 Found 8420 hits with Last Name = 'ren' and Initial = 's'
Found 8420 hits with Last Name = 'ren' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50273292
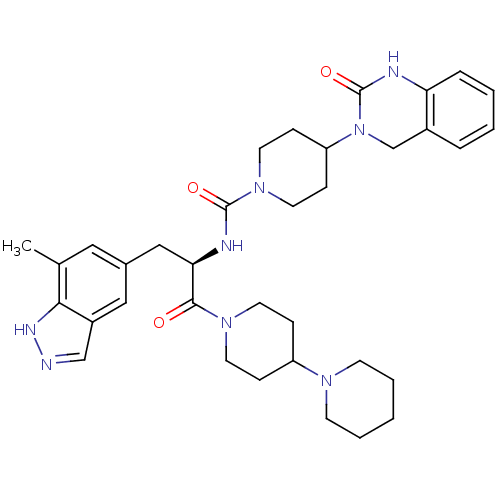
((R)-N-(1-(1,4'-bipiperidin-1'-yl)-3-(7-methyl-1H-i...)Show SMILES Cc1cc(C[C@@H](NC(=O)N2CCC(CC2)N2Cc3ccccc3NC2=O)C(=O)N2CCC(CC2)N2CCCCC2)cc2cn[nH]c12 |r| Show InChI InChI=1S/C35H46N8O3/c1-24-19-25(20-27-22-36-39-32(24)27)21-31(33(44)41-15-9-28(10-16-41)40-13-5-2-6-14-40)38-34(45)42-17-11-29(12-18-42)43-23-26-7-3-4-8-30(26)37-35(43)46/h3-4,7-8,19-20,22,28-29,31H,2,5-6,9-18,21,23H2,1H3,(H,36,39)(H,37,46)(H,38,45)/t31-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [I125]CGRP from human CGRP receptor in SK-N-MC cells |
J Med Chem 51: 4858-61 (2008)
Article DOI: 10.1021/jm800546t
BindingDB Entry DOI: 10.7270/Q2N016BV |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor
(Homo sapiens (Human)) | BDBM50268484
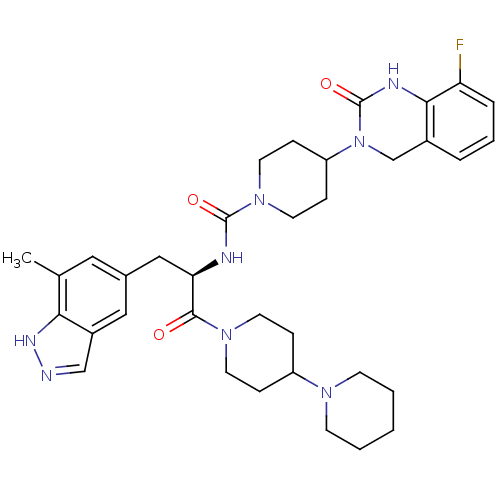
((R)-4-(8-Fluoro-2-oxo-1,2-dihydroquinazolin-3(4H)-...)Show SMILES Cc1cc(C[C@@H](NC(=O)N2CCC(CC2)N2Cc3cccc(F)c3NC2=O)C(=O)N2CCC(CC2)N2CCCCC2)cc2cn[nH]c12 |r| Show InChI InChI=1S/C35H45FN8O3/c1-23-18-24(19-26-21-37-40-31(23)26)20-30(33(45)42-14-8-27(9-15-42)41-12-3-2-4-13-41)38-34(46)43-16-10-28(11-17-43)44-22-25-6-5-7-29(36)32(25)39-35(44)47/h5-7,18-19,21,27-28,30H,2-4,8-17,20,22H2,1H3,(H,37,40)(H,38,46)(H,39,47)/t30-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [I125]CGRP from human CGRP receptor in SK-N-MC cells |
J Med Chem 51: 4858-61 (2008)
Article DOI: 10.1021/jm800546t
BindingDB Entry DOI: 10.7270/Q2N016BV |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50240440
(4-P-PDOT | CHEMBL285718 | N-(4-Phenyl-1,2,3,4-tetr...)Show InChI InChI=1S/C19H21NO/c1-2-19(21)20-16-12-15-10-6-7-11-17(15)18(13-16)14-8-4-3-5-9-14/h3-11,16,18H,2,12-13H2,1H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Parma
Curated by ChEMBL
| Assay Description
Inhibition of 2-[125I]iodomelatonin binding to human melatonin receptor MT2 expressed in NIH3T3 rat fibroblast cells |
J Med Chem 48: 4049-60 (2005)
Article DOI: 10.1021/jm048956y
BindingDB Entry DOI: 10.7270/Q2319ZND |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340384
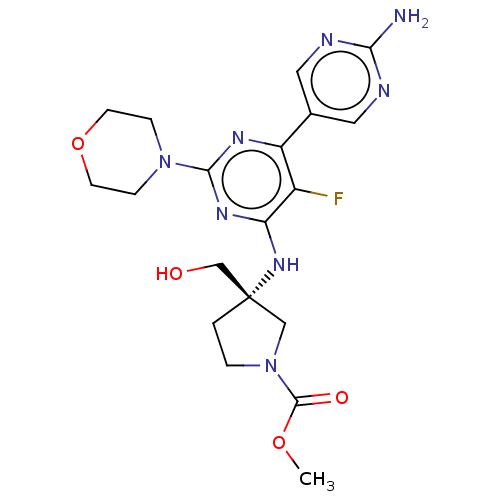
(US9758538, Example 72)Show SMILES COC(=O)N1CC[C@@](CO)(C1)Nc1nc(nc(c1F)-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C19H25FN8O4/c1-31-18(30)28-3-2-19(10-28,11-29)26-15-13(20)14(12-8-22-16(21)23-9-12)24-17(25-15)27-4-6-32-7-5-27/h8-9,29H,2-7,10-11H2,1H3,(H2,21,22,23)(H,24,25,26)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207217
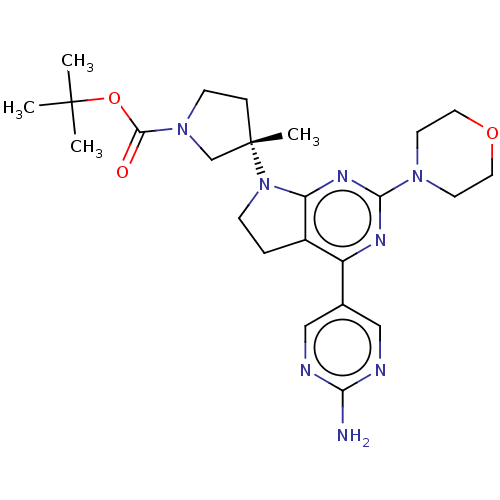
(US9260439, 194 | US9260439, 238 | US9260439, 239)Show SMILES CC(C)(C)OC(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C24H34N8O3/c1-23(2,3)35-22(33)31-8-6-24(4,15-31)32-7-5-17-18(16-13-26-20(25)27-14-16)28-21(29-19(17)32)30-9-11-34-12-10-30/h13-14H,5-12,15H2,1-4H3,(H2,25,26,27)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207378
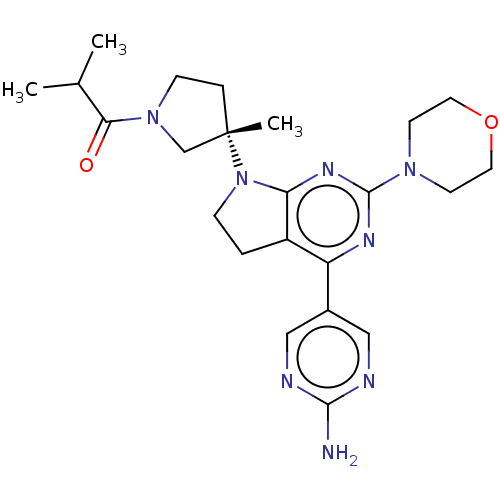
(US9260439, 262)Show SMILES CC(C)C(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C23H32N8O2/c1-15(2)20(32)30-7-5-23(3,14-30)31-6-4-17-18(16-12-25-21(24)26-13-16)27-22(28-19(17)31)29-8-10-33-11-9-29/h12-13,15H,4-11,14H2,1-3H3,(H2,24,25,26)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207196
