 Found 76 hits with Last Name = 'reidy' and Initial = 's'
Found 76 hits with Last Name = 'reidy' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholinesterase
(Homo sapiens (Human)) | BDBM50303940
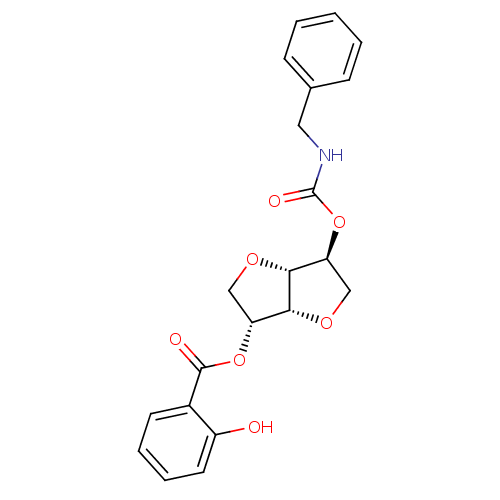
(2-(Benzylaminocarbonyloxy-)5-O-Salicyloyl-1,4:3,6-...)Show SMILES Oc1ccccc1C(=O)O[C@@H]1CO[C@@H]2[C@H](CO[C@H]12)OC(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C21H21NO7/c23-15-9-5-4-8-14(15)20(24)28-16-11-26-19-17(12-27-18(16)19)29-21(25)22-10-13-6-2-1-3-7-13/h1-9,16-19,23H,10-12H2,(H,22,25)/t16-,17+,18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in human liver microsomes |
J Med Chem 53: 1190-9 (2010)
Article DOI: 10.1021/jm9014845
BindingDB Entry DOI: 10.7270/Q26M37S1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50303950
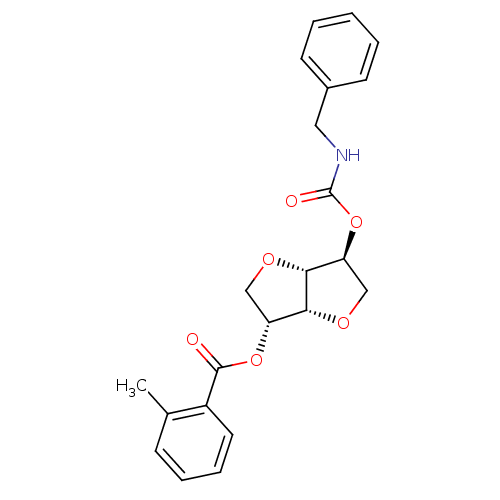
(CHEMBL583687 | Isosorbide-2-benzylcarbamate-5-(o-t...)Show SMILES Cc1ccccc1C(=O)O[C@@H]1CO[C@@H]2[C@H](CO[C@H]12)OC(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C22H23NO6/c1-14-7-5-6-10-16(14)21(24)28-17-12-26-20-18(13-27-19(17)20)29-22(25)23-11-15-8-3-2-4-9-15/h2-10,17-20H,11-13H2,1H3,(H,23,25)/t17-,18+,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in human liver microsomes |
J Med Chem 53: 1190-9 (2010)
Article DOI: 10.1021/jm9014845
BindingDB Entry DOI: 10.7270/Q26M37S1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50253229

(CHEMBL445695 | Isosorbide-2-(benzylcarbamate)-5-mo...)Show SMILES [O-][N+](=O)O[C@@H]1CO[C@@H]2[C@H](CO[C@H]12)OC(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C14H16N2O7/c17-14(15-6-9-4-2-1-3-5-9)22-10-7-20-13-11(23-16(18)19)8-21-12(10)13/h1-5,10-13H,6-8H2,(H,15,17)/t10-,11+,12+,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuchE after 60 mins by carbamylation assay |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50253231
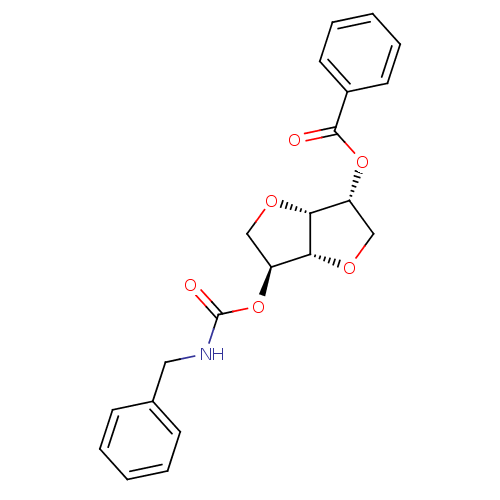
((3R,3aR,6S,6aR)-6-(benzylcarbamoyloxy)hexahydrofur...)Show SMILES O=C(NCc1ccccc1)O[C@H]1CO[C@@H]2[C@@H](CO[C@H]12)OC(=O)c1ccccc1 |r| Show InChI InChI=1S/C21H21NO6/c23-20(15-9-5-2-6-10-15)27-16-12-25-19-17(13-26-18(16)19)28-21(24)22-11-14-7-3-1-4-8-14/h1-10,16-19H,11-13H2,(H,22,24)/t16-,17+,18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in human liver microsomes |
J Med Chem 53: 1190-9 (2010)
Article DOI: 10.1021/jm9014845
BindingDB Entry DOI: 10.7270/Q26M37S1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50253231

((3R,3aR,6S,6aR)-6-(benzylcarbamoyloxy)hexahydrofur...)Show SMILES O=C(NCc1ccccc1)O[C@H]1CO[C@@H]2[C@@H](CO[C@H]12)OC(=O)c1ccccc1 |r| Show InChI InChI=1S/C21H21NO6/c23-20(15-9-5-2-6-10-15)27-16-12-25-19-17(13-26-18(16)19)28-21(24)22-11-14-7-3-1-4-8-14/h1-10,16-19H,11-13H2,(H,22,24)/t16-,17+,18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50252786
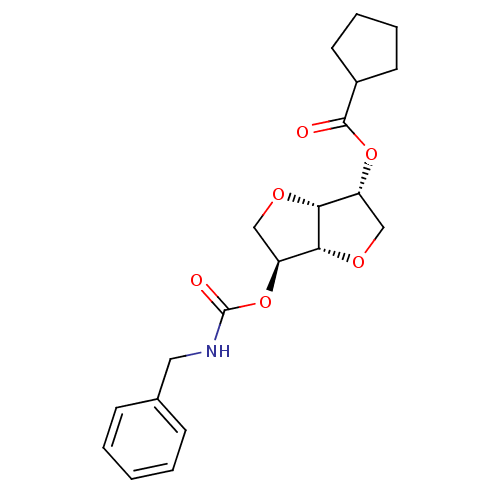
