 Found 116 hits with Last Name = 'rigat' and Initial = 'k'
Found 116 hits with Last Name = 'rigat' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239250

(CHEMBL4086080)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccncc1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-3-4-22(30(37)36-32(13-14-32)23-11-15-35-16-12-23)18-25(19)21-7-10-27-26(17-21)28(31(38)34-2)29(39-27)20-5-8-24(33)9-6-20/h3-12,15-18H,13-14H2,1-2H3,(H,34,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50239251

(CHEMBL4096241)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1cccnc1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-5-6-22(30(37)36-32(13-14-32)23-4-3-15-35-18-23)17-25(19)21-9-12-27-26(16-21)28(31(38)34-2)29(39-27)20-7-10-24(33)11-8-20/h3-12,15-18H,13-14H2,1-2H3,(H,34,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP3A4 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50239250

(CHEMBL4086080)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccncc1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-3-4-22(30(37)36-32(13-14-32)23-11-15-35-16-12-23)18-25(19)21-7-10-27-26(17-21)28(31(38)34-2)29(39-27)20-5-8-24(33)9-6-20/h3-12,15-18H,13-14H2,1-2H3,(H,34,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP3A4 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239251

(CHEMBL4096241)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1cccnc1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-5-6-22(30(37)36-32(13-14-32)23-4-3-15-35-18-23)17-25(19)21-9-12-27-26(16-21)28(31(38)34-2)29(39-27)20-7-10-24(33)11-8-20/h3-12,15-18H,13-14H2,1-2H3,(H,34,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50254772
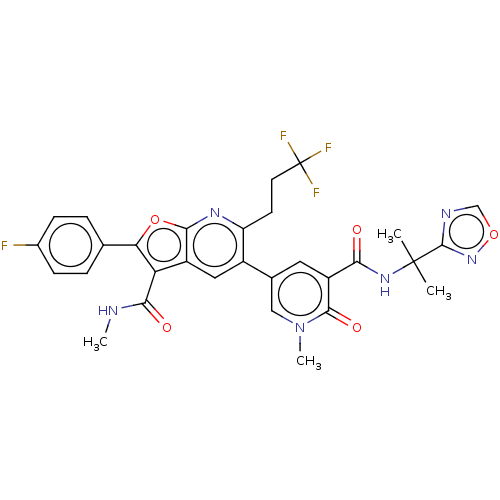
(CHEMBL4061621)Show SMILES CNC(=O)c1c(oc2nc(CCC(F)(F)F)c(cc12)-c1cc(C(=O)NC(C)(C)c2ncon2)c(=O)n(C)c1)-c1ccc(F)cc1 Show InChI InChI=1S/C30H26F4N6O5/c1-29(2,28-36-14-44-39-28)38-24(41)20-11-16(13-40(4)27(20)43)18-12-19-22(25(42)35-3)23(15-5-7-17(31)8-6-15)45-26(19)37-21(18)9-10-30(32,33)34/h5-8,11-14H,9-10H2,1-4H3,(H,35,42)(H,38,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, Connecticut 06492, United States.
Curated by ChEMBL
| Assay Description
Competitive inhibition of CYP3A4 in human liver microsomes in presence of NADPH by LC-MS/MS analysis |
ACS Med Chem Lett 8: 771-774 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00211
BindingDB Entry DOI: 10.7270/Q2KK9F7P |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepacivirus C) | BDBM50484982

(CHEMBL2017865)Show SMILES OC(=O)\C=C\c1ccc(NC(=O)C2(CCCC2)NC(=O)c2ccc3c(C4CCCCC4)c4-c5ncccc5OCCn4c3c2)cc1 Show InChI InChI=1S/C37H38N4O5/c42-31(43)17-12-24-10-14-27(15-11-24)39-36(45)37(18-4-5-19-37)40-35(44)26-13-16-28-29(23-26)41-21-22-46-30-9-6-20-38-33(30)34(41)32(28)25-7-2-1-3-8-25/h6,9-17,20,23,25H,1-5,7-8,18-19,21-22H2,(H,39,45)(H,40,44)(H,42,43)/b17-12+ | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus NS5B RNA-dependent RNA polymerase P495L mutant |
Bioorg Med Chem Lett 22: 2866-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.063
BindingDB Entry DOI: 10.7270/Q2NK3HWC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239252

