

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50145198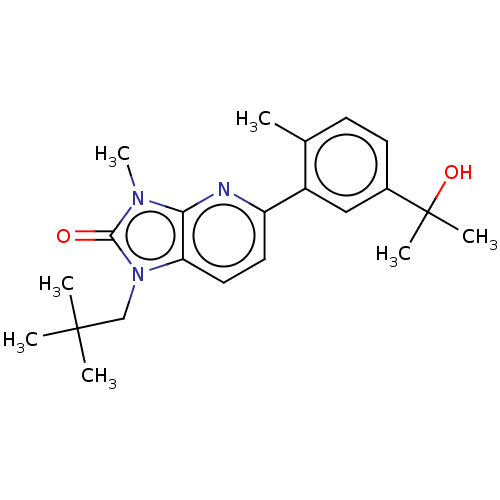 (CHEMBL3765778) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Positive allosteric modulation of 5-HT2B receptor (unknown origin) | Bioorg Med Chem Lett 26: 1260-4 (2016) Article DOI: 10.1016/j.bmcl.2016.01.021 BindingDB Entry DOI: 10.7270/Q2NV9M4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50145198 (CHEMBL3765778) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Positive allosteric modulation of platelet-activating factor receptor (unknown origin) | Bioorg Med Chem Lett 26: 1260-4 (2016) Article DOI: 10.1016/j.bmcl.2016.01.021 BindingDB Entry DOI: 10.7270/Q2NV9M4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132G] (Homo sapiens (Human)) | BDBM195601 (GSK321 | US11311527, Cpd ID GSK321 | US11376246, C...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | 25 |
Albert Einstein College of Medicine | Assay Description RapidFire-MS/MS measurements of mutant IDH1 and IDH2 reactions were conducted at room temperature in 384-well Greiner polypropylene microtiter plates... | Nat Chem Biol 11: 878-86 (2015) Article DOI: 10.1038/nchembio.1930 BindingDB Entry DOI: 10.7270/Q2RR1X2G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM128366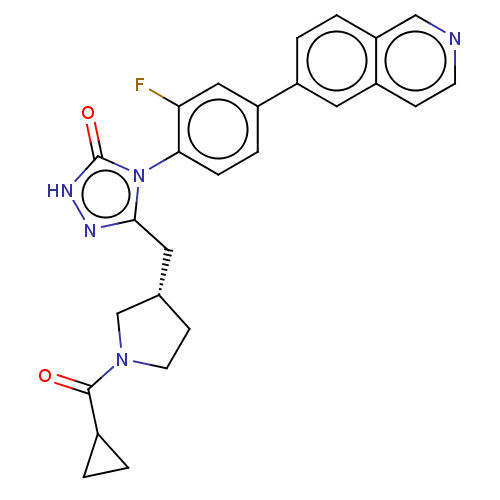 (US8802864, 132) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.0 | n/a |
GlaxoSmithKline LLC US Patent | Assay Description Assay #1:Inhibition of FAS activity can be measured based on the detection of residual NADPH substrate after the FAS assay is quenched. This assay is... | US Patent US8802864 (2014) BindingDB Entry DOI: 10.7270/Q2N58K26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132C] (Homo sapiens (Human)) | BDBM195601 (GSK321 | US11311527, Cpd ID GSK321 | US11376246, C...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | 25 |