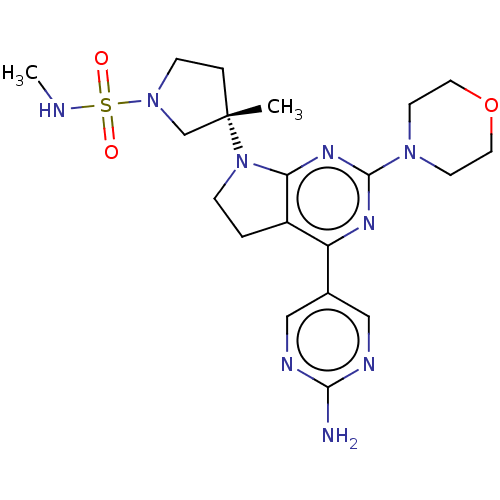
(US9260439, 173)Show SMILES CNS(=O)(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C20H29N9O3S/c1-20(4-6-28(13-20)33(30,31)22-2)29-5-3-15-16(14-11-23-18(21)24-12-14)25-19(26-17(15)29)27-7-9-32-10-8-27/h11-12,22H,3-10,13H2,1-2H3,(H2,21,23,24)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50007522
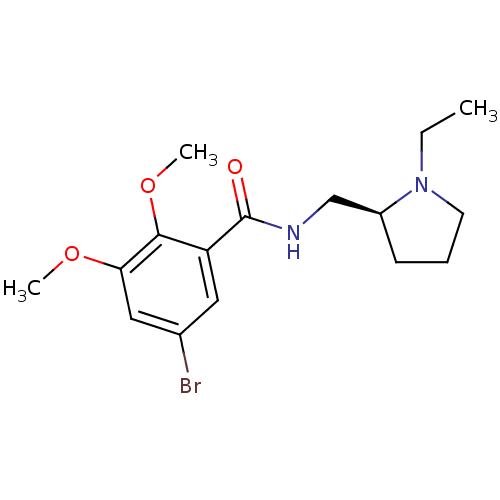
(5-Bromo-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2,3-dime...)Show InChI InChI=1S/C16H23BrN2O3/c1-4-19-7-5-6-12(19)10-18-16(20)13-8-11(17)9-14(21-2)15(13)22-3/h8-9,12H,4-7,10H2,1-3H3,(H,18,20)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB
Curated by ChEMBL
| Assay Description
Inhibition of [3H]raclopride binding to rat striatal dopamine receptor D2 |
J Med Chem 34: 948-55 (1991)
BindingDB Entry DOI: 10.7270/Q2GT5NS9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340336
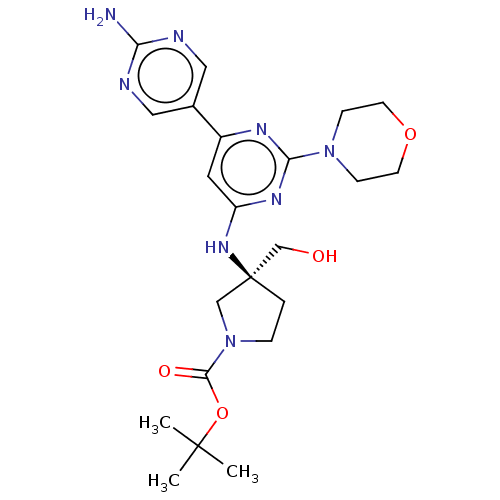
(US9758538, Example 24)Show SMILES CC(C)(C)OC(=O)N1CC[C@@](CO)(C1)Nc1cc(nc(n1)N1CCOCC1)-c1cnc(N)nc1 |r| Show InChI InChI=1S/C22H32N8O4/c1-21(2,3)34-20(32)30-5-4-22(13-30,14-31)28-17-10-16(15-11-24-18(23)25-12-15)26-19(27-17)29-6-8-33-9-7-29/h10-12,31H,4-9,13-14H2,1-3H3,(H2,23,24,25)(H,26,27,28)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340314
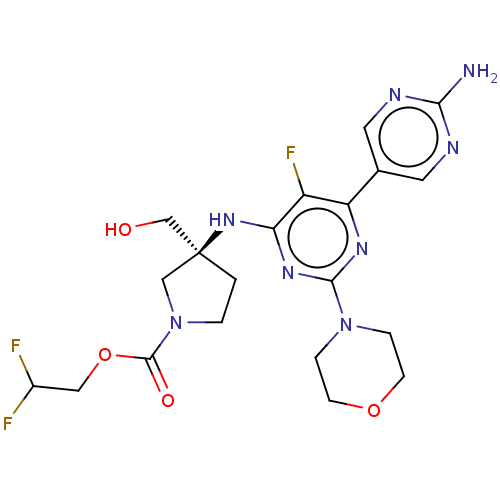
((Scheme A): Preparation of 2,2-difluoroethyl (3S)-...)Show SMILES Nc1ncc(cn1)-c1nc(nc(N[C@@]2(CO)CCN(C2)C(=O)OCC(F)F)c1F)N1CCOCC1 |r| Show InChI InChI=1S/C20H25F3N8O4/c21-13(22)9-35-19(33)31-2-1-20(10-31,11-32)29-16-14(23)15(12-7-25-17(24)26-8-12)27-18(28-16)30-3-5-34-6-4-30/h7-8,13,32H,1-6,9-11H2,(H2,24,25,26)(H,27,28,29)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340346

(US9758538, Example 34)Show SMILES COC(=O)N1CC[C@@](CO)(C1)Nc1cc(nc(n1)N1CCOCC1)-c1cnc(N)nc1 |r| Show InChI InChI=1S/C19H26N8O4/c1-30-18(29)27-3-2-19(11-27,12-28)25-15-8-14(13-9-21-16(20)22-10-13)23-17(24-15)26-4-6-31-7-5-26/h8-10,28H,2-7,11-12H2,1H3,(H2,20,21,22)(H,23,24,25)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340391
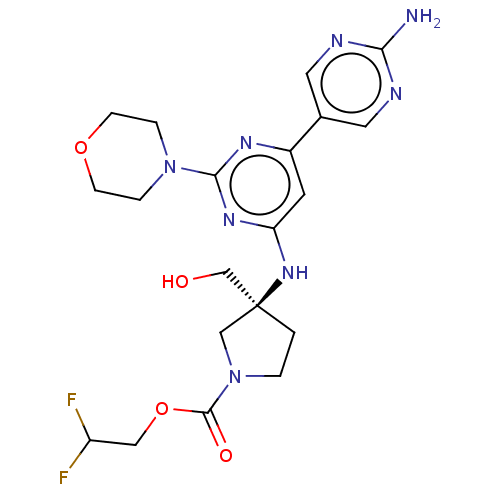
(US9758538, Example 79)Show SMILES Nc1ncc(cn1)-c1cc(N[C@@]2(CO)CCN(C2)C(=O)OCC(F)F)nc(n1)N1CCOCC1 |r| Show InChI InChI=1S/C20H26F2N8O4/c21-15(22)10-34-19(32)30-2-1-20(11-30,12-31)28-16-7-14(13-8-24-17(23)25-9-13)26-18(27-16)29-3-5-33-6-4-29/h7-9,15,31H,1-6,10-12H2,(H2,23,24,25)(H,26,27,28)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50368060
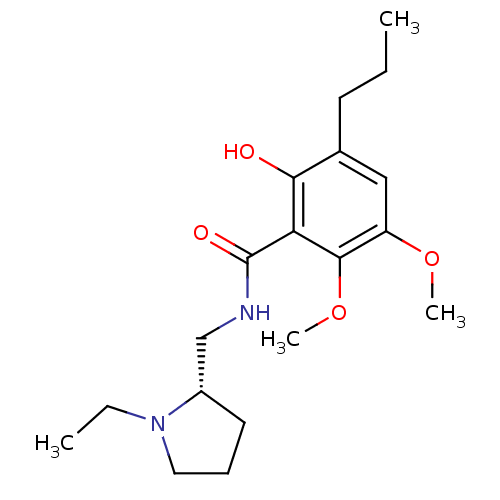
(CHEMBL1907695)Show SMILES CCCc1cc(OC)c(OC)c(C(=O)NC[C@@H]2CCCN2CC)c1O |r| Show InChI InChI=1S/C19H30N2O4/c1-5-8-13-11-15(24-3)18(25-4)16(17(13)22)19(23)20-12-14-9-7-10-21(14)6-2/h11,14,22H,5-10,12H2,1-4H3,(H,20,23)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB
Curated by ChEMBL
| Assay Description
Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 |
J Med Chem 33: 1155-63 (1990)
BindingDB Entry DOI: 10.7270/Q24X58DR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207236
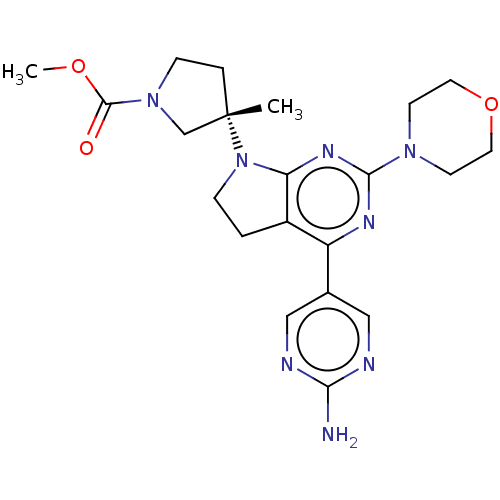
(US9260439, 213)Show SMILES COC(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C21H28N8O3/c1-21(4-6-28(13-21)20(30)31-2)29-5-3-15-16(14-11-23-18(22)24-12-14)25-19(26-17(15)29)27-7-9-32-10-8-27/h11-12H,3-10,13H2,1-2H3,(H2,22,23,24)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207028