(CHEMBL492635 | Isosorbide-2-benzylcarbamate-5-cycl...)Show SMILES O=C(NCc1ccccc1)O[C@H]1CO[C@@H]2[C@@H](CO[C@H]12)OC(=O)C1CCCC1 |r| Show InChI InChI=1S/C20H25NO6/c22-19(14-8-4-5-9-14)26-15-11-24-18-16(12-25-17(15)18)27-20(23)21-10-13-6-2-1-3-7-13/h1-3,6-7,14-18H,4-5,8-12H2,(H,21,23)/t15-,16+,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50303952
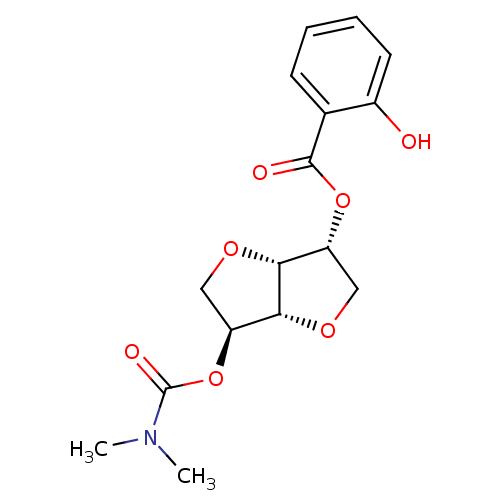
(2-(Dimethylaminocarbonyloxy-)5-O-Salicyloyl-1,4:3,...)Show SMILES CN(C)C(=O)O[C@H]1CO[C@@H]2[C@@H](CO[C@H]12)OC(=O)c1ccccc1O |r| Show InChI InChI=1S/C16H19NO7/c1-17(2)16(20)24-12-8-22-13-11(7-21-14(12)13)23-15(19)9-5-3-4-6-10(9)18/h3-6,11-14,18H,7-8H2,1-2H3/t11-,12+,13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in human liver microsomes |
J Med Chem 53: 1190-9 (2010)
Article DOI: 10.1021/jm9014845
BindingDB Entry DOI: 10.7270/Q26M37S1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50303951
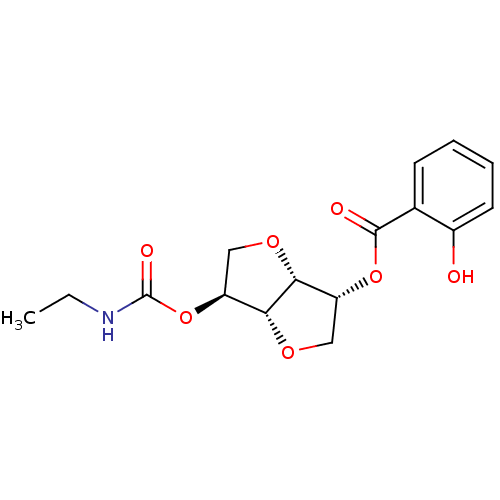
(2-(Ethylaminocarbonyloxy-)5-O-Salicyloyl-1,4:3,6-d...)Show SMILES CCNC(=O)O[C@H]1CO[C@@H]2[C@@H](CO[C@H]12)OC(=O)c1ccccc1O |r| Show InChI InChI=1S/C16H19NO7/c1-2-17-16(20)24-12-8-22-13-11(7-21-14(12)13)23-15(19)9-5-3-4-6-10(9)18/h3-6,11-14,18H,2,7-8H2,1H3,(H,17,20)/t11-,12+,13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in human liver microsomes |
J Med Chem 53: 1190-9 (2010)
Article DOI: 10.1021/jm9014845
BindingDB Entry DOI: 10.7270/Q26M37S1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50253266
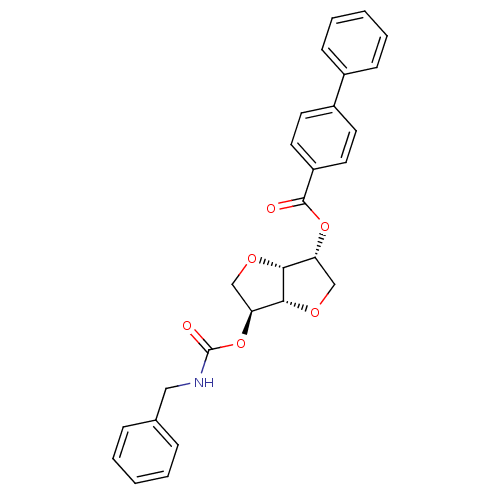
((3R,3aR,6S,6aR)-6-(benzylcarbamoyloxy)hexahydrofur...)Show SMILES O=C(NCc1ccccc1)O[C@H]1CO[C@@H]2[C@@H](CO[C@H]12)OC(=O)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C27H25NO6/c29-26(21-13-11-20(12-14-21)19-9-5-2-6-10-19)33-22-16-31-25-23(17-32-24(22)25)34-27(30)28-15-18-7-3-1-4-8-18/h1-14,22-25H,15-17H2,(H,28,30)/t22-,23+,24-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50253266

((3R,3aR,6S,6aR)-6-(benzylcarbamoyloxy)hexahydrofur...)Show SMILES O=C(NCc1ccccc1)O[C@H]1CO[C@@H]2[C@@H](CO[C@H]12)OC(=O)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C27H25NO6/c29-26(21-13-11-20(12-14-21)19-9-5-2-6-10-19)33-22-16-31-25-23(17-32-24(22)25)34-27(30)28-15-18-7-3-1-4-8-18/h1-14,22-25H,15-17H2,(H,28,30)/t22-,23+,24-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in human liver microsomes |
J Med Chem 53: 1190-9 (2010)
Article DOI: 10.1021/jm9014845
BindingDB Entry DOI: 10.7270/Q26M37S1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50303941
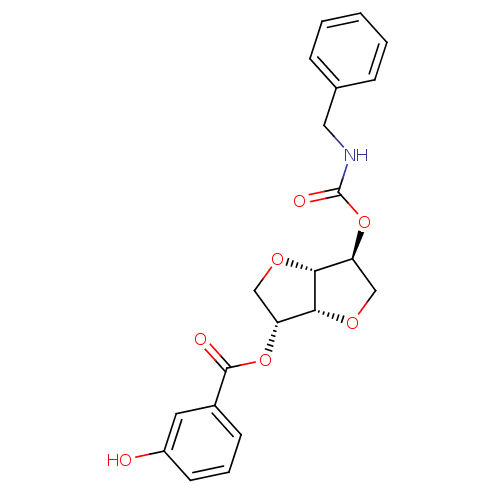
(2-(Benzylaminocarbonyloxy-)5-O-(m-hydroxybenzoyl)-...)Show SMILES Oc1cccc(c1)C(=O)O[C@@H]1CO[C@@H]2[C@H](CO[C@H]12)OC(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C21H21NO7/c23-15-8-4-7-14(9-15)20(24)28-16-11-26-19-17(12-27-18(16)19)29-21(25)22-10-13-5-2-1-3-6-13/h1-9,16-19,23H,10-12H2,(H,22,25)/t16-,17+,18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in human liver microsomes |
J Med Chem 53: 1190-9 (2010)
Article DOI: 10.1021/jm9014845
BindingDB Entry DOI: 10.7270/Q26M37S1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50303943
