

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2307 (5- or 6-Substituted Indole BHAP analogue 21 | N-[2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2309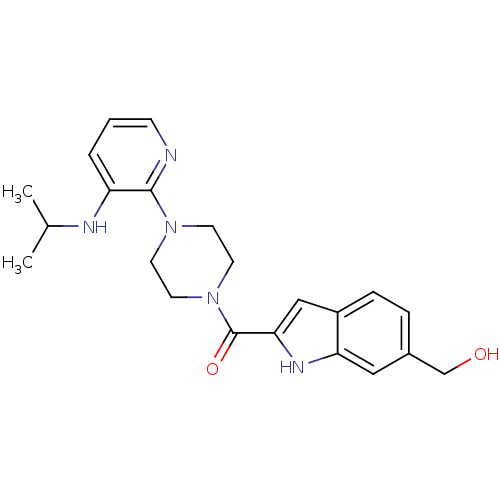 (5- or 6-Substituted Indole BHAP analogue 23 | [2-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2295 (2-({4-[3-(propan-2-ylamino)pyridin-2-yl]piperazin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1944 (BHAP deriv. | CHEMBL593 | DELAVIRDINE MESYLATE | D...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50055471 (CHEMBL149055 | N-{2-[4-(3-Isopropoxy-pyridin-2-yl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description In vitro for inhibition of HIV-1 reverse transcriptase. | J Med Chem 39: 5267-75 (1997) Article DOI: 10.1021/jm960269m BindingDB Entry DOI: 10.7270/Q2XK8DPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1944 (BHAP deriv. | CHEMBL593 | DELAVIRDINE MESYLATE | D...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description In vitro for inhibition of HIV-1 reverse transcriptase. | J Med Chem 39: 5267-75 (1997) Article DOI: 10.1021/jm960269m BindingDB Entry DOI: 10.7270/Q2XK8DPT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2291 (2-[4-(1H-indol-2-ylcarbonyl)piperazin-1-yl]-N-(pro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2299 (2-({4-[3-(propan-2-ylamino)pyridin-2-yl]piperazin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2296 (2-{4-[(6-methoxy-1H-indol-2-yl)carbonyl]piperazin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2292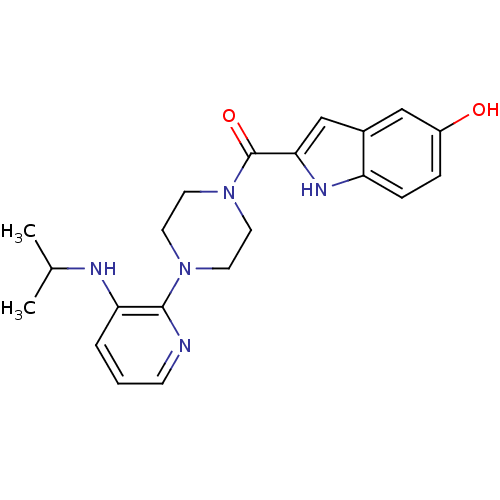 (2-({4-[3-(propan-2-ylamino)pyridin-2-yl]piperazin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2301 (2-({4-[3-(propan-2-ylamino)pyridin-2-yl]piperazin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50055473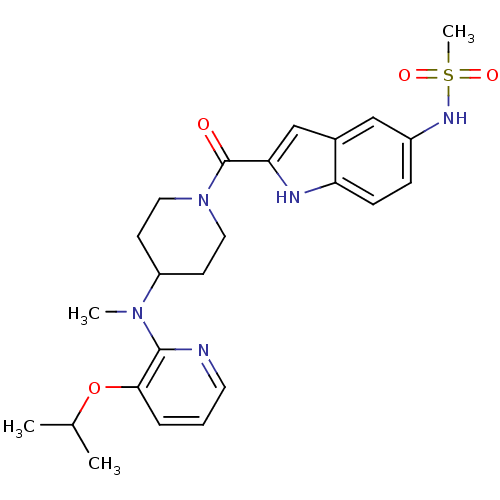 (CHEMBL151955 | N-(2-{4-[(3-Isopropoxy-pyridin-2-yl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description In vitro for inhibition of HIV-1 reverse transcriptase. | J Med Chem 39: 5267-75 (1997) Article DOI: 10.1021/jm960269m BindingDB Entry DOI: 10.7270/Q2XK8DPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50055478 ((1H-Indol-2-yl)-[4-(3-isopropoxy-pyridin-2-yl)-pip...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description In vitro for inhibition of HIV-1 reverse transcriptase. | J Med Chem 39: 5267-75 (1997) Article DOI: 10.1021/jm960269m BindingDB Entry DOI: 10.7270/Q2XK8DPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2303 (5- or 6-Substituted Indole BHAP analogue 17 | N-[2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50055476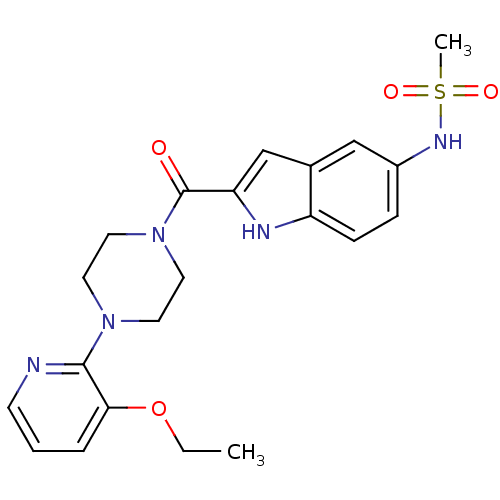 (CHEMBL151173 | N-{2-[4-(3-Ethoxy-pyridin-2-yl)-pip...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description In vitro for inhibition of HIV-1 reverse transcriptase. | J Med Chem 39: 5267-75 (1997) Article DOI: 10.1021/jm960269m BindingDB Entry DOI: 10.7270/Q2XK8DPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1945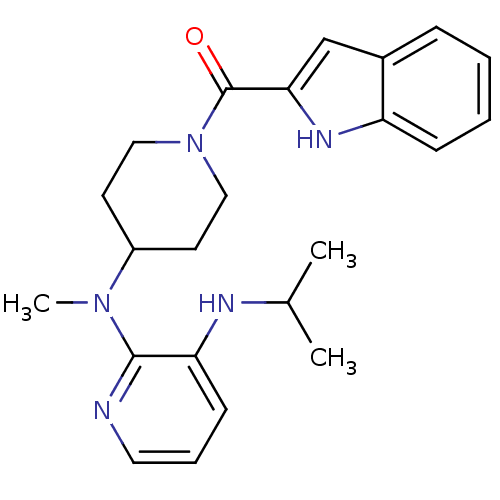 ((Alkylamino)piperidine BHAP Analog 1 | 2-N-[1-(1H-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description In vitro for inhibition of HIV-1 reverse transcriptase. | J Med Chem 39: 5267-75 (1997) Article DOI: 10.1021/jm960269m BindingDB Entry DOI: 10.7270/Q2XK8DPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2290 (2-({4-[3-(ethylamino)pyridin-2-yl]piperazin-1-yl}c...