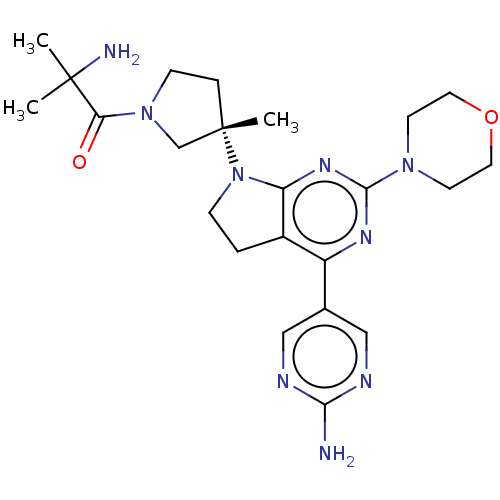
(US9260439, 10 | US9260439, 4)Show SMILES CC(C)(N)C(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C23H33N9O2/c1-22(2,25)19(33)31-7-5-23(3,14-31)32-6-4-16-17(15-12-26-20(24)27-13-15)28-21(29-18(16)32)30-8-10-34-11-9-30/h12-13H,4-11,14,25H2,1-3H3,(H2,24,26,27)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50007508
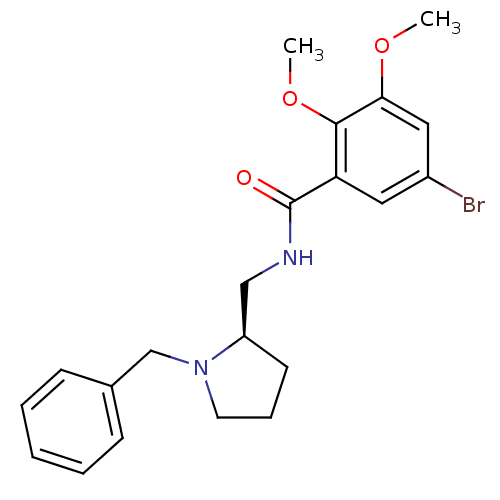
((R) N-(1-Benzyl-pyrrolidin-2-ylmethyl)-5-bromo-2,3...)Show SMILES COc1cc(Br)cc(C(=O)NC[C@H]2CCCN2Cc2ccccc2)c1OC Show InChI InChI=1S/C21H25BrN2O3/c1-26-19-12-16(22)11-18(20(19)27-2)21(25)23-13-17-9-6-10-24(17)14-15-7-4-3-5-8-15/h3-5,7-8,11-12,17H,6,9-10,13-14H2,1-2H3,(H,23,25)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB
Curated by ChEMBL
| Assay Description
Inhibition of [3H]raclopride binding to rat striatal dopamine receptor D2 |
J Med Chem 34: 948-55 (1991)
BindingDB Entry DOI: 10.7270/Q2GT5NS9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207172
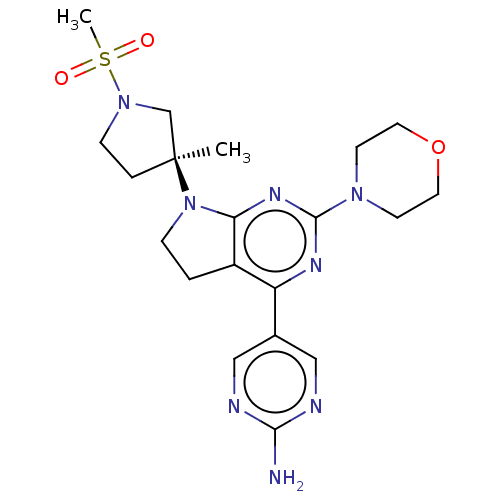
(US9260439, 149)Show SMILES C[C@@]1(CCN(C1)S(C)(=O)=O)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C20H28N8O3S/c1-20(4-6-27(13-20)32(2,29)30)28-5-3-15-16(14-11-22-18(21)23-12-14)24-19(25-17(15)28)26-7-9-31-10-8-26/h11-12H,3-10,13H2,1-2H3,(H2,21,22,23)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| <0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207391
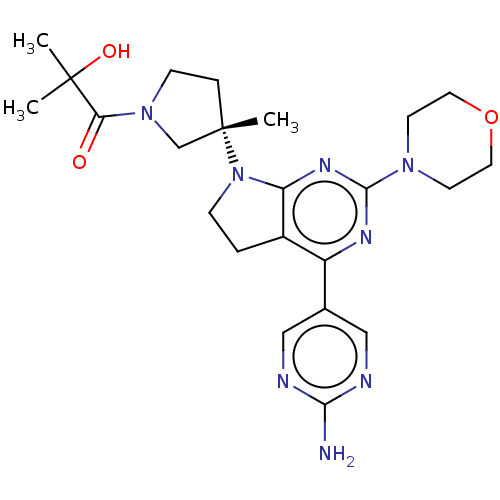
(US9260439, 275)Show SMILES CC(C)(O)C(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C23H32N8O3/c1-22(2,33)19(32)30-7-5-23(3,14-30)31-6-4-16-17(15-12-25-20(24)26-13-15)27-21(28-18(16)31)29-8-10-34-11-9-29/h12-13,33H,4-11,14H2,1-3H3,(H2,24,25,26)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50368067
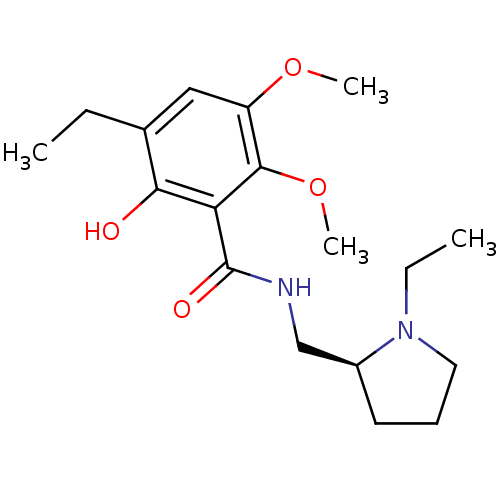
(CHEMBL1907702)Show SMILES CCN1CCC[C@H]1CNC(=O)c1c(O)c(CC)cc(OC)c1OC |r| Show InChI InChI=1S/C18H28N2O4/c1-5-12-10-14(23-3)17(24-4)15(16(12)21)18(22)19-11-13-8-7-9-20(13)6-2/h10,13,21H,5-9,11H2,1-4H3,(H,19,22)/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB
Curated by ChEMBL
| Assay Description
Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 |
J Med Chem 33: 1155-63 (1990)
BindingDB Entry DOI: 10.7270/Q24X58DR |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50007517
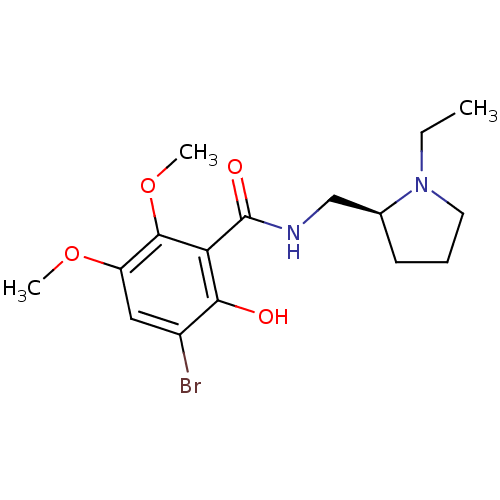
((S)-3-bromo-N-((1-ethylpyrrolidin-2-yl)methyl)-2-h...)Show InChI InChI=1S/C16H23BrN2O4/c1-4-19-7-5-6-10(19)9-18-16(21)13-14(20)11(17)8-12(22-2)15(13)23-3/h8,10,20H,4-7,9H2,1-3H3,(H,18,21)/t10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB
Curated by ChEMBL
| Assay Description
Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 |
J Med Chem 33: 1155-63 (1990)
BindingDB Entry DOI: 10.7270/Q24X58DR |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50007517

((S)-3-bromo-N-((1-ethylpyrrolidin-2-yl)methyl)-2-h...)Show InChI InChI=1S/C16H23BrN2O4/c1-4-19-7-5-6-10(19)9-18-16(21)13-14(20)11(17)8-12(22-2)15(13)23-3/h8,10,20H,4-7,9H2,1-3H3,(H,18,21)/t10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB
Curated by ChEMBL
| Assay Description
Inhibition of [3H]raclopride binding to rat striatal dopamine receptor D2 |
J Med Chem 34: 948-55 (1991)
BindingDB Entry DOI: 10.7270/Q2GT5NS9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(MOUSE) | BDBM81766
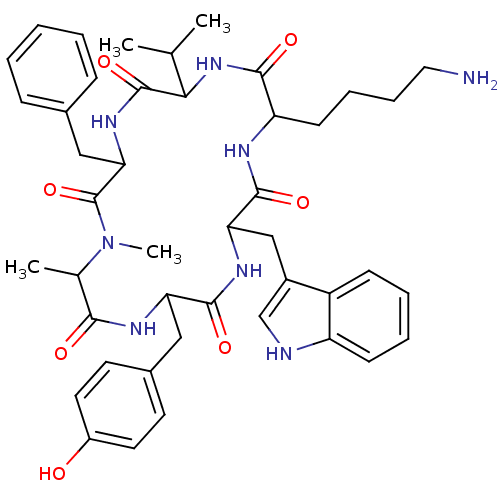