(CHEMBL4078188)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-6-7-22(30(37)36-32(14-15-32)27-5-3-4-16-35-27)18-24(19)21-10-13-26-25(17-21)28(31(38)34-2)29(39-26)20-8-11-23(33)12-9-20/h3-13,16-18H,14-15H2,1-2H3,(H,34,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239253
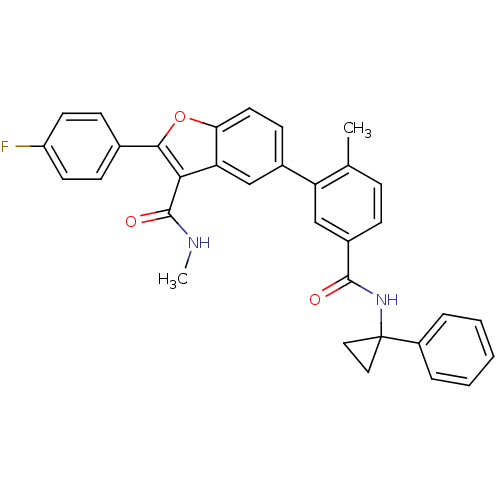
(CHEMBL4088517)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C33H27FN2O3/c1-20-8-9-23(31(37)36-33(16-17-33)24-6-4-3-5-7-24)19-26(20)22-12-15-28-27(18-22)29(32(38)35-2)30(39-28)21-10-13-25(34)14-11-21/h3-15,18-19H,16-17H2,1-2H3,(H,35,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepacivirus C) | BDBM50484986
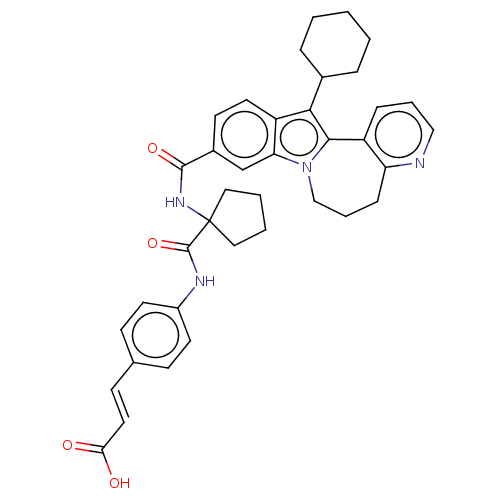
(CHEMBL2017863)Show SMILES OC(=O)\C=C\c1ccc(NC(=O)C2(CCCC2)NC(=O)c2ccc3c(C4CCCCC4)c4-c5cccnc5CCCn4c3c2)cc1 Show InChI InChI=1S/C38H40N4O4/c43-33(44)19-14-25-12-16-28(17-13-25)40-37(46)38(20-4-5-21-38)41-36(45)27-15-18-30-32(24-27)42-23-7-11-31-29(10-6-22-39-31)35(42)34(30)26-8-2-1-3-9-26/h6,10,12-19,22,24,26H,1-5,7-9,11,20-21,23H2,(H,40,46)(H,41,45)(H,43,44)/b19-14+ | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus NS5B RNA-dependent RNA polymerase P495L mutant |
Bioorg Med Chem Lett 22: 2866-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.063
BindingDB Entry DOI: 10.7270/Q2NK3HWC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50239253