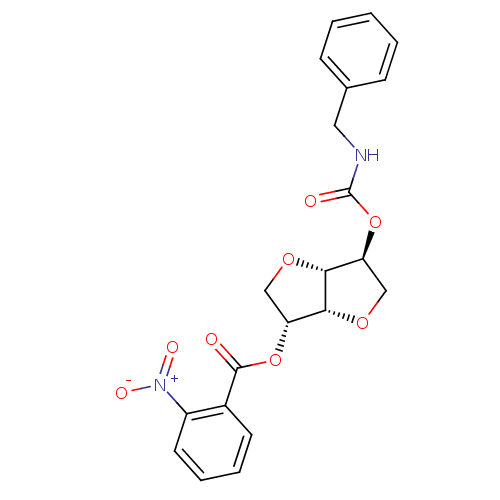
(2-(Benzylaminocarbonyloxy-)5-O-(o-nitro-benzoyl)-1...)Show SMILES [O-][N+](=O)c1ccccc1C(=O)O[C@@H]1CO[C@@H]2[C@H](CO[C@H]12)OC(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C21H20N2O8/c24-20(14-8-4-5-9-15(14)23(26)27)30-16-11-28-19-17(12-29-18(16)19)31-21(25)22-10-13-6-2-1-3-7-13/h1-9,16-19H,10-12H2,(H,22,25)/t16-,17+,18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in human liver microsomes |
J Med Chem 53: 1190-9 (2010)
Article DOI: 10.1021/jm9014845
BindingDB Entry DOI: 10.7270/Q26M37S1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in human liver microsomes |
J Med Chem 53: 1190-9 (2010)
Article DOI: 10.1021/jm9014845
BindingDB Entry DOI: 10.7270/Q26M37S1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50303946

(2-(benzylaminocarbonyloxy-)5-O-(o-amino-benzoyl)-1...)Show SMILES Nc1ccccc1C(=O)O[C@@H]1CO[C@@H]2[C@H](CO[C@H]12)OC(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C21H22N2O6/c22-15-9-5-4-8-14(15)20(24)28-16-11-26-19-17(12-27-18(16)19)29-21(25)23-10-13-6-2-1-3-7-13/h1-9,16-19H,10-12,22H2,(H,23,25)/t16-,17+,18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in human liver microsomes |
J Med Chem 53: 1190-9 (2010)
Article DOI: 10.1021/jm9014845
BindingDB Entry DOI: 10.7270/Q26M37S1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50253264
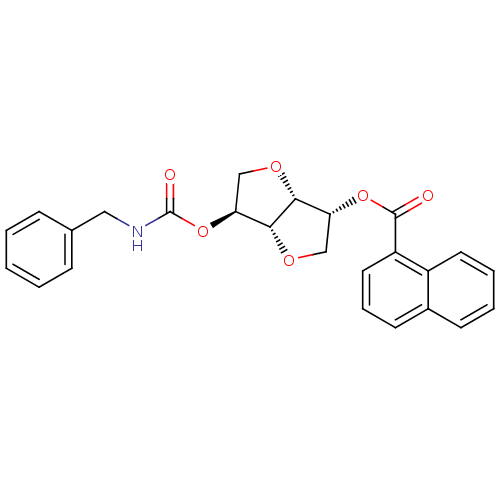
(2-(Benzylaminocarbonyloxy-)-5-O-(1-naphthoyl)-1,4:...)Show SMILES O=C(NCc1ccccc1)O[C@H]1CO[C@@H]2[C@@H](CO[C@H]12)OC(=O)c1cccc2ccccc12 |r| Show InChI InChI=1S/C25H23NO6/c27-24(19-12-6-10-17-9-4-5-11-18(17)19)31-20-14-29-23-21(15-30-22(20)23)32-25(28)26-13-16-7-2-1-3-8-16/h1-12,20-23H,13-15H2,(H,26,28)/t20-,21+,22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50253265

(2-(Benzylaminocarbonyloxy-)-5-O-(2-naphthoyl)-1,4:...)Show SMILES O=C(NCc1ccccc1)O[C@H]1CO[C@@H]2[C@@H](CO[C@H]12)OC(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C25H23NO6/c27-24(19-11-10-17-8-4-5-9-18(17)12-19)31-20-14-29-23-21(15-30-22(20)23)32-25(28)26-13-16-6-2-1-3-7-16/h1-12,20-23H,13-15H2,(H,26,28)/t20-,21+,22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50303944
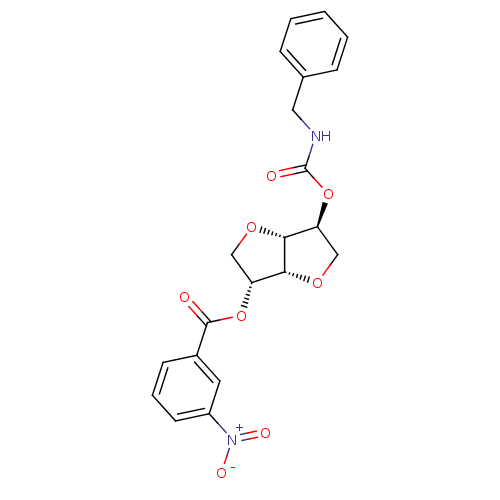
(2-(Benzylaminocarbonyloxy-)5-O-(m-nitro-benzoyl)-1...)Show SMILES [O-][N+](=O)c1cccc(c1)C(=O)O[C@@H]1CO[C@@H]2[C@H](CO[C@H]12)OC(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C21H20N2O8/c24-20(14-7-4-8-15(9-14)23(26)27)30-16-11-28-19-17(12-29-18(16)19)31-21(25)22-10-13-5-2-1-3-6-13/h1-9,16-19H,10-12H2,(H,22,25)/t16-,17+,18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in human liver microsomes |
J Med Chem 53: 1190-9 (2010)
Article DOI: 10.1021/jm9014845
BindingDB Entry DOI: 10.7270/Q26M37S1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10620

((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...)Show InChI InChI=1S/C14H22N2O2/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4/h7-11H,6H2,1-5H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of BuChE |