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50055470 ((1H-Indol-2-yl)-{4-[(3-isopropoxy-pyridin-2-yl)-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description In vitro for inhibition of HIV-1 reverse transcriptase. | J Med Chem 39: 5267-75 (1997) Article DOI: 10.1021/jm960269m BindingDB Entry DOI: 10.7270/Q2XK8DPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50055472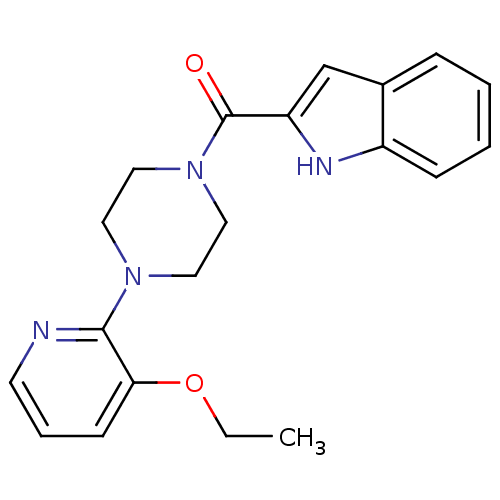 (CHEMBL356173 | [4-(3-Ethoxy-pyridin-2-yl)-piperazi...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description In vitro for inhibition of HIV-1 reverse transcriptase. | J Med Chem 39: 5267-75 (1997) Article DOI: 10.1021/jm960269m BindingDB Entry DOI: 10.7270/Q2XK8DPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2308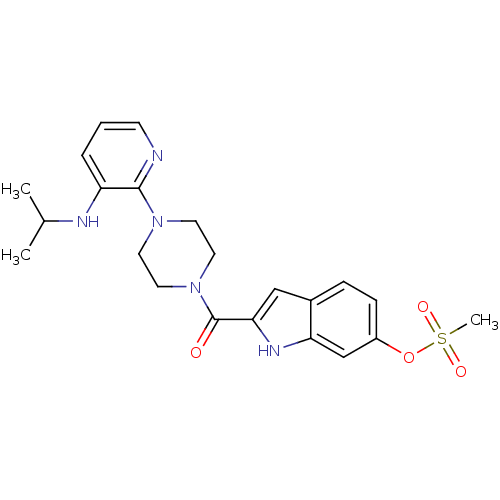 (2-({4-[3-(propan-2-ylamino)pyridin-2-yl]piperazin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1947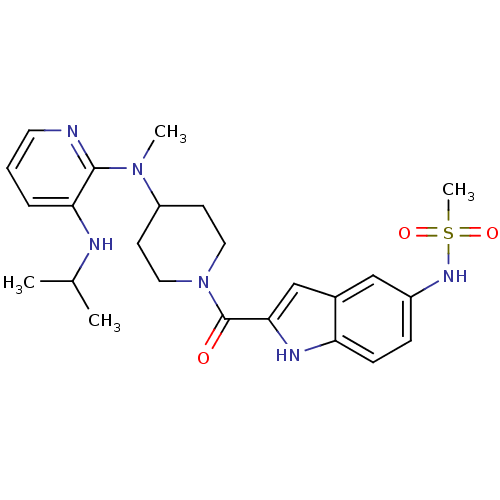 ((Alkylamino)piperidine BHAP Analog 3 | CHEMBL12433...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description In vitro for inhibition of HIV-1 reverse transcriptase. | J Med Chem 39: 5267-75 (1997) Article DOI: 10.1021/jm960269m BindingDB Entry DOI: 10.7270/Q2XK8DPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50055475 (CHEMBL424141 | {4-[(3-Ethylamino-pyridin-2-yl)-met...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description In vitro for inhibition of HIV-1 reverse transcriptase. | J Med Chem 39: 5267-75 (1997) Article DOI: 10.1021/jm960269m BindingDB Entry DOI: 10.7270/Q2XK8DPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2304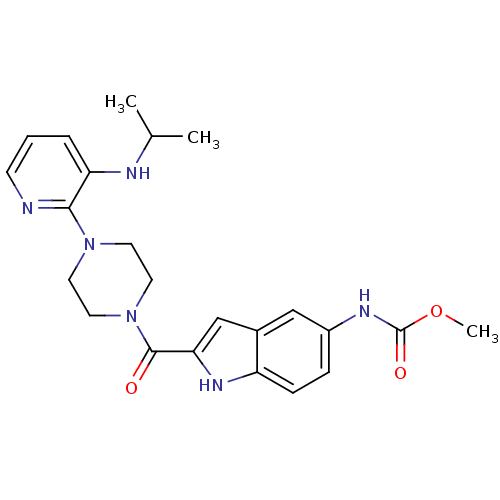 (5- or 6-Substituted Indole BHAP analogue 18 | meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50055469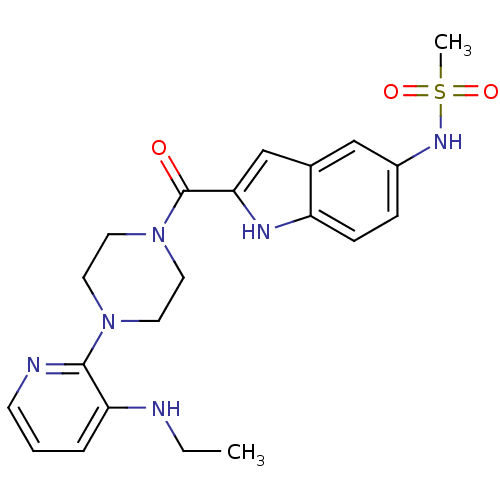 (CHEMBL151192 | N-{2-[4-(3-Ethylamino-pyridin-2-yl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description In vitro for inhibition of HIV-1 reverse