(CHEMBL4088517)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C33H27FN2O3/c1-20-8-9-23(31(37)36-33(16-17-33)24-6-4-3-5-7-24)19-26(20)22-12-15-28-27(18-22)29(32(38)35-2)30(39-28)21-10-13-25(34)14-11-21/h3-15,18-19H,16-17H2,1-2H3,(H,35,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP3A4 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepacivirus C) | BDBM50484981
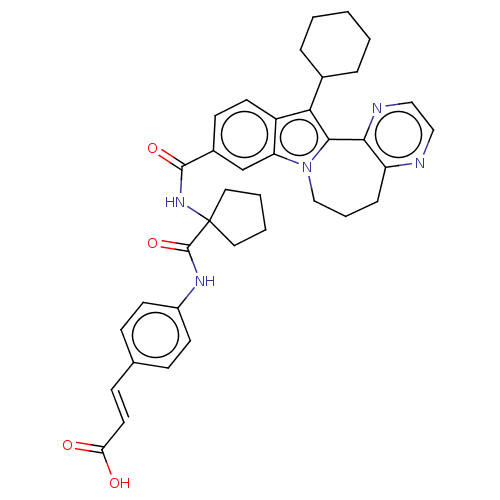
(CHEMBL2017862)Show SMILES OC(=O)\C=C\c1ccc(NC(=O)C2(CCCC2)NC(=O)c2ccc3c(C4CCCCC4)c4-c5nccnc5CCCn4c3c2)cc1 Show InChI InChI=1S/C37H39N5O4/c43-31(44)17-12-24-10-14-27(15-11-24)40-36(46)37(18-4-5-19-37)41-35(45)26-13-16-28-30(23-26)42-22-6-9-29-33(39-21-20-38-29)34(42)32(28)25-7-2-1-3-8-25/h10-17,20-21,23,25H,1-9,18-19,22H2,(H,40,46)(H,41,45)(H,43,44)/b17-12+ | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus NS5B RNA-dependent RNA polymerase P495L mutant |
Bioorg Med Chem Lett 22: 2866-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.063
BindingDB Entry DOI: 10.7270/Q2NK3HWC |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepacivirus C) | BDBM50484983

(CHEMBL2017866)Show SMILES OC(=O)\C=C\c1ccc(NC(=O)C2(CCCC2)NC(=O)c2ccc3c(C4CCCCC4)c4-c5cccnc5OCCn4c3c2)cc1 Show InChI InChI=1S/C37H38N4O5/c42-31(43)17-12-24-10-14-27(15-11-24)39-36(45)37(18-4-5-19-37)40-34(44)26-13-16-28-30(23-26)41-21-22-46-35-29(9-6-20-38-35)33(41)32(28)25-7-2-1-3-8-25/h6,9-17,20,23,25H,1-5,7-8,18-19,21-22H2,(H,39,45)(H,40,44)(H,42,43)/b17-12+ | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus NS5B RNA-dependent RNA polymerase P495L mutant |
Bioorg Med Chem Lett 22: 2866-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.063
BindingDB Entry DOI: 10.7270/Q2NK3HWC |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepacivirus C) | BDBM50484985

(CHEMBL2017864)Show SMILES OC(=O)\C=C\c1ccc(NC(=O)C2(CCCC2)NC(=O)c2ccc3c(C4CCCCC4)c4-c5ncccc5NC(=O)Cn4c3c2)cc1 Show InChI InChI=1S/C37H37N5O5/c43-30-22-42-29-21-25(13-16-27(29)32(24-7-2-1-3-8-24)34(42)33-28(40-30)9-6-20-38-33)35(46)41-37(18-4-5-19-37)36(47)39-26-14-10-23(11-15-26)12-17-31(44)45/h6,9-17,20-21,24H,1-5,7-8,18-19,22H2,(H,39,47)(H,40,43)(H,41,46)(H,44,45)/b17-12+ | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus NS5B RNA-dependent RNA polymerase P495L mutant |
Bioorg Med Chem Lett 22: 2866-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.063
BindingDB Entry DOI: 10.7270/Q2NK3HWC |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepacivirus C) | BDBM50484977

(CHEMBL2017856)Show SMILES OC(=O)\C=C\c1ccc(NC(=O)C2(CCCC2)NC(=O)c2ccc3c(C4CCCCC4)c4-c5ccccc5CCCn4c3c2)cc1 Show InChI InChI=1S/C39H41N3O4/c43-34(44)21-16-26-14-18-30(19-15-26)40-38(46)39(22-6-7-23-39)41-37(45)29-17-20-32-33(25-29)42-24-8-12-27-9-4-5-13-31(27)36(42)35(32)28-10-2-1-3-11-28/h4-5,9,13-21,25,28H,1-3,6-8,10-12,22-24H2,(H,40,46)(H,41,45)(H,43,44)/b21-16+ | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus NS5B RNA-dependent RNA polymerase P495L mutant |
Bioorg Med Chem Lett 22: 2866-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.063
BindingDB Entry DOI: 10.7270/Q2NK3HWC |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepacivirus C) | BDBM50484984