Albert Einstein College of Medicine | Assay Description RapidFire-MS/MS measurements of mutant IDH1 and IDH2 reactions were conducted at room temperature in 384-well Greiner polypropylene microtiter plates... | Nat Chem Biol 11: 878-86 (2015) Article DOI: 10.1038/nchembio.1930 BindingDB Entry DOI: 10.7270/Q2RR1X2G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic [R132H] (Homo sapiens (Human)) | BDBM195601 (GSK321 | US11311527, Cpd ID GSK321 | US11376246, C...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | 25 |
Albert Einstein College of Medicine | Assay Description RapidFire-MS/MS measurements of mutant IDH1 and IDH2 reactions were conducted at room temperature in 384-well Greiner polypropylene microtiter plates... | Nat Chem Biol 11: 878-86 (2015) Article DOI: 10.1038/nchembio.1930 BindingDB Entry DOI: 10.7270/Q2RR1X2G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM128362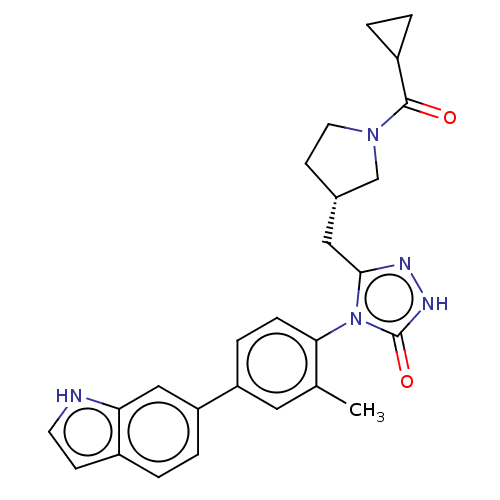 (US8802864, 42) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
GlaxoSmithKline LLC US Patent | Assay Description Assay #1:Inhibition of FAS activity can be measured based on the detection of residual NADPH substrate after the FAS assay is quenched. This assay is... | US Patent US8802864 (2014) BindingDB Entry DOI: 10.7270/Q2N58K26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM128359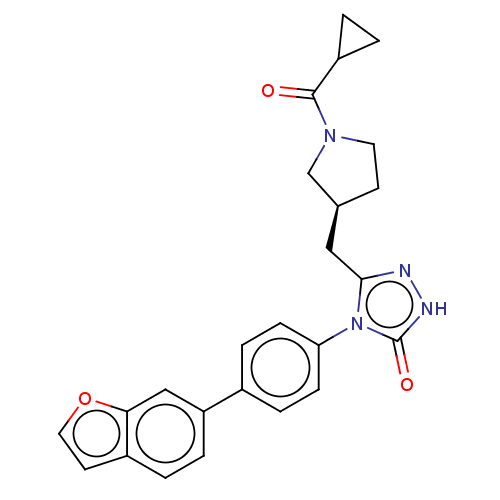 (US8802864, 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.0 | n/a |
GlaxoSmithKline LLC US Patent | Assay Description Assay #1:Inhibition of FAS activity can be measured based on the detection of residual NADPH substrate after the FAS assay is quenched. This assay is... | US Patent US8802864 (2014) BindingDB Entry DOI: 10.7270/Q2N58K26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM119133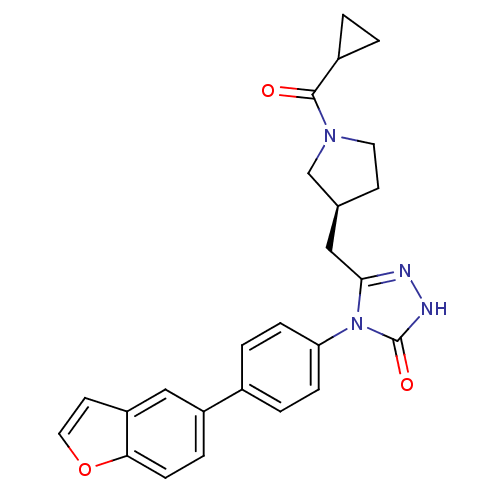 ((S)-4-(4-(Benzofuran-5-yl)phenyl)-3-((1-(cycloprop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.0 | n/a |