transcriptase. | J Med Chem 39: 5267-75 (1997) Article DOI: 10.1021/jm960269m BindingDB Entry DOI: 10.7270/Q2XK8DPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1317 (3-[[(4,7-Dichlorobenzoxazol-2-yl)-methyl]amino]-5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1951 ((Alkylamino)piperidine BHAP Analog 7 | 1-[(5-Metha...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description In vitro for inhibition of HIV-1 reverse transcriptase. | J Med Chem 39: 5267-75 (1997) Article DOI: 10.1021/jm960269m BindingDB Entry DOI: 10.7270/Q2XK8DPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50055468 (CHEMBL147224 | {4-[(3-Ethoxy-pyridin-2-yl)-methyl-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description In vitro for inhibition of HIV-1 reverse transcriptase. | J Med Chem 39: 5267-75 (1997) Article DOI: 10.1021/jm960269m BindingDB Entry DOI: 10.7270/Q2XK8DPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50055474 (CHEMBL151316 | N-(2-{4-[(3-Ethoxy-pyridin-2-yl)-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description In vitro for inhibition of HIV-1 reverse transcriptase. | J Med Chem 39: 5267-75 (1997) Article DOI: 10.1021/jm960269m BindingDB Entry DOI: 10.7270/Q2XK8DPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2300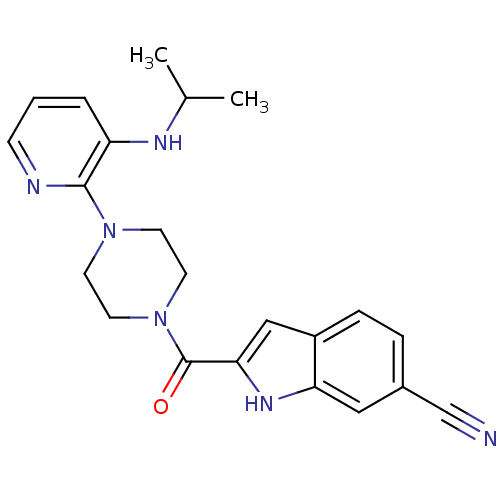 (2-({4-[3-(propan-2-ylamino)pyridin-2-yl]piperazin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2289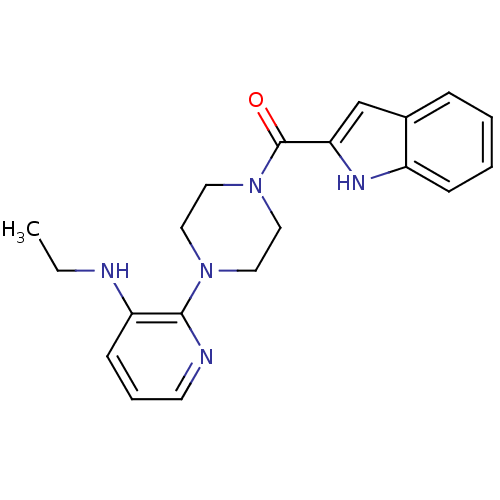 (BHAP analogue 1 | N-ethyl-2-[4-(1H-indol-2-ylcarbo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2293 (2-(4-{[5-(benzyloxy)-1H-indol-2-yl]carbonyl}pipera...