(CHEMBL2017858)Show SMILES OC(=O)\C=C\c1ccc(NC(=O)C2(CCCC2)NC(=O)c2ccc3c(C4CCCCC4)c4-c5ccoc5CCCn4c3c2)cc1 Show InChI InChI=1S/C37H39N3O5/c41-32(42)17-12-24-10-14-27(15-11-24)38-36(44)37(19-4-5-20-37)39-35(43)26-13-16-28-30(23-26)40-21-6-9-31-29(18-22-45-31)34(40)33(28)25-7-2-1-3-8-25/h10-18,22-23,25H,1-9,19-21H2,(H,38,44)(H,39,43)(H,41,42)/b17-12+ | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus NS5B RNA-dependent RNA polymerase P495L mutant |
Bioorg Med Chem Lett 22: 2866-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.063
BindingDB Entry DOI: 10.7270/Q2NK3HWC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239249

(CHEMBL4104000)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H25FN4O3/c1-18-4-5-21(28(37)36-31(12-13-31)30-34-14-3-15-35-30)17-23(18)20-8-11-25-24(16-20)26(29(38)33-2)27(39-25)19-6-9-22(32)10-7-19/h3-11,14-17H,12-13H2,1-2H3,(H,33,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50239252

(CHEMBL4078188)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-6-7-22(30(37)36-32(14-15-32)27-5-3-4-16-35-27)18-24(19)21-10-13-26-25(17-21)28(31(38)34-2)29(39-26)20-8-11-23(33)12-9-20/h3-13,16-18H,14-15H2,1-2H3,(H,34,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP3A4 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50239249

(CHEMBL4104000)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H25FN4O3/c1-18-4-5-21(28(37)36-31(12-13-31)30-34-14-3-15-35-30)17-23(18)20-8-11-25-24(16-20)26(29(38)33-2)27(39-25)19-6-9-22(32)10-7-19/h3-11,14-17H,12-13H2,1-2H3,(H,33,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by thallium flux assay |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepacivirus C) | BDBM50484980
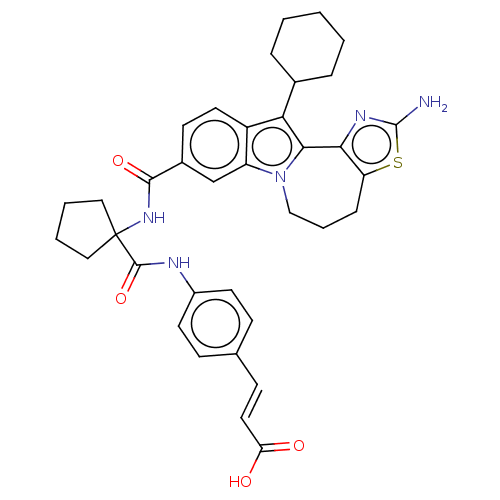
(CHEMBL2017861)Show SMILES Nc1nc-2c(CCCn3c-2c(C2CCCCC2)c2ccc(cc32)C(=O)NC2(CCCC2)C(=O)Nc2ccc(\C=C\C(O)=O)cc2)s1 Show InChI InChI=1S/C36H39N5O4S/c37-35-39-31-28(46-35)9-6-20-41-27-21-24(13-16-26(27)30(32(31)41)23-7-2-1-3-8-23)33(44)40-36(18-4-5-19-36)34(45)38-25-14-10-22(11-15-25)12-17-29(42)43/h10-17,21,23H,1-9,18-20H2,(H2,37,39)(H,38,45)(H,40,44)(H,42,43)/b17-12+ | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus NS5B RNA-dependent RNA polymerase P495L mutant |
Bioorg Med Chem Lett 22: 2866-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.063
BindingDB Entry DOI: 10.7270/Q2NK3HWC |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepacivirus C) | BDBM50484979

