US10882838, Example 1.28) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50233613 (CHEMBL4092136 | US10882838, Example 1.33) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from mouse CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50029959 (CHEMBL3353442 | US11564928, Compound 61) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... | J Med Chem 57: 8777-91 (2014) Article DOI: 10.1021/jm500807e BindingDB Entry DOI: 10.7270/Q2QC054S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50233600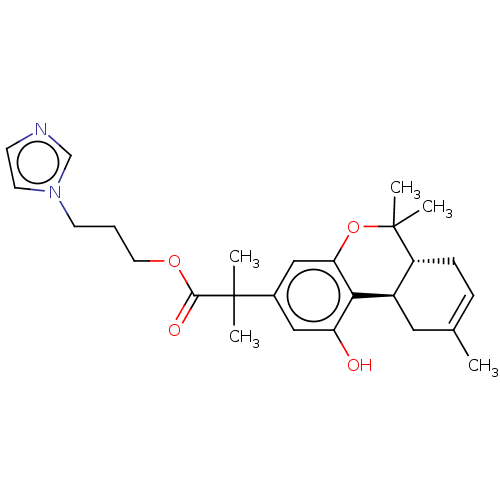 (CHEMBL4098428 | US10882838, Example 1.10) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Displacement of [3H]CP-55,940 from human CB2 receptor expressed in HEK293 cells by scintillation counting method | J Med Chem 58: 665-81 (2015) Article DOI: 10.1021/jm501165d BindingDB Entry DOI: 10.7270/Q2K076HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 886 total ) | Next | Last >> |