(CAS_3086456 | MK 678 | NSC_3086456)Show SMILES CC(C)C1NC(=O)C(CCCCN)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(C)N(C)C(=O)C(Cc2ccccc2)NC1=O Show InChI InChI=1S/C44H56N8O7/c1-26(2)38-43(58)50-37(23-28-12-6-5-7-13-28)44(59)52(4)27(3)39(54)48-35(22-29-17-19-31(53)20-18-29)41(56)49-36(24-30-25-46-33-15-9-8-14-32(30)33)42(57)47-34(40(55)51-38)16-10-11-21-45/h5-9,12-15,17-20,25-27,34-38,46,53H,10-11,16,21-24,45H2,1-4H3,(H,47,57)(H,48,54)(H,49,56)(H,50,58)(H,51,55) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chicago
Curated by PDSP Ki Database
| |
J Biol Chem 267: 20422-8 (1992)
BindingDB Entry DOI: 10.7270/Q2DF6PPB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207234
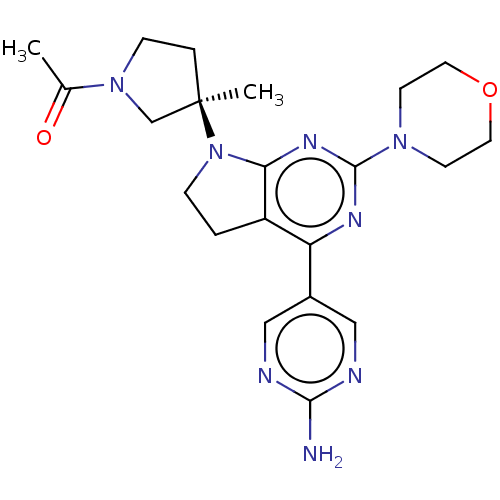
(US9260439, 211)Show SMILES CC(=O)N1CC[C@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C21H28N8O2/c1-14(30)28-6-4-21(2,13-28)29-5-3-16-17(15-11-23-19(22)24-12-15)25-20(26-18(16)29)27-7-9-31-10-8-27/h11-12H,3-10,13H2,1-2H3,(H2,22,23,24)/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(MOUSE) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chicago
Curated by PDSP Ki Database
| |
J Biol Chem 267: 20422-8 (1992)
BindingDB Entry DOI: 10.7270/Q2DF6PPB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207239
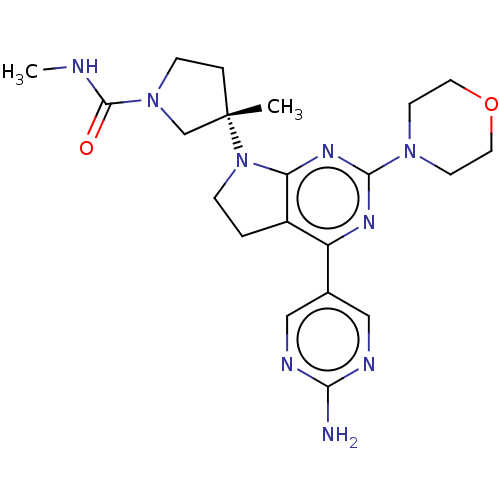
(US9260439, 216)Show SMILES CNC(=O)N1CC[C@@](C)(C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C21H29N9O2/c1-21(4-6-29(13-21)20(31)23-2)30-5-3-15-16(14-11-24-18(22)25-12-14)26-19(27-17(15)30)28-7-9-32-10-8-28/h11-12H,3-10,13H2,1-2H3,(H,23,31)(H2,22,24,25)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50368065

(CHEMBL1907692)Show SMILES CCN1CCC[C@H]1CNC(=O)c1c(O)c(Cl)cc(OC)c1OC |r| Show InChI InChI=1S/C16H23ClN2O4/c1-4-19-7-5-6-10(19)9-18-16(21)13-14(20)11(17)8-12(22-2)15(13)23-3/h8,10,20H,4-7,9H2,1-3H3,(H,18,21)/t10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB
Curated by ChEMBL
| Assay Description
Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 |
J Med Chem 33: 1155-63 (1990)
BindingDB Entry DOI: 10.7270/Q24X58DR |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599]
(Human immunodeficiency virus type 1) | BDBM854
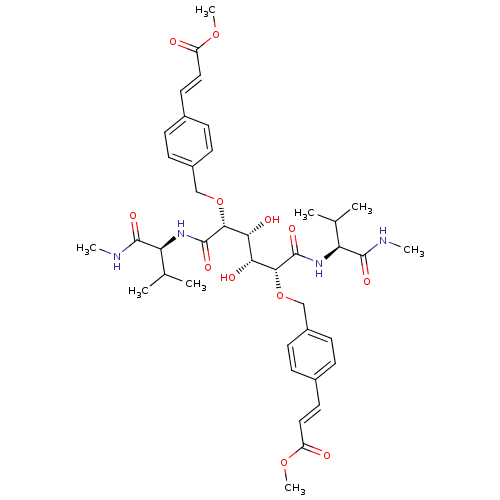
(C2-Symmetric inhibitor 12 | CHEMBL127045 | N1,N6-B...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](OCc1ccc(\C=C\C(=O)OC)cc1)[C@H](O)[C@@H](O)[C@@H](OCc1ccc(\C=C\C(=O)OC)cc1)C(=O)N[C@@H](C(C)C)C(=O)NC)C(C)C |r| Show InChI InChI=1S/C40H54N4O12/c1-23(2)31(37(49)41-5)43-39(51)35(55-21-27-13-9-25(10-14-27)17-19-29(45)53-7)33(47)34(48)36(40(52)44-32(24(3)4)38(50)42-6)56-22-28-15-11-26(12-16-28)18-20-30(46)54-8/h9-20,23-24,31-36,47-48H,21-22H2,1-8H3,(H,41,49)(H,42,50)(H,43,51)(H,44,52)/b19-17+,20-18+/t31-,32-,33+,34+,35+,36+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | -58.3 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University
| Assay Description
Ki values were determined by using a fluorescent substrate (DABCYL-gamma-Abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS). All incubations were performed a... |
J Med Chem 42: 3835-44 (1999)
Article DOI: 10.1021/jm9910371
BindingDB Entry DOI: 10.7270/Q2T151VX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340352
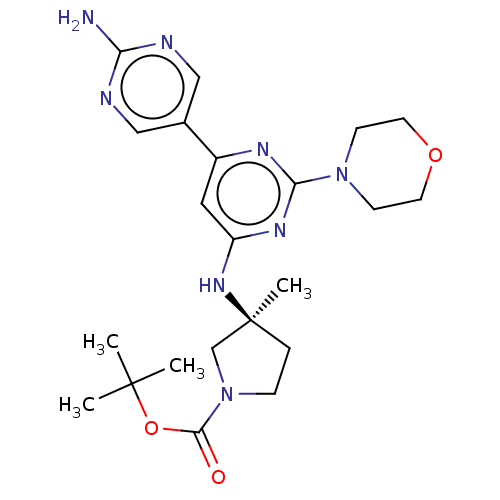