(CHEMBL2017860)Show SMILES Cc1nc-2c(CCCn3c-2c(C2CCCCC2)c2ccc(cc32)C(=O)NC2(CCCC2)C(=O)Nc2ccc(\C=C\C(O)=O)cc2)s1 Show InChI InChI=1S/C37H40N4O4S/c1-23-38-33-30(46-23)10-7-21-41-29-22-26(14-17-28(29)32(34(33)41)25-8-3-2-4-9-25)35(44)40-37(19-5-6-20-37)36(45)39-27-15-11-24(12-16-27)13-18-31(42)43/h11-18,22,25H,2-10,19-21H2,1H3,(H,39,45)(H,40,44)(H,42,43)/b18-13+ | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus NS5B RNA-dependent RNA polymerase P495L mutant |
Bioorg Med Chem Lett 22: 2866-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.063
BindingDB Entry DOI: 10.7270/Q2NK3HWC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50239252

(CHEMBL4078188)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-6-7-22(30(37)36-32(14-15-32)27-5-3-4-16-35-27)18-24(19)21-10-13-26-25(17-21)28(31(38)34-2)29(39-26)20-8-11-23(33)12-9-20/h3-13,16-18H,14-15H2,1-2H3,(H,34,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by thallium flux assay |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepacivirus C) | BDBM50484978

(CHEMBL2017857)Show SMILES OC(=O)\C=C\c1ccc(NC(=O)C2(CCCC2)NC(=O)c2ccc3c(C4CCCCC4)c4-c5ncoc5CCCn4c3c2)cc1 Show InChI InChI=1S/C36H38N4O5/c41-30(42)17-12-23-10-14-26(15-11-23)38-35(44)36(18-4-5-19-36)39-34(43)25-13-16-27-28(21-25)40-20-6-9-29-32(37-22-45-29)33(40)31(27)24-7-2-1-3-8-24/h10-17,21-22,24H,1-9,18-20H2,(H,38,44)(H,39,43)(H,41,42)/b17-12+ | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus NS5B RNA-dependent RNA polymerase P495L mutant |
Bioorg Med Chem Lett 22: 2866-71 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.063
BindingDB Entry DOI: 10.7270/Q2NK3HWC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239248
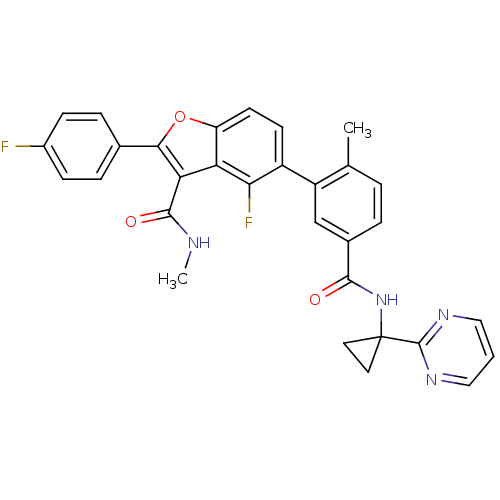
(CHEMBL4093031)Show SMILES CNC(=O)c1c(oc2ccc(c(F)c12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H24F2N4O3/c1-17-4-5-19(28(38)37-31(12-13-31)30-35-14-3-15-36-30)16-22(17)21-10-11-23-24(26(21)33)25(29(39)34-2)27(40-23)18-6-8-20(32)9-7-18/h3-11,14-16H,12-13H2,1-2H3,(H,34,39)(H,37,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50239249

(CHEMBL4104000)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H25FN4O3/c1-18-4-5-21(28(37)36-31(12-13-31)30-34-14-3-15-35-30)17-23(18)20-8-11-25-24(16-20)26(29(38)33-2)27(39-25)19-6-9-22(32)10-7-19/h3-11,14-17H,12-13H2,1-2H3,(H,33,38)(H,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP3A4 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50239248

(CHEMBL4093031)Show SMILES CNC(=O)c1c(oc2ccc(c(F)c12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H24F2N4O3/c1-17-4-5-19(28(38)37-31(12-13-31)30-35-14-3-15-36-30)16-22(17)21-10-11-23-24(26(21)33)25(29(39)34-2)27(40-23)18-6-8-20(32)9-7-18/h3-11,14-16H,12-13H2,1-2H3,(H,34,39)(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP3A4 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50142902