J Med Chem 53: 1190-9 (2010)
Article DOI: 10.1021/jm9014845
BindingDB Entry DOI: 10.7270/Q26M37S1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50303949
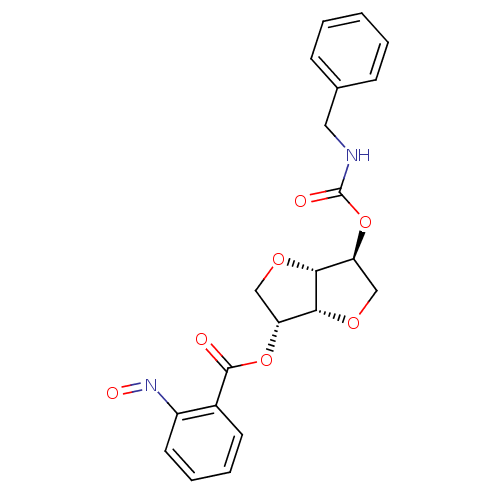
(2-(Benzylaminocarbonyloxy-)5-O-(o-nitroso-benzoyl)...)Show SMILES O=Nc1ccccc1C(=O)O[C@@H]1CO[C@@H]2[C@H](CO[C@H]12)OC(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C21H20N2O7/c24-20(14-8-4-5-9-15(14)23-26)29-16-11-27-19-17(12-28-18(16)19)30-21(25)22-10-13-6-2-1-3-7-13/h1-9,16-19H,10-12H2,(H,22,25)/t16-,17+,18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in human liver microsomes |
J Med Chem 53: 1190-9 (2010)
Article DOI: 10.1021/jm9014845
BindingDB Entry DOI: 10.7270/Q26M37S1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50253229

(CHEMBL445695 | Isosorbide-2-(benzylcarbamate)-5-mo...)Show SMILES [O-][N+](=O)O[C@@H]1CO[C@@H]2[C@H](CO[C@H]12)OC(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C14H16N2O7/c17-14(15-6-9-4-2-1-3-5-9)22-10-7-20-13-11(23-16(18)19)8-21-12(10)13/h1-5,10-13H,6-8H2,(H,15,17)/t10-,11+,12+,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50306692
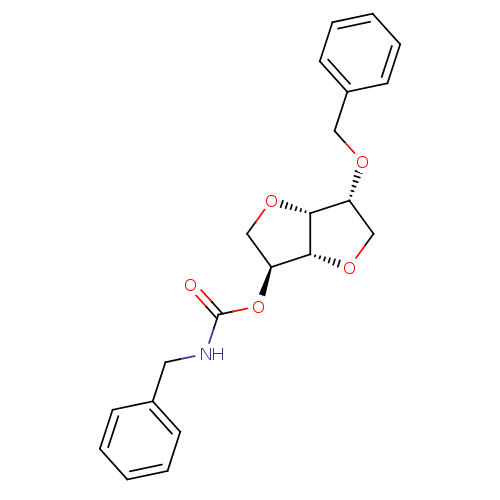
(2-(Benzylaminocarbonyloxy)-5-O-benzyl-1,4:3,6-dian...)Show SMILES O=C(NCc1ccccc1)O[C@H]1CO[C@@H]2[C@@H](CO[C@H]12)OCc1ccccc1 |r| Show InChI InChI=1S/C21H23NO5/c23-21(22-11-15-7-3-1-4-8-15)27-18-14-26-19-17(13-25-20(18)19)24-12-16-9-5-2-6-10-16/h1-10,17-20H,11-14H2,(H,22,23)/t17-,18+,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE preincubated for 30 mins before substrate addition by Ellman's assay |
Bioorg Med Chem 18: 1045-53 (2010)
Article DOI: 10.1016/j.bmc.2009.12.052
BindingDB Entry DOI: 10.7270/Q2CZ3789 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE type 6S |
J Med Chem 53: 1190-9 (2010)
Article DOI: 10.1021/jm9014845
BindingDB Entry DOI: 10.7270/Q26M37S1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50253230
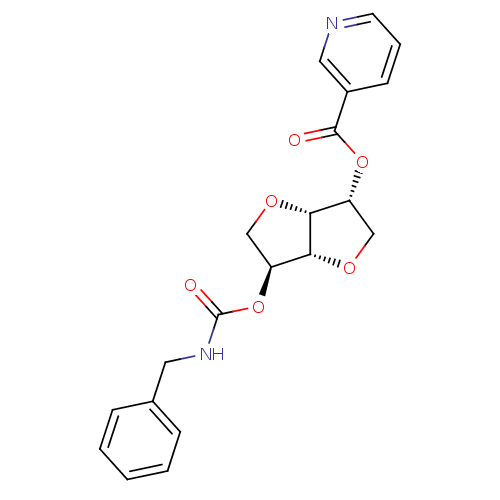
((3R,3aR,6S,6aR)-6-(benzylcarbamoyloxy)hexahydrofur...)Show SMILES O=C(NCc1ccccc1)O[C@H]1CO[C@@H]2[C@@H](CO[C@H]12)OC(=O)c1cccnc1 |r| Show InChI InChI=1S/C20H20N2O6/c23-19(14-7-4-8-21-10-14)27-15-11-25-18-16(12-26-17(15)18)28-20(24)22-9-13-5-2-1-3-6-13/h1-8,10,15-18H,9,11-12H2,(H,22,24)/t15-,16+,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in human liver microsomes |
J Med Chem 53: 1190-9 (2010)
Article DOI: 10.1021/jm9014845
BindingDB Entry DOI: 10.7270/Q26M37S1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50253230

((3R,3aR,6S,6aR)-6-(benzylcarbamoyloxy)hexahydrofur...)Show SMILES O=C(NCc1ccccc1)O[C@H]1CO[C@@H]2[C@@H](CO[C@H]12)OC(=O)c1cccnc1 |r| Show InChI InChI=1S/C20H20N2O6/c23-19(14-7-4-8-21-10-14)27-15-11-25-18-16(12-26-17(15)18)28-20(24)22-9-13-5-2-1-3-6-13/h1-8,10,15-18H,9,11-12H2,(H,22,24)/t15-,16+,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM10624