(US9758538, Example 40)Show SMILES CC(C)(C)OC(=O)N1CC[C@@](C)(C1)Nc1cc(nc(n1)N1CCOCC1)-c1cnc(N)nc1 |r| Show InChI InChI=1S/C22H32N8O3/c1-21(2,3)33-20(31)30-6-5-22(4,14-30)28-17-11-16(15-12-24-18(23)25-13-15)26-19(27-17)29-7-9-32-10-8-29/h11-13H,5-10,14H2,1-4H3,(H2,23,24,25)(H,26,27,28)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM12218

((2R,3R,4R,5R)-N-benzyl-2,5-bis(benzyloxy)-3,4-dihy...)Show SMILES O[C@H]([C@@H](O)[C@@H](OCc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)[C@@H](OCc1ccccc1)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C36H38N2O7/c39-29-20-27-18-10-11-19-28(27)30(29)38-36(43)34(45-23-26-16-8-3-9-17-26)32(41)31(40)33(44-22-25-14-6-2-7-15-25)35(42)37-21-24-12-4-1-5-13-24/h1-19,29-34,39-41H,20-23H2,(H,37,42)(H,38,43)/t29-,30+,31-,32-,33-,34-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University
| Assay Description
A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... |
Eur J Biochem 270: 1746-58 (2003)
Article DOI: 10.1046/j.1432-1033.2003.03533.x
BindingDB Entry DOI: 10.7270/Q2NZ85WX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50246967
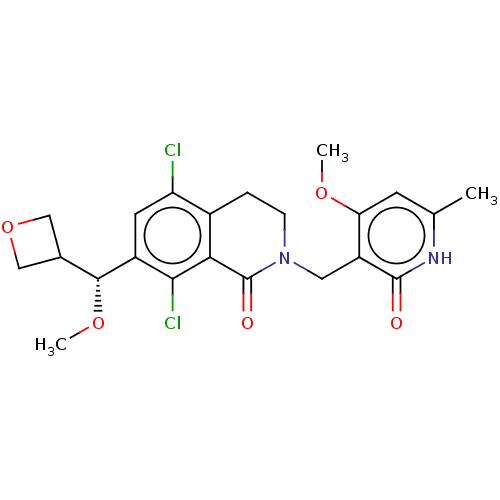
(CHEMBL4080228 | US10570121, Example 81)Show SMILES CO[C@H](C1COC1)c1cc(Cl)c2CCN(Cc3c(OC)cc(C)[nH]c3=O)C(=O)c2c1Cl |r| Show InChI InChI=1S/C22H24Cl2N2O5/c1-11-6-17(29-2)15(21(27)25-11)8-26-5-4-13-16(23)7-14(19(24)18(13)22(26)28)20(30-3)12-9-31-10-12/h6-7,12,20H,4-5,8-10H2,1-3H3,(H,25,27)/t20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec
Curated by ChEMBL
| Assay Description
Binding affinity to EZH2 (unknown origin) |
J Med Chem 61: 650-665 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01375
BindingDB Entry DOI: 10.7270/Q2X069G8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein kinase C delta type
(Mus musculus) | BDBM50057514
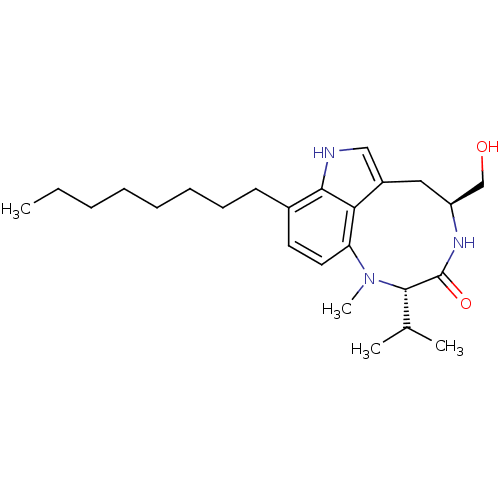
((10S,13S)-13-Hydroxymethyl-10-isopropyl-9-methyl-5...)Show SMILES CCCCCCCCc1ccc2N(C)[C@@H](C(C)C)C(=O)N[C@H](CO)Cc3c[nH]c1c23 Show InChI InChI=1S/C25H39N3O2/c1-5-6-7-8-9-10-11-18-12-13-21-22-19(15-26-23(18)22)14-20(16-29)27-25(30)24(17(2)3)28(21)4/h12-13,15,17,20,24,26,29H,5-11,14,16H2,1-4H3,(H,27,30)/t20-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]- PDBu from Protein kinase C delta C1b domain |
J Med Chem 45: 853-60 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V25 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM851
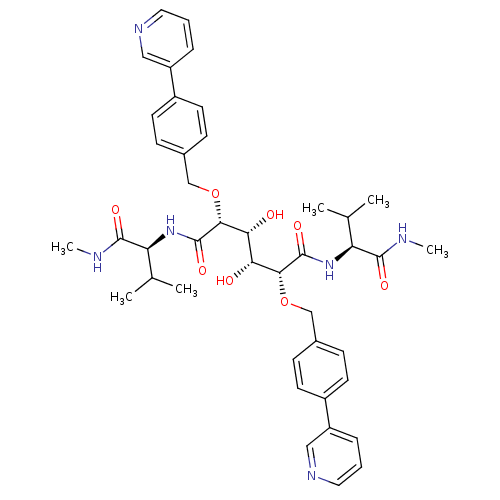
((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S)-2-methyl...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](OCc1ccc(cc1)-c1cccnc1)[C@H](O)[C@@H](O)[C@@H](OCc1ccc(cc1)-c1cccnc1)C(=O)N[C@@H](C(C)C)C(=O)NC)C(C)C |r| Show InChI InChI=1S/C42H52N6O8/c1-25(2)33(39(51)43-5)47-41(53)37(55-23-27-11-15-29(16-12-27)31-9-7-19-45-21-31)35(49)36(50)38(42(54)48-34(26(3)4)40(52)44-6)56-24-28-13-17-30(18-14-28)32-10-8-20-46-22-32/h7-22,25-26,33-38,49-50H,23-24H2,1-6H3,(H,43,51)(H,44,52)(H,47,53)(H,48,54)/t33-,34-,35+,36+,37+,38+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University
| Assay Description
A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... |
Eur J Biochem 270: 1746-58 (2003)
Article DOI: 10.1046/j.1432-1033.2003.03533.x
BindingDB Entry DOI: 10.7270/Q2NZ85WX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Reversible binding affinity to human EGFR L858R/ T790M double mutant expressed in baculovirus by fluorometric analysis |