GlaxoSmithKline LLC US Patent | Assay Description Assay #1:Inhibition of FAS activity can be measured based on the detection of residual NADPH substrate after the FAS assay is quenched. This assay is... | US Patent US8802864 (2014) BindingDB Entry DOI: 10.7270/Q2N58K26 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM128360 (US8802864, 23) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.0 | n/a |
GlaxoSmithKline LLC US Patent | Assay Description Assay #1:Inhibition of FAS activity can be measured based on the detection of residual NADPH substrate after the FAS assay is quenched. This assay is... | US Patent US8802864 (2014) BindingDB Entry DOI: 10.7270/Q2N58K26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM128363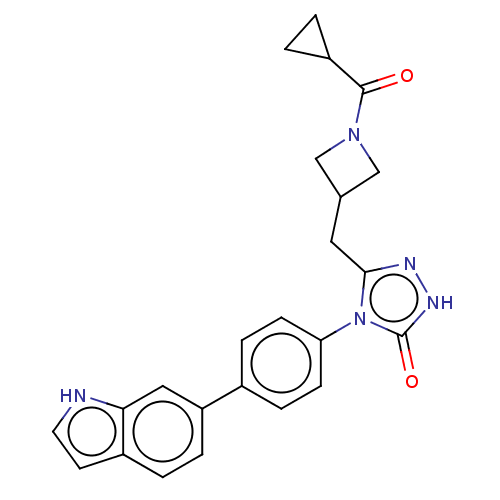 (US8802864, 67) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.0 | n/a |
GlaxoSmithKline LLC US Patent | Assay Description Assay #1:Inhibition of FAS activity can be measured based on the detection of residual NADPH substrate after the FAS assay is quenched. This assay is... | US Patent US8802864 (2014) BindingDB Entry DOI: 10.7270/Q2N58K26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM128358 (US8802864, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.0 | n/a |
GlaxoSmithKline LLC US Patent | Assay Description Assay #1:Inhibition of FAS activity can be measured based on the detection of residual NADPH substrate after the FAS assay is quenched. This assay is... | US Patent US8802864 (2014) BindingDB Entry DOI: 10.7270/Q2N58K26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM128365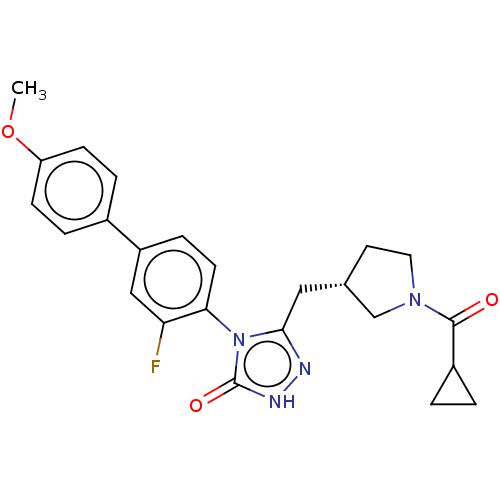 (US8802864, 116) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.0 | n/a |