(1,5-bis(4-allyldimethylammoniumphenyl)-pentan-3-on...)Show SMILES C[N+](C)(CC=C)c1ccc(CCC(=O)CCc2ccc(cc2)[N+](C)(C)CC=C)cc1 Show InChI InChI=1S/C27H38N2O/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3/q+2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50252789
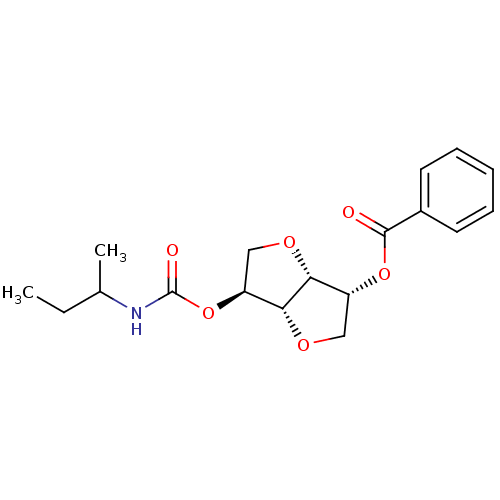
(CHEMBL494684 | Isosorbide-2-(butylcarbamate)-5-ben...)Show SMILES CCC(C)NC(=O)O[C@H]1CO[C@@H]2[C@@H](CO[C@H]12)OC(=O)c1ccccc1 |r| Show InChI InChI=1S/C18H23NO6/c1-3-11(2)19-18(21)25-14-10-23-15-13(9-22-16(14)15)24-17(20)12-7-5-4-6-8-12/h4-8,11,13-16H,3,9-10H2,1-2H3,(H,19,21)/t11?,13-,14+,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50303939
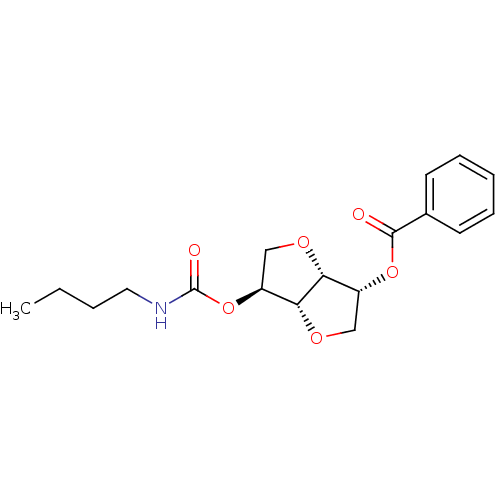
((3R,3aR,6S,6aR)-6-(butylcarbamoyloxy)hexahydrofuro...)Show SMILES CCCCNC(=O)O[C@H]1CO[C@@H]2[C@@H](CO[C@H]12)OC(=O)c1ccccc1 |r| Show InChI InChI=1S/C18H23NO6/c1-2-3-9-19-18(21)25-14-11-23-15-13(10-22-16(14)15)24-17(20)12-7-5-4-6-8-12/h4-8,13-16H,2-3,9-11H2,1H3,(H,19,21)/t13-,14+,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in human liver microsomes |
J Med Chem 53: 1190-9 (2010)
Article DOI: 10.1021/jm9014845
BindingDB Entry DOI: 10.7270/Q26M37S1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50252745
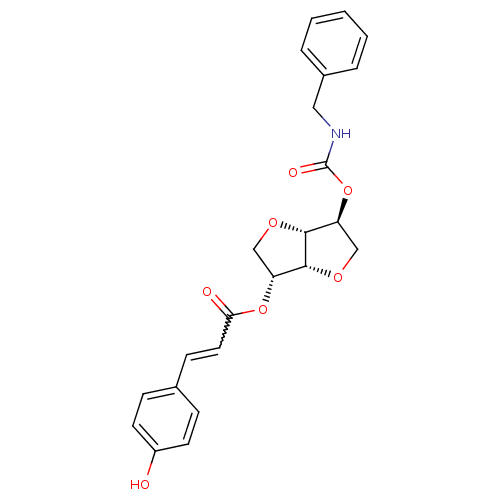
(2-(Benzylaminocarbonyloxy-) 5-(coumarincarbonyloxy...)Show SMILES Oc1ccc(C=CC(=O)O[C@@H]2CO[C@@H]3[C@H](CO[C@H]23)OC(=O)NCc2ccccc2)cc1 |r,w:6.6| Show InChI InChI=1S/C23H23NO7/c25-17-9-6-15(7-10-17)8-11-20(26)30-18-13-28-22-19(14-29-21(18)22)31-23(27)24-12-16-4-2-1-3-5-16/h1-11,18-19,21-22,25H,12-14H2,(H,24,27)/t18-,19+,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50303947
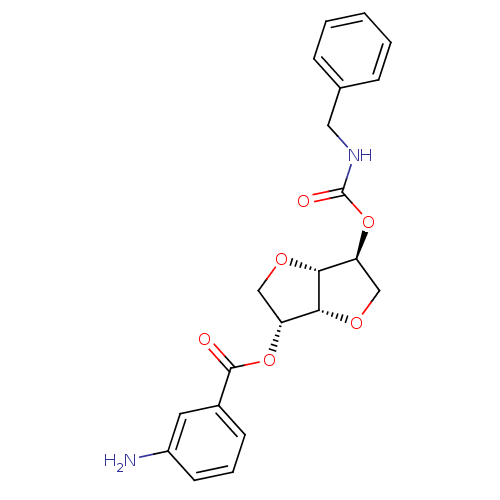
(2-(Benzylaminocarbonyloxy-)5-O-(m-amino-benzoyl)-1...)Show SMILES Nc1cccc(c1)C(=O)O[C@@H]1CO[C@@H]2[C@H](CO[C@H]12)OC(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C21H22N2O6/c22-15-8-4-7-14(9-15)20(24)28-16-11-26-19-17(12-27-18(16)19)29-21(25)23-10-13-5-2-1-3-6-13/h1-9,16-19H,10-12,22H2,(H,23,25)/t16-,17+,18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in human liver microsomes |
J Med Chem 53: 1190-9 (2010)
Article DOI: 10.1021/jm9014845
BindingDB Entry DOI: 10.7270/Q26M37S1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50253263
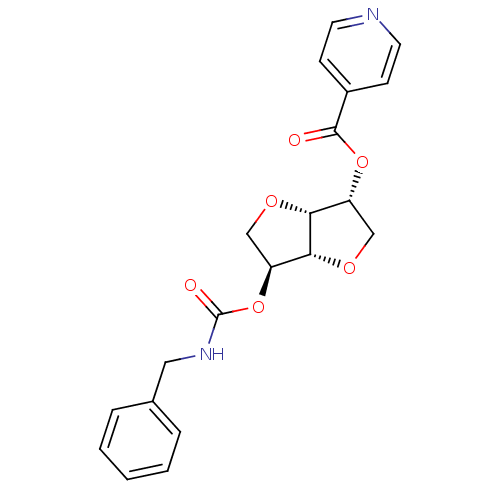
(CHEMBL492481 | Isosorbide-2-benzylcarbamate-5-ison...)Show SMILES O=C(NCc1ccccc1)O[C@H]1CO[C@@H]2[C@@H](CO[C@H]12)OC(=O)c1ccncc1 |r| Show InChI InChI=1S/C20H20N2O6/c23-19(14-6-8-21-9-7-14)27-15-11-25-18-16(12-26-17(15)18)28-20(24)22-10-13-4-2-1-3-5-13/h1-9,15-18H,10-12H2,(H,22,24)/t15-,16+,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50303945
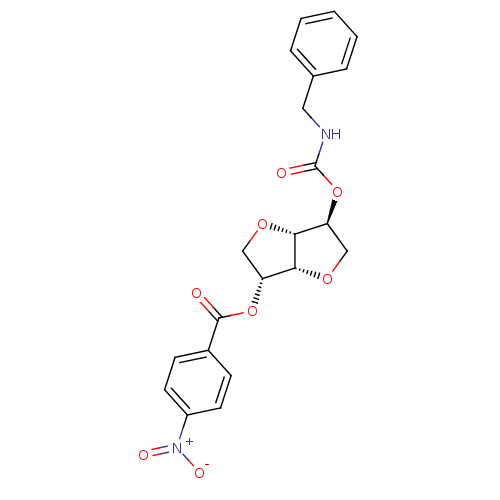
(2-(Benzylaminocarbonyloxy-)5-O-(p-nitro-benzoyl)-1...)Show SMILES [O-][N+](=O)c1ccc(cc1)C(=O)O[C@@H]1CO[C@@H]2[C@H](CO[C@H]12)OC(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C21H20N2O8/c24-20(14-6-8-15(9-7-14)23(26)27)30-16-11-28-19-17(12-29-18(16)19)31-21(25)22-10-13-4-2-1-3-5-13/h1-9,16-19H,10-12H2,(H,22,25)/t16-,17+,18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in human liver microsomes |