J Med Chem 59: 2005-24 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01633
BindingDB Entry DOI: 10.7270/Q2KS6TDD |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207061
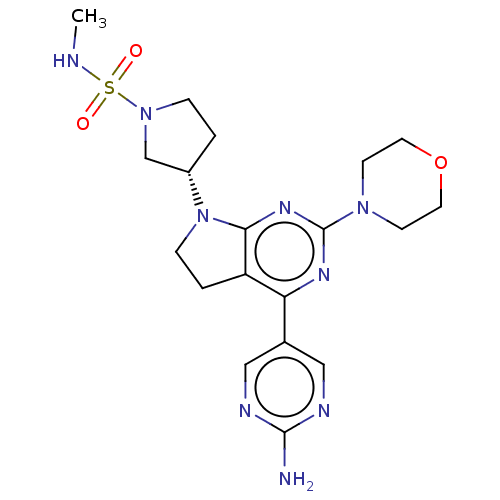
(US9260439, 38)Show SMILES CNS(=O)(=O)N1CC[C@@H](C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C19H27N9O3S/c1-21-32(29,30)27-4-2-14(12-27)28-5-3-15-16(13-10-22-18(20)23-11-13)24-19(25-17(15)28)26-6-8-31-9-7-26/h10-11,14,21H,2-9,12H2,1H3,(H2,20,22,23)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50007518

((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...)Show InChI InChI=1S/C17H25ClN2O3/c1-4-11-9-13(18)16(23-3)14(15(11)21)17(22)19-10-12-7-6-8-20(12)5-2/h9,12,21H,4-8,10H2,1-3H3,(H,19,22)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB
Curated by ChEMBL
| Assay Description
Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 |
J Med Chem 33: 1155-63 (1990)
BindingDB Entry DOI: 10.7270/Q24X58DR |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Mus musculus) | BDBM50099646
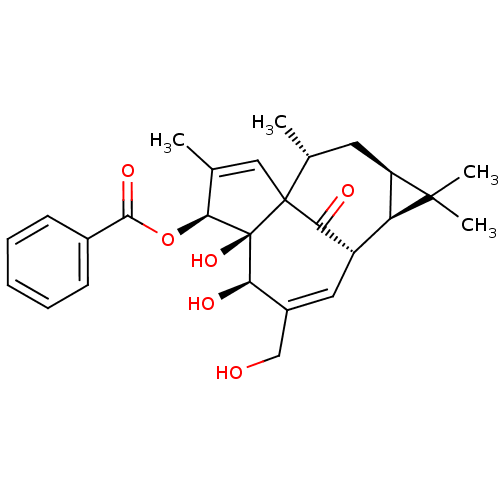
(5,6-dihydroxy-7-hydroxymethyl-3,11,11,14-tetrameth...)Show SMILES C[C@@H]1C[C@@H]2[C@H]([C@@H]3C=C(CO)[C@@H](O)[C@]4(O)[C@@H](OC(=O)c5ccccc5)C(C)=CC14C3=O)C2(C)C |c:26,t:6| Show InChI InChI=1S/C27H32O6/c1-14-12-26-15(2)10-19-20(25(19,3)4)18(22(26)30)11-17(13-28)21(29)27(26,32)23(14)33-24(31)16-8-6-5-7-9-16/h5-9,11-12,15,18-21,23,28-29,32H,10,13H2,1-4H3/t15-,18+,19-,20?,21-,23+,26?,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from protein kinase C delta C1b domain (Wild type) |
J Med Chem 44: 1690-701 (2001)
BindingDB Entry DOI: 10.7270/Q2RR1ZX3 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor
(RABBIT) | BDBM82372
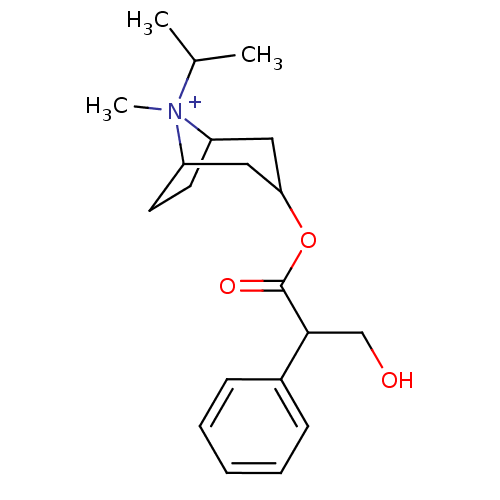
(CAS_22254-24-6 | Ipratropium | NSC_3746)Show SMILES CC(C)[N+]1(C)C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |TLB:1:3:6.7:9.10.11,THB:4:3:6.7:9.10.11,12:10:3:6.7,(-1.51,-1.3,;-.6,-.05,;-1.22,1.36,;.93,-.22,;1.51,1.21,;1.98,-1.04,;1.38,-2.36,;.22,-3.07,;1.19,-1.74,;3.01,-1.74,;3.97,-2.54,;3.71,-1.01,;4.74,-3.87,;3.97,-5.2,;2.43,-5.2,;4.74,-6.54,;6.28,-6.54,;7.05,-7.87,;3.97,-7.87,;4.73,-9.21,;3.96,-10.54,;2.42,-10.54,;1.65,-9.2,;2.43,-7.87,)| Show InChI InChI=1S/C20H30NO3/c1-14(2)21(3)16-9-10-17(21)12-18(11-16)24-20(23)19(13-22)15-7-5-4-6-8-15/h4-8,14,16-19,22H,9-13H2,1-3H3/q+1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by PDSP Ki Database
| |
Mol Pharmacol 38: 805-15 (1990)
BindingDB Entry DOI: 10.7270/Q2H993P0 |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus (rat)) | BDBM50308250
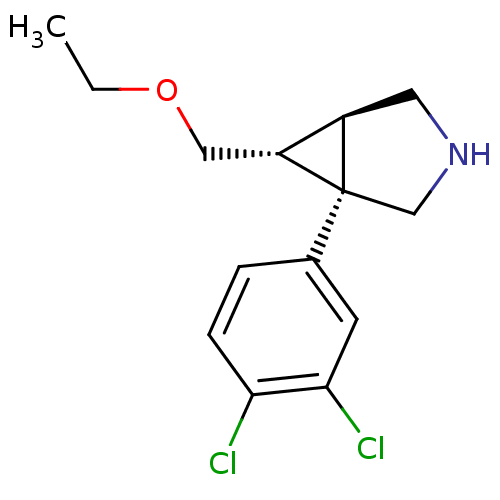
((1R,5R,6R)-1-(3,4-dichlorophenyl)-6-(ethoxymethyl)...)Show SMILES CCOC[C@@H]1[C@H]2CNC[C@@]12c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C14H17Cl2NO/c1-2-18-7-11-10-6-17-8-14(10,11)9-3-4-12(15)13(16)5-9/h3-5,10-11,17H,2,6-8H2,1H3/t10-,11-,14+/m1/s1 | Reactome pathway
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicine Research Centre
Curated by ChEMBL
| Assay Description
Displacement of [N-methyl-3H]nisoxetine from rat hippocampus NET by filtration binding assay |
J Med Chem 53: 2534-51 (2010)
Article DOI: 10.1021/jm901818u
BindingDB Entry DOI: 10.7270/Q2ST7PX2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM340347
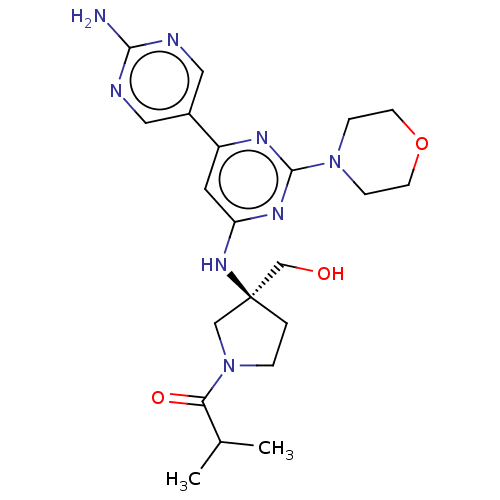