GlaxoSmithKline LLC US Patent | Assay Description Assay #1:Inhibition of FAS activity can be measured based on the detection of residual NADPH substrate after the FAS assay is quenched. This assay is... | US Patent US8802864 (2014) BindingDB Entry DOI: 10.7270/Q2N58K26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isocitrate dehydrogenase [NADP] cytoplasmic (Homo sapiens (Human)) | BDBM195601 (GSK321 | US11311527, Cpd ID GSK321 | US11376246, C...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | 25 |
Albert Einstein College of Medicine | Assay Description For WT IDH1 and IDH2, reactions were conducted at room temperature in 384-well Greiner black microtiter plates in a total volume of 10 μL of ass... | Nat Chem Biol 11: 878-86 (2015) Article DOI: 10.1038/nchembio.1930 BindingDB Entry DOI: 10.7270/Q2RR1X2G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM128357 (US8802864, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 126 | n/a | n/a | n/a | n/a | 7.0 | n/a |
GlaxoSmithKline LLC US Patent | Assay Description Assay #1:Inhibition of FAS activity can be measured based on the detection of residual NADPH substrate after the FAS assay is quenched. This assay is... | US Patent US8802864 (2014) BindingDB Entry DOI: 10.7270/Q2N58K26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM128361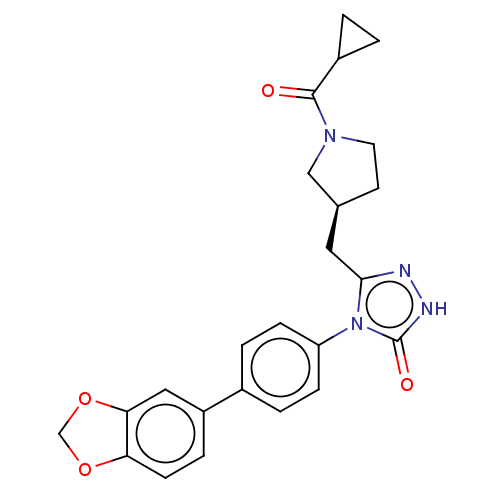 (US8802864, 38) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 158 | n/a | n/a | n/a | n/a | 7.0 | n/a |
GlaxoSmithKline LLC US Patent | Assay Description Assay #1:Inhibition of FAS activity can be measured based on the detection of residual NADPH substrate after the FAS assay is quenched. This assay is... | US Patent US8802864 (2014) BindingDB Entry DOI: 10.7270/Q2N58K26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM128364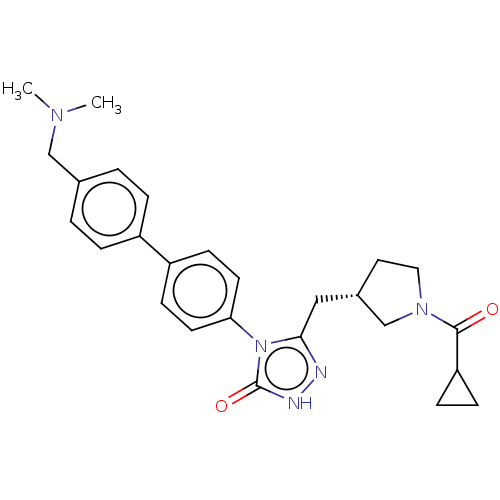 (US8802864, 95) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.0 | n/a |