J Med Chem 53: 1190-9 (2010)
Article DOI: 10.1021/jm9014845
BindingDB Entry DOI: 10.7270/Q26M37S1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50252743
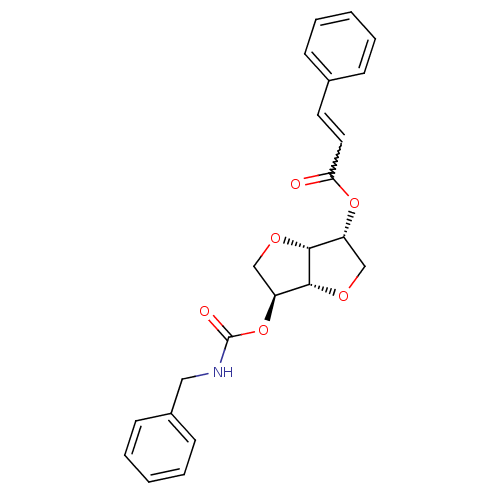
(2-(Benzylaminocarbonyloxy-) 5-O-cinnamoyl-1,4:3,6-...)Show SMILES O=C(NCc1ccccc1)O[C@H]1CO[C@@H]2[C@@H](CO[C@H]12)OC(=O)C=Cc1ccccc1 |r,w:22.24| Show InChI InChI=1S/C23H23NO6/c25-20(12-11-16-7-3-1-4-8-16)29-18-14-27-22-19(15-28-21(18)22)30-23(26)24-13-17-9-5-2-6-10-17/h1-12,18-19,21-22H,13-15H2,(H,24,26)/t18-,19+,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50306693
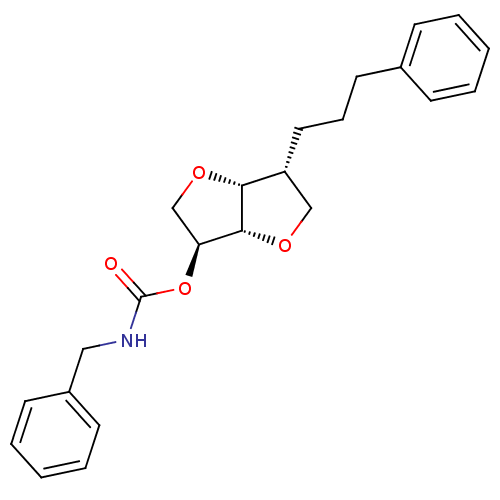
(2-(Benzylaminocarbonyloxy)-5-O-(phenylpropyloxy)-1...)Show SMILES O=C(NCc1ccccc1)O[C@H]1CO[C@@H]2[C@H](CCCc3ccccc3)CO[C@H]12 |r| Show InChI InChI=1S/C23H27NO4/c25-23(24-14-18-10-5-2-6-11-18)28-20-16-27-21-19(15-26-22(20)21)13-7-12-17-8-3-1-4-9-17/h1-6,8-11,19-22H,7,12-16H2,(H,24,25)/t19-,20+,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 201 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE preincubated for 30 mins before substrate addition by Ellman's assay |
Bioorg Med Chem 18: 1045-53 (2010)
Article DOI: 10.1016/j.bmc.2009.12.052
BindingDB Entry DOI: 10.7270/Q2CZ3789 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50303948
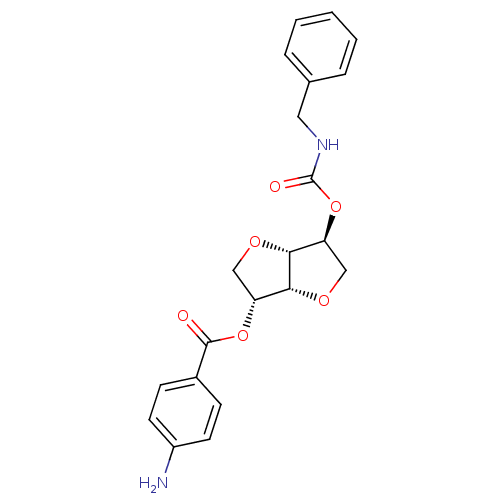
(2-(Benzylaminocarbonyloxy-)5-O-(p-amino-benzoyl)-1...)Show SMILES Nc1ccc(cc1)C(=O)O[C@@H]1CO[C@@H]2[C@H](CO[C@H]12)OC(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C21H22N2O6/c22-15-8-6-14(7-9-15)20(24)28-16-11-26-19-17(12-27-18(16)19)29-21(25)23-10-13-4-2-1-3-5-13/h1-9,16-19H,10-12,22H2,(H,23,25)/t16-,17+,18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 297 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in human liver microsomes |
J Med Chem 53: 1190-9 (2010)
Article DOI: 10.1021/jm9014845
BindingDB Entry DOI: 10.7270/Q26M37S1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50303942
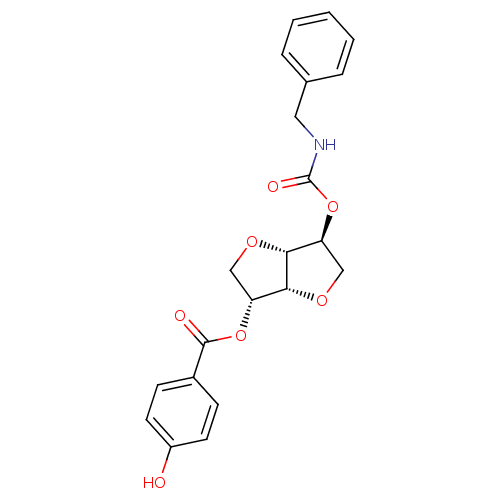
(2-(Benzylaminocarbonyloxy-)5-O-(p-hydroxy-benzoyl)...)Show SMILES Oc1ccc(cc1)C(=O)O[C@@H]1CO[C@@H]2[C@H](CO[C@H]12)OC(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C21H21NO7/c23-15-8-6-14(7-9-15)20(24)28-16-11-26-19-17(12-27-18(16)19)29-21(25)22-10-13-4-2-1-3-5-13/h1-9,16-19,23H,10-12H2,(H,22,25)/t16-,17+,18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 323 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in human liver microsomes |
J Med Chem 53: 1190-9 (2010)
Article DOI: 10.1021/jm9014845
BindingDB Entry DOI: 10.7270/Q26M37S1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50252785
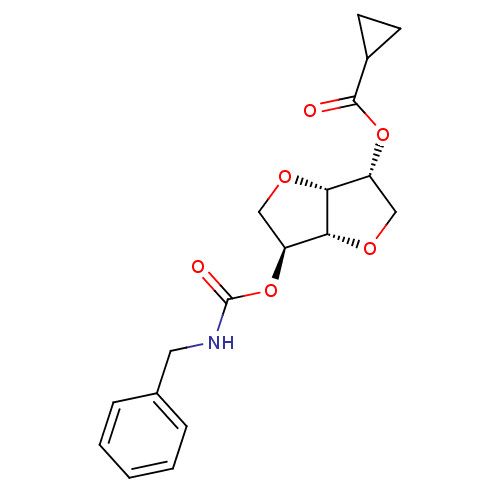