(US9758538, Example 35)Show SMILES CC(C)C(=O)N1CC[C@@](CO)(C1)Nc1cc(nc(n1)N1CCOCC1)-c1cnc(N)nc1 |r| Show InChI InChI=1S/C21H30N8O3/c1-14(2)18(31)29-4-3-21(12-29,13-30)27-17-9-16(15-10-23-19(22)24-11-15)25-20(26-17)28-5-7-32-8-6-28/h9-11,14,30H,3-8,12-13H2,1-2H3,(H2,22,23,24)(H,25,26,27)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Mus musculus) | BDBM50099646

(5,6-dihydroxy-7-hydroxymethyl-3,11,11,14-tetrameth...)Show SMILES C[C@@H]1C[C@@H]2[C@H]([C@@H]3C=C(CO)[C@@H](O)[C@]4(O)[C@@H](OC(=O)c5ccccc5)C(C)=CC14C3=O)C2(C)C |c:26,t:6| Show InChI InChI=1S/C27H32O6/c1-14-12-26-15(2)10-19-20(25(19,3)4)18(22(26)30)11-17(13-28)21(29)27(26,32)23(14)33-24(31)16-8-6-5-7-9-16/h5-9,11-12,15,18-21,23,28-29,32H,10,13H2,1-4H3/t15-,18+,19-,20?,21-,23+,26?,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from protein kinase C delta C1b domain mutant (F13G) |
J Med Chem 44: 1690-701 (2001)
BindingDB Entry DOI: 10.7270/Q2RR1ZX3 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM207043
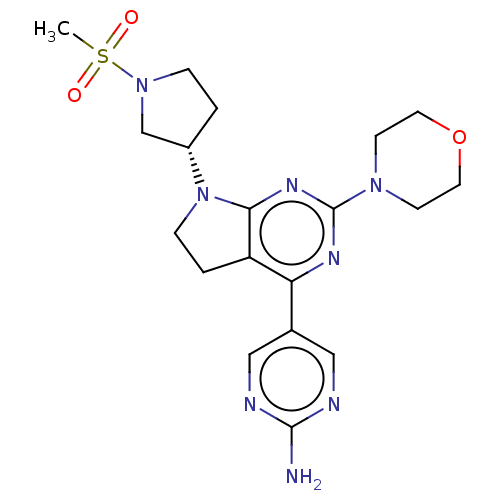
(US9260439, 20)Show SMILES CS(=O)(=O)N1CC[C@@H](C1)N1CCc2c1nc(nc2-c1cnc(N)nc1)N1CCOCC1 |r| Show InChI InChI=1S/C19H26N8O3S/c1-31(28,29)26-4-2-14(12-26)27-5-3-15-16(13-10-21-18(20)22-11-13)23-19(24-17(15)27)25-6-8-30-9-7-25/h10-11,14H,2-9,12H2,1H3,(H2,20,21,22)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human full-length PI3K p110alpha/p85alpha (322 to 600) expressed in baculovirus infected Sf21 cells using phosphatidylinosi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01652
BindingDB Entry DOI: 10.7270/Q2TH8RC9 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50007509
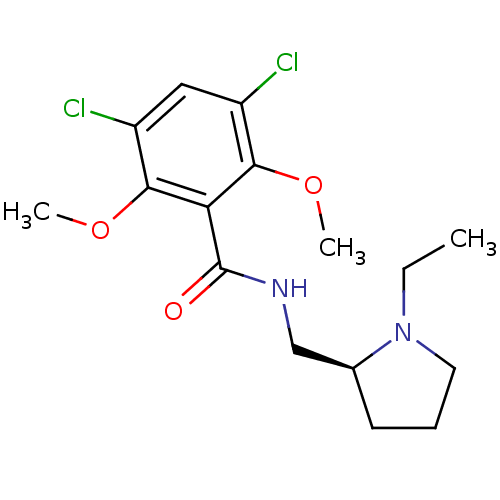
(3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2,6...)Show InChI InChI=1S/C16H22Cl2N2O3/c1-4-20-7-5-6-10(20)9-19-16(21)13-14(22-2)11(17)8-12(18)15(13)23-3/h8,10H,4-7,9H2,1-3H3,(H,19,21)/t10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB
Curated by ChEMBL
| Assay Description
Inhibition of [3H]raclopride binding to rat striatal dopamine receptor D2 |
J Med Chem 34: 948-55 (1991)
BindingDB Entry DOI: 10.7270/Q2GT5NS9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by PDSP Ki Database
| |
Neuropsychopharmacology 28: 519-26 (2003)
Article DOI: 10.1038/sj.npp.1300027
BindingDB Entry DOI: 10.7270/Q2833QKK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50015720
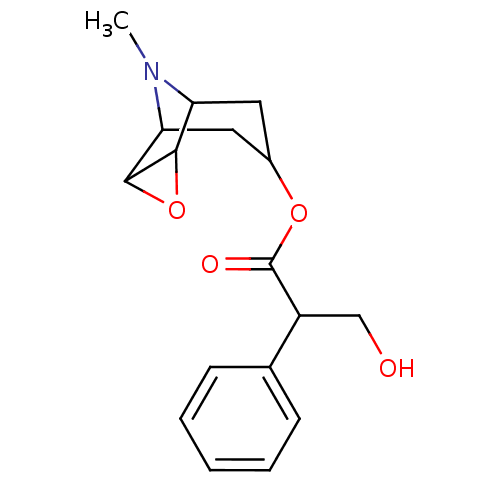
((hyoscine)3-Hydroxy-2-phenyl-propionic acid 9-meth...)Show SMILES CN1C2CC(CC1C1OC21)OC(=O)C(CO)c1ccccc1 |TLB:8:9:1:3.5.4,8:7:1:3.5.4,0:1:9.7:3.5.4,THB:10:4:9.7:1| Show InChI InChI=1S/C17H21NO4/c1-18-13-7-11(8-14(18)16-15(13)22-16)21-17(20)12(9-19)10-5-3-2-4-6-10/h2-6,11-16,19H,7-9H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by PDSP Ki Database
| |
Mol Pharmacol 38: 805-15 (1990)
BindingDB Entry DOI: 10.7270/Q2H993P0 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50007510
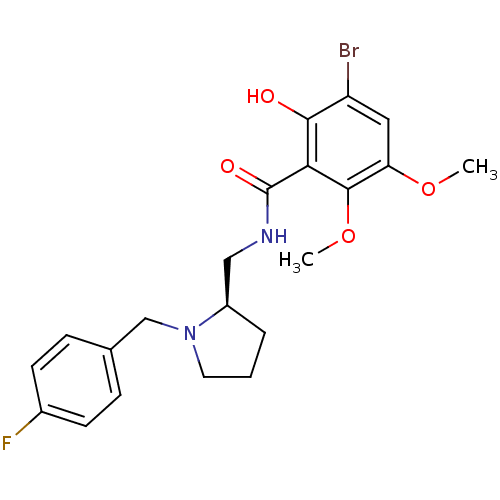
((R) 3-Bromo-N-[1-(4-fluoro-benzyl)-pyrrolidin-2-yl...)Show SMILES COc1cc(Br)c(O)c(C(=O)NC[C@H]2CCCN2Cc2ccc(F)cc2)c1OC Show InChI InChI=1S/C21H24BrFN2O4/c1-28-17-10-16(22)19(26)18(20(17)29-2)21(27)24-11-15-4-3-9-25(15)12-13-5-7-14(23)8-6-13/h5-8,10,15,26H,3-4,9,11-12H2,1-2H3,(H,24,27)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB
Curated by ChEMBL
| Assay Description