GlaxoSmithKline LLC US Patent | Assay Description Assay #1:Inhibition of FAS activity can be measured based on the detection of residual NADPH substrate after the FAS assay is quenched. This assay is... | US Patent US8802864 (2014) BindingDB Entry DOI: 10.7270/Q2N58K26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491060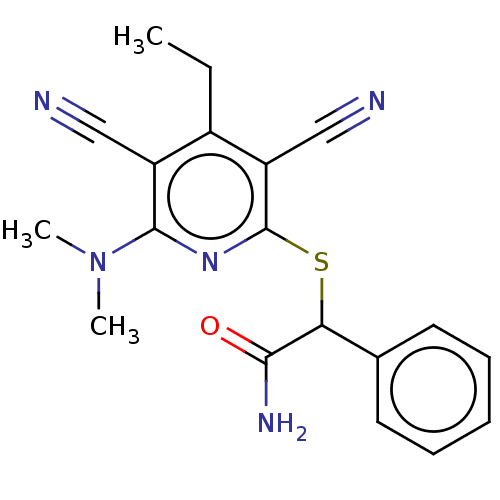 (BDBM491430 | US10975056, Example 3 | US10975056, E...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491061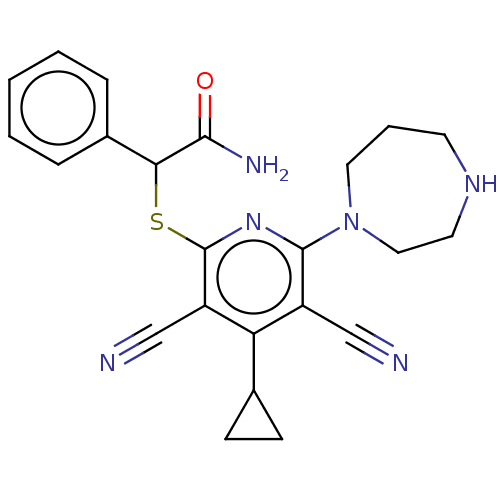 (US10975056, Example 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491062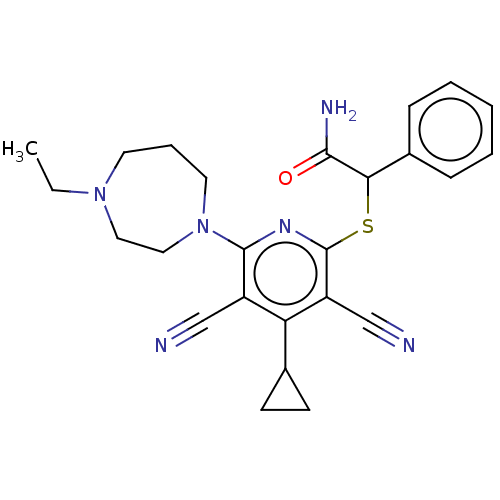 (2-{[3,5-dicyano-4-cyclopropyl-6-(4-ethyl-1,4-diaze...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491063 (US10975056, Example 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491064 (2-{[3,5-dicyano-4-ethyl-6-(4-ethyl-1,4-diazepan-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491071 (US10975056, Example 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491073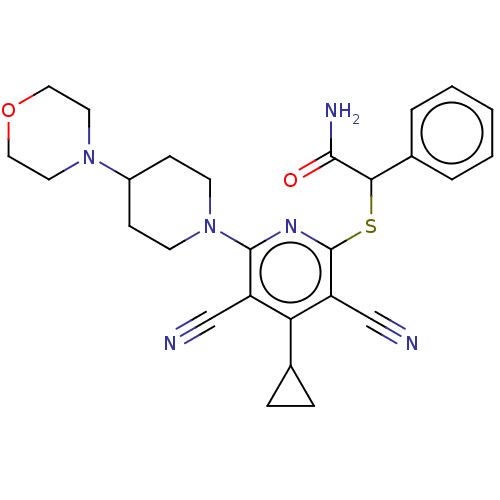 (US10975056, Example 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491074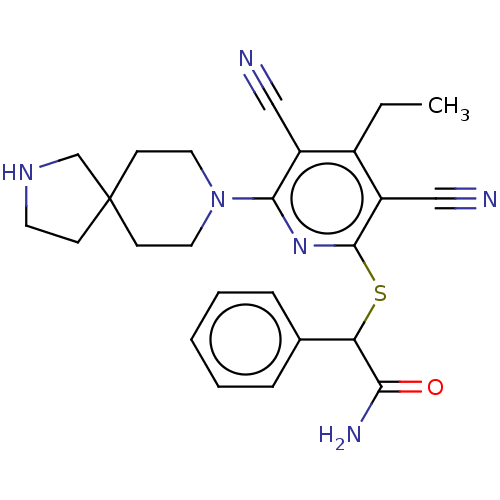 (2-((3,5-dicyano-4-ethyl-6-(2,8-diazaspiro[4.5]deca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491080 (US10975056, Example 23) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491081 (US10975056, Example 24) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491082 (US10975056, Example 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491075 (US10975056, Example 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491077 (US10975056, Example 20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491084 (US10975056, Example 27) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491204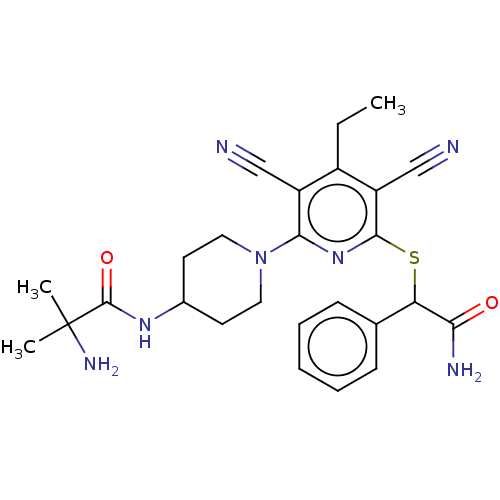 (US10975056, Example 148) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491205 (US10975056, Example 149) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491209 (US10975056, Example 153) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491212 (US10975056, Example 156) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491223 (US10975056, Example 167) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491224 (US10975056, Example 168 | US11771711, Reference co...