(CHEMBL492432 | Isosorbide-2-benzylcarbamate-5-cycl...)Show SMILES O=C(NCc1ccccc1)O[C@H]1CO[C@@H]2[C@@H](CO[C@H]12)OC(=O)C1CC1 |r| Show InChI InChI=1S/C18H21NO6/c20-17(12-6-7-12)24-13-9-22-16-14(10-23-15(13)16)25-18(21)19-8-11-4-2-1-3-5-11/h1-5,12-16H,6-10H2,(H,19,21)/t13-,14+,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 334 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
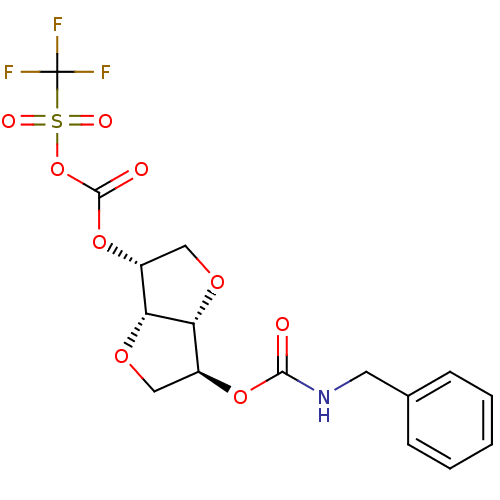
Cholinesterase
(Homo sapiens (Human)) | BDBM50252787
(CHEMBL492636 | Isosorbide-2-benzylcarbamate-5-trif...)Show SMILES FC(F)(F)S(=O)(=O)OC(=O)O[C@@H]1CO[C@@H]2[C@H](CO[C@H]12)OC(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C16H16F3NO9S/c17-16(18,19)30(23,24)29-15(22)28-11-8-26-12-10(7-25-13(11)12)27-14(21)20-6-9-4-2-1-3-5-9/h1-5,10-13H,6-8H2,(H,20,21)/t10-,11+,12+,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 359 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
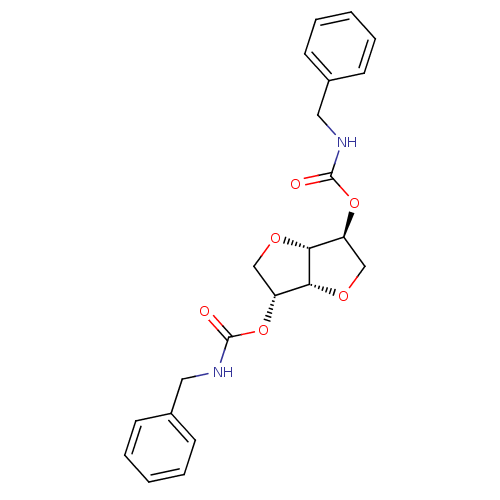
Cholinesterase
(Homo sapiens (Human)) | BDBM50306689
(CHEMBL600013 | Isosorbide-di-(benzylcarbamate))Show SMILES O=C(NCc1ccccc1)O[C@@H]1CO[C@@H]2[C@H](CO[C@H]12)OC(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C22H24N2O6/c25-21(23-11-15-7-3-1-4-8-15)29-17-13-27-20-18(14-28-19(17)20)30-22(26)24-12-16-9-5-2-6-10-16/h1-10,17-20H,11-14H2,(H,23,25)(H,24,26)/t17-,18+,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE preincubated for 30 mins before substrate addition by Ellman's assay |
Bioorg Med Chem 18: 1045-53 (2010)
Article DOI: 10.1016/j.bmc.2009.12.052
BindingDB Entry DOI: 10.7270/Q2CZ3789 |
More data for this
Ligand-Target Pair | |
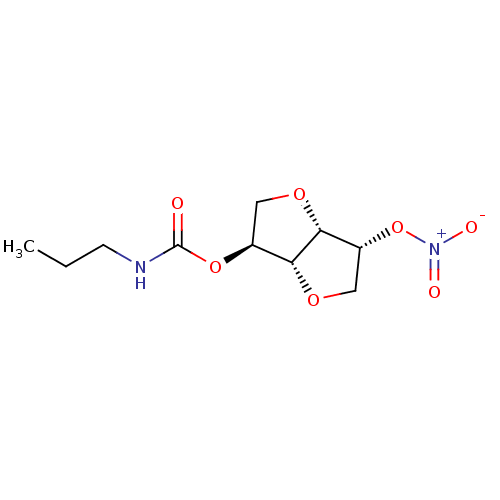
Cholinesterase
(Homo sapiens (Human)) | BDBM50253226
(CHEMBL494519 | Isosorbide-2-(propylcarbamate)-5-mo...)Show SMILES CCCNC(=O)O[C@H]1CO[C@@H]2[C@@H](CO[C@H]12)O[N+]([O-])=O |r| Show InChI InChI=1S/C10H16N2O7/c1-2-3-11-10(13)18-6-4-16-9-7(19-12(14)15)5-17-8(6)9/h6-9H,2-5H2,1H3,(H,11,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 639 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50252788
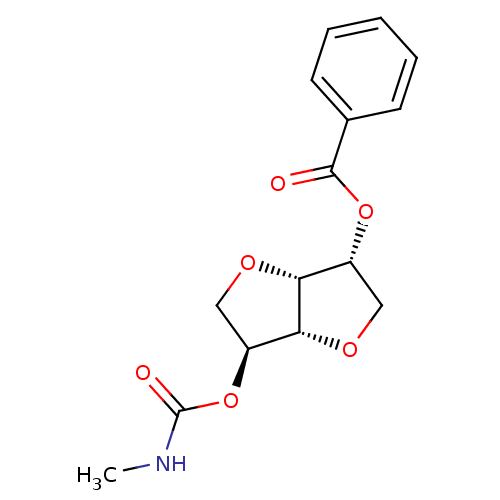
(CHEMBL523500 | Isosorbide-2-(methylcarbamate)-5-be...)Show SMILES CNC(=O)O[C@H]1CO[C@@H]2[C@@H](CO[C@H]12)OC(=O)c1ccccc1 |r| Show InChI InChI=1S/C15H17NO6/c1-16-15(18)22-11-8-20-12-10(7-19-13(11)12)21-14(17)9-5-3-2-4-6-9/h2-6,10-13H,7-8H2,1H3,(H,16,18)/t10-,11+,12-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 669 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50252784
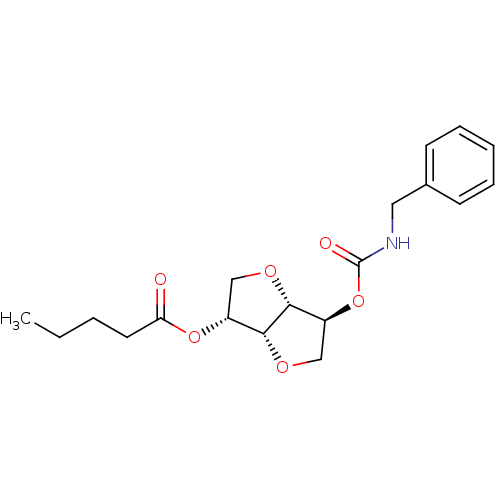
(CHEMBL521666 | Isosorbide-2-benzylcarbamate-5-pent...)Show SMILES CCCCC(=O)O[C@@H]1CO[C@@H]2[C@H](CO[C@H]12)OC(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C19H25NO6/c1-2-3-9-16(21)25-14-11-23-18-15(12-24-17(14)18)26-19(22)20-10-13-7-5-4-6-8-13/h4-8,14-15,17-18H,2-3,9-12H2,1H3,(H,20,22)/t14-,15+,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM10625
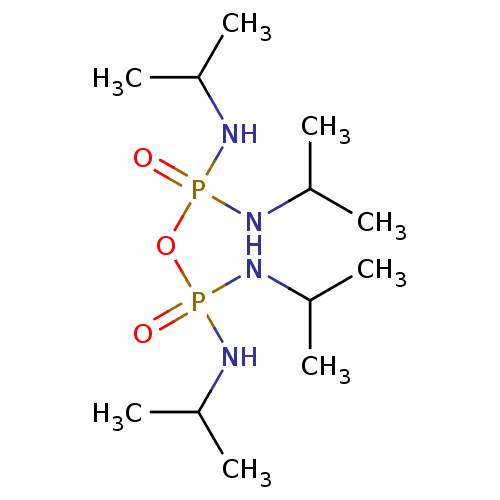
(({[bis(propan-2-ylamino)phosphoryl]oxy}(propan-2-y...)Show InChI InChI=1S/C12H32N4O3P2/c1-9(2)13-20(17,14-10(3)4)19-21(18,15-11(5)6)16-12(7)8/h9-12H,1-8H3,(H2,13,14,17)(H2,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 733 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50253227