Inhibition of [3H]spiperone binding to rat striatal dopamine receptor D2 was determined in vitro |
J Med Chem 34: 948-55 (1991)
BindingDB Entry DOI: 10.7270/Q2GT5NS9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50015720

((hyoscine)3-Hydroxy-2-phenyl-propionic acid 9-meth...)Show SMILES CN1C2CC(CC1C1OC21)OC(=O)C(CO)c1ccccc1 |TLB:8:9:1:3.5.4,8:7:1:3.5.4,0:1:9.7:3.5.4,THB:10:4:9.7:1| Show InChI InChI=1S/C17H21NO4/c1-18-13-7-11(8-14(18)16-15(13)22-16)21-17(20)12(9-19)10-5-3-2-4-6-10/h2-6,11-16,19H,7-9H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by PDSP Ki Database
| |
Mol Pharmacol 38: 805-15 (1990)
BindingDB Entry DOI: 10.7270/Q2H993P0 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM358

((2R,3R,4R,5R)-2,5-bis(benzyloxy)-3,4-dihydroxy-N,N...)Show SMILES O[C@H]([C@@H](O)[C@@H](OCc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)[C@@H](OCc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C38H40N2O8/c41-29-19-25-15-7-9-17-27(25)31(29)39-37(45)35(47-21-23-11-3-1-4-12-23)33(43)34(44)36(48-22-24-13-5-2-6-14-24)38(46)40-32-28-18-10-8-16-26(28)20-30(32)42/h1-18,29-36,41-44H,19-22H2,(H,39,45)(H,40,46)/t29-,30-,31+,32+,33-,34-,35-,36-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.200 | -56.3 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University
| Assay Description
A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... |
Eur J Biochem 270: 1746-58 (2003)
Article DOI: 10.1046/j.1432-1033.2003.03533.x
BindingDB Entry DOI: 10.7270/Q2NZ85WX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M4
(Chick) | BDBM82372

(CAS_22254-24-6 | Ipratropium | NSC_3746)Show SMILES CC(C)[N+]1(C)C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |TLB:1:3:6.7:9.10.11,THB:4:3:6.7:9.10.11,12:10:3:6.7,(-1.51,-1.3,;-.6,-.05,;-1.22,1.36,;.93,-.22,;1.51,1.21,;1.98,-1.04,;1.38,-2.36,;.22,-3.07,;1.19,-1.74,;3.01,-1.74,;3.97,-2.54,;3.71,-1.01,;4.74,-3.87,;3.97,-5.2,;2.43,-5.2,;4.74,-6.54,;6.28,-6.54,;7.05,-7.87,;3.97,-7.87,;4.73,-9.21,;3.96,-10.54,;2.42,-10.54,;1.65,-9.2,;2.43,-7.87,)| Show InChI InChI=1S/C20H30NO3/c1-14(2)21(3)16-9-10-17(21)12-18(11-16)24-20(23)19(13-22)15-7-5-4-6-8-15/h4-8,14,16-19,22H,9-13H2,1-3H3/q+1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by PDSP Ki Database
| |
Mol Pharmacol 38: 805-15 (1990)
BindingDB Entry DOI: 10.7270/Q2H993P0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor
(RABBIT) | BDBM50015720

((hyoscine)3-Hydroxy-2-phenyl-propionic acid 9-meth...)Show SMILES CN1C2CC(CC1C1OC21)OC(=O)C(CO)c1ccccc1 |TLB:8:9:1:3.5.4,8:7:1:3.5.4,0:1:9.7:3.5.4,THB:10:4:9.7:1| Show InChI InChI=1S/C17H21NO4/c1-18-13-7-11(8-14(18)16-15(13)22-16)21-17(20)12(9-19)10-5-3-2-4-6-10/h2-6,11-16,19H,7-9H2,1H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by PDSP Ki Database
| |
Mol Pharmacol 38: 805-15 (1990)
BindingDB Entry DOI: 10.7270/Q2H993P0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM86698
(BENZTROPINE | CAS_132-17-2 | CHEMBL116590 | NSC_23...)Show SMILES CN1C2CCC1CC(C2)OC(c1ccccc1)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C21H25NO/c1-22-18-12-13-19(22)15-20(14-18)23-21(16-8-4-2-5-9-16)17-10-6-3-7-11-17/h2-11,18-21H,12-15H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by PDSP Ki Database
| |
Mol Pharmacol 38: 805-15 (1990)
BindingDB Entry DOI: 10.7270/Q2H993P0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data