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491225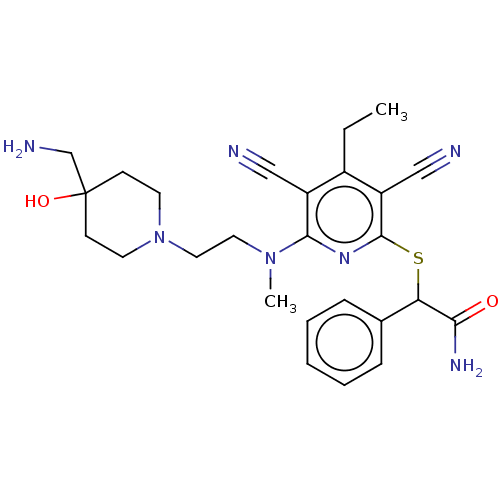 (US10975056, Example 170) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491233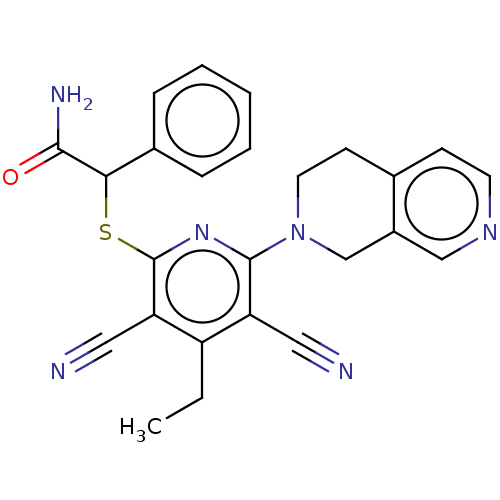 (2-((3,5-Dicyano-6-(3,4-dihydro-2,7-naphthyridin-2 ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491239 (US10975056, Example 186) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491244 (2-((3,5-Dicyano-6-(dimethylamino)-4-methoxypyridin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491245 (US10975056, Example 192) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491246 (US10975056, Example 193) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491249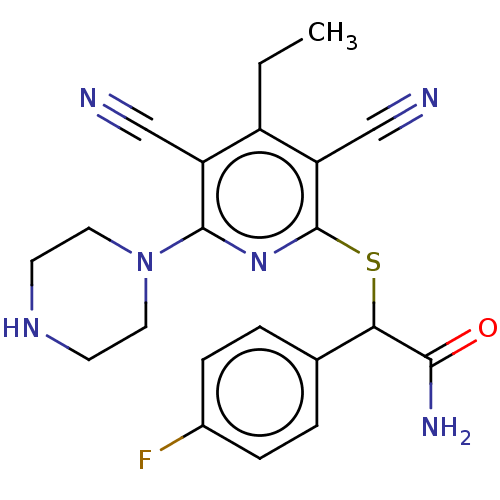 (US10975056, Example 196) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491251 (US10975056, Example 198) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491252 (US10975056, Example 199) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491534 (US10975056, Example 517) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491535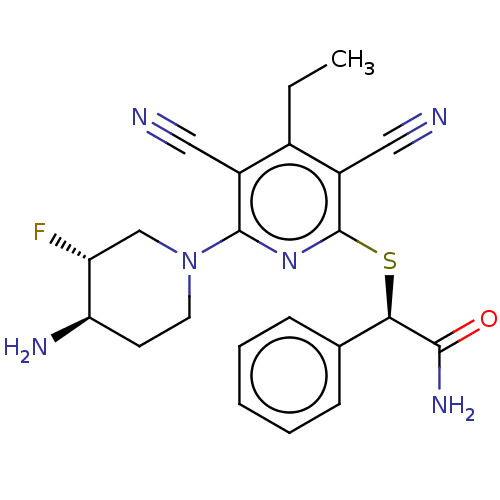 (US10975056, Example 518) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491536 (US10975056, Example 519) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM491537 (US10975056, Example 520) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Intellectual Property Development Limited; Cancer Research Technology Ltd. US Patent | Assay Description Biochemical activity of DNMT1 analyzed using SPA technology. Plates (Griener 784075) were pre-stamped with 100 nL/well of compound (11-point, 3-fold ... | US Patent US10975056 (2021) BindingDB Entry DOI: 10.7270/Q2VM4GCT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 754 total ) | Next | Last >> |