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2297 (2-{4-[(6-fluoro-1H-indol-2-yl)carbonyl]piperazin-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50055477 (CHEMBL148532 | N-{2-[4-(3-tert-Butylamino-pyridin-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description In vitro for inhibition of HIV-1 reverse transcriptase. | J Med Chem 39: 5267-75 (1997) Article DOI: 10.1021/jm960269m BindingDB Entry DOI: 10.7270/Q2XK8DPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1437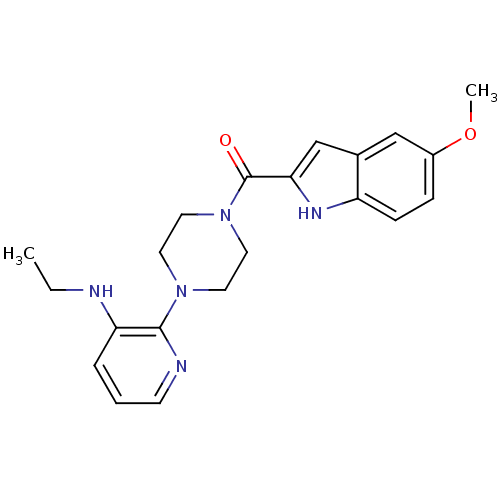 (5-methoxyindole-2-carboxylic acid [N -[3-(aminoeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1437 (5-methoxyindole-2-carboxylic acid [N -[3-(aminoeth...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia & Upjohn Curated by ChEMBL | Assay Description In vitro for inhibition of HIV-1 reverse transcriptase. | J Med Chem 39: 5267-75 (1997) Article DOI: 10.1021/jm960269m BindingDB Entry DOI: 10.7270/Q2XK8DPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2302 (2-({4-[3-(propan-2-ylamino)pyridin-2-yl]piperazin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2310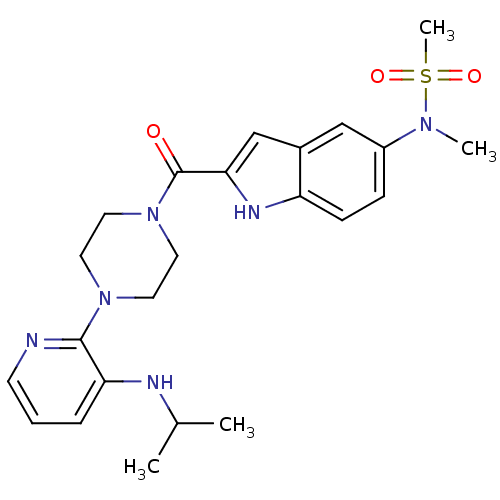 (5- or 6-Substituted Indole BHAP analogue 24 | N-me...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2306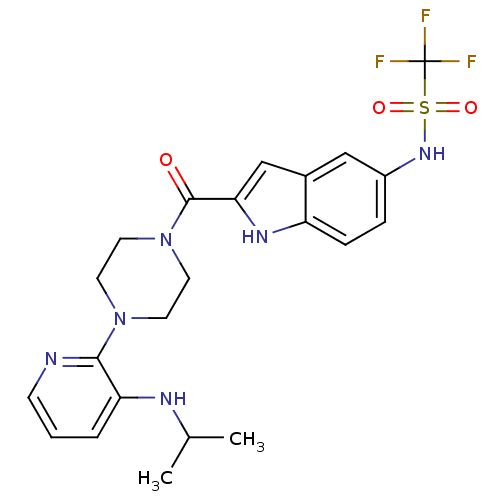 (5- or 6-Substituted Indole BHAP analogue 20 | trif...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2305 (2,2,2-trifluoro-N-[2-({4-[3-(propan-2-ylamino)pyri...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2294 (2-{4-[(5-methyl-1H-indol-2-yl)carbonyl]piperazin-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2298 (2-(4-{[6-(benzyloxy)-1H-indol-2-yl]carbonyl}pipera...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | 8.3 | 37 |
Upjohn | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1505-8 (1993) Article DOI: 10.1021/jm00062a027 BindingDB Entry DOI: 10.7270/Q2N58JJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||