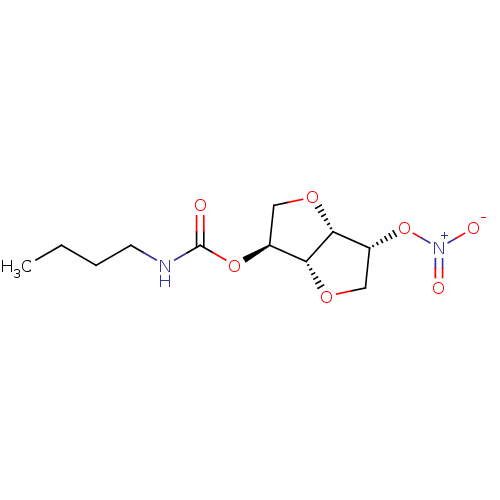
(CHEMBL494520 | Isosorbide-2-(butylcarbamate)-5-mon...)Show SMILES CCCCNC(=O)O[C@H]1CO[C@@H]2[C@@H](CO[C@H]12)O[N+]([O-])=O |r| Show InChI InChI=1S/C11H18N2O7/c1-2-3-4-12-11(14)19-7-5-17-10-8(20-13(15)16)6-18-9(7)10/h7-10H,2-6H2,1H3,(H,12,14)/t7-,8+,9+,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 894 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50252783

(CHEMBL493672 | Isosorbide-2-benzylcarbamate-5-prop...)Show SMILES CCC(=O)O[C@@H]1CO[C@@H]2[C@H](CO[C@H]12)OC(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C17H21NO6/c1-2-14(19)23-12-9-21-16-13(10-22-15(12)16)24-17(20)18-8-11-6-4-3-5-7-11/h3-7,12-13,15-16H,2,8-10H2,1H3,(H,18,20)/t12-,13+,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50303954
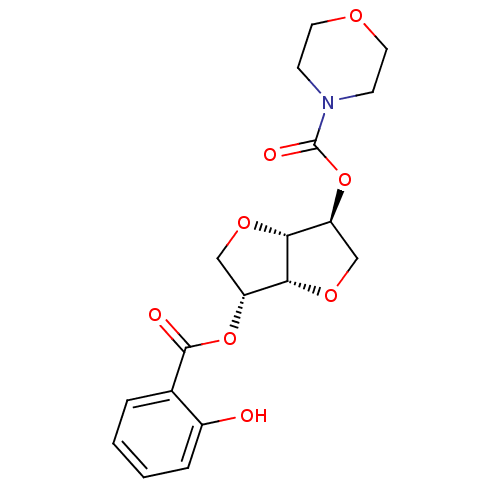
(2-(Morpholinocarbonyloxy-)5-O-salicyloyl-1,4:3,6-d...)Show SMILES Oc1ccccc1C(=O)O[C@@H]1CO[C@@H]2[C@H](CO[C@H]12)OC(=O)N1CCOCC1 |r| Show InChI InChI=1S/C18H21NO8/c20-12-4-2-1-3-11(12)17(21)26-13-9-24-16-14(10-25-15(13)16)27-18(22)19-5-7-23-8-6-19/h1-4,13-16,20H,5-10H2/t13-,14+,15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of BuChE in human liver microsomes |
J Med Chem 53: 1190-9 (2010)
Article DOI: 10.1021/jm9014845
BindingDB Entry DOI: 10.7270/Q26M37S1 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50306688
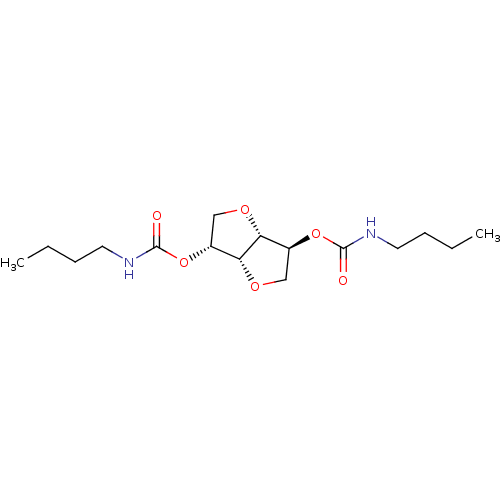
(CHEMBL606050 | Isosorbide-di-(butylcarbamate))Show SMILES CCCCNC(=O)O[C@@H]1CO[C@@H]2[C@H](CO[C@H]12)OC(=O)NCCCC |r| Show InChI InChI=1S/C16H28N2O6/c1-3-5-7-17-15(19)23-11-9-21-14-12(10-22-13(11)14)24-16(20)18-8-6-4-2/h11-14H,3-10H2,1-2H3,(H,17,19)(H,18,20)/t11-,12+,13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuChE preincubated for 30 mins before substrate addition by Ellman's assay |
Bioorg Med Chem 18: 1045-53 (2010)
Article DOI: 10.1016/j.bmc.2009.12.052
BindingDB Entry DOI: 10.7270/Q2CZ3789 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50253228
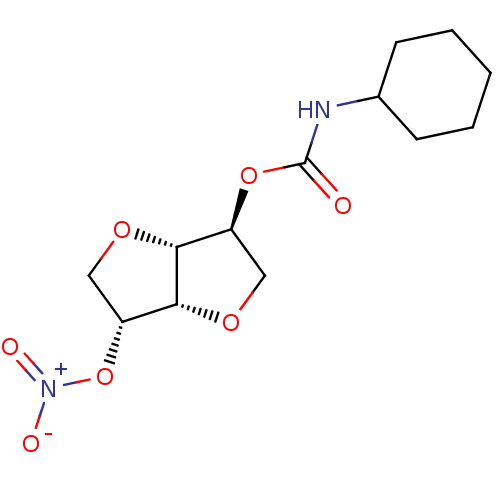
(CHEMBL521852 | Isosorbide-2-(cyclohexylcarbamate)-...)Show SMILES [O-][N+](=O)O[C@@H]1CO[C@@H]2[C@H](CO[C@H]12)OC(=O)NC1CCCCC1 |r| Show InChI InChI=1S/C13H20N2O7/c16-13(14-8-4-2-1-3-5-8)21-9-6-19-12-10(22-15(17)18)7-20-11(9)12/h8-12H,1-7H2,(H,14,16)/t9-,10+,11+,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of human plasma BuchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM10625

(({[bis(propan-2-ylamino)phosphoryl]oxy}(propan-2-y...)Show InChI InChI=1S/C12H32N4O3P2/c1-9(2)13-20(17,14-10(3)4)19-21(18,15-11(5)6)16-12(7)8/h9-12H,1-8H3,(H2,13,14,17)(H2,15,16,18) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AchE by Ellman's method |
J Med Chem 51: 6400-9 (2008)
Article DOI: 10.1021/jm800564y
BindingDB Entry DOI: 10.7270/Q2RV0NJ3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data