(CHEMBL3760108)Show SMILES [H][C@@]12C[C@@]1(Cn1c(c(C3CCCCC3)c3ccc(cc13)C(=O)NS(=O)(=O)CC(C)C)-c1ccc(OC)cc21)C(=O)N1C2CCC1CN(C)C2 |r| Show InChI InChI=1S/C38H48N4O5S/c1-23(2)21-48(45,46)39-36(43)25-10-14-30-33(16-25)41-22-38(37(44)42-26-11-12-27(42)20-40(3)19-26)18-32(38)31-17-28(47-4)13-15-29(31)35(41)34(30)24-8-6-5-7-9-24/h10,13-17,23-24,26-27,32H,5-9,11-12,18-22H2,1-4H3,(H,39,43)/t26?,27?,32-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by FLIPR assay |
Bioorg Med Chem Lett 26: 936-40 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.058
BindingDB Entry DOI: 10.7270/Q2ZG6V33 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50239248

(CHEMBL4093031)Show SMILES CNC(=O)c1c(oc2ccc(c(F)c12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H24F2N4O3/c1-17-4-5-19(28(38)37-31(12-13-31)30-35-14-3-15-36-30)16-22(17)21-10-11-23-24(26(21)33)25(29(39)34-2)27(40-23)18-6-8-20(32)9-7-18/h3-11,14-16H,12-13H2,1-2H3,(H,34,39)(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp assay |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50239248

(CHEMBL4093031)Show SMILES CNC(=O)c1c(oc2ccc(c(F)c12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H24F2N4O3/c1-17-4-5-19(28(38)37-31(12-13-31)30-35-14-3-15-36-30)16-22(17)21-10-11-23-24(26(21)33)25(29(39)34-2)27(40-23)18-6-8-20(32)9-7-18/h3-11,14-16H,12-13H2,1-2H3,(H,34,39)(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by thallium flux assay |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
DNA polymerase alpha catalytic subunit
(Homo sapiens (Human)) | BDBM50268397

(CHEMBL4067852)Show SMILES [H][C@@]12C[C@@]1(Cn1c(c(C3CCCCC3)c3ccc(cc13)C(=O)NS(=O)(=O)C1(C)CC1)-c1ccc(F)cc21)C(=O)N1C2CCC1CN(C)C2 |r,TLB:37:39:47.45.44:41.42,THB:46:45:39:41.42| Show InChI InChI=1S/C37H43FN4O4S/c1-36(14-15-36)47(45,46)39-34(43)23-8-12-28-31(16-23)41-21-37(35(44)42-25-10-11-26(42)20-40(2)19-25)18-30(37)29-17-24(38)9-13-27(29)33(41)32(28)22-6-4-3-5-7-22/h8-9,12-13,16-17,22,25-26,30H,3-7,10-11,14-15,18-21H2,1-2H3,(H,39,43)/t25?,26?,30-,37-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Discovery Chemistry and Molecular Technologies, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT 06492, United States. Electronic address: zhizhenZheng
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase alpha |
Bioorg Med Chem Lett 27: 3294-3300 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.024
BindingDB Entry DOI: 10.7270/Q20004KW |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50268397

(CHEMBL4067852)Show SMILES [H][C@@]12C[C@@]1(Cn1c(c(C3CCCCC3)c3ccc(cc13)C(=O)NS(=O)(=O)C1(C)CC1)-c1ccc(F)cc21)C(=O)N1C2CCC1CN(C)C2 |r,TLB:37:39:47.45.44:41.42,THB:46:45:39:41.42| Show InChI InChI=1S/C37H43FN4O4S/c1-36(14-15-36)47(45,46)39-34(43)23-8-12-28-31(16-23)41-21-37(35(44)42-25-10-11-26(42)20-40(2)19-25)18-30(37)29-17-24(38)9-13-27(29)33(41)32(28)22-6-4-3-5-7-22/h8-9,12-13,16-17,22,25-26,30H,3-7,10-11,14-15,18-21H2,1-2H3,(H,39,43)/t25?,26?,30-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Discovery Chemistry and Molecular Technologies, Bristol-Myers Squibb Research and Development, 5 Research Parkway, Wallingford, CT 06492, United States. Electronic address: zhizhenZheng
Curated by ChEMBL
| Assay Description
Inhibition of human DNA polymerase beta |
Bioorg Med Chem Lett 27: 3294-3300 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.024
BindingDB Entry DOI: 10.7270/Q20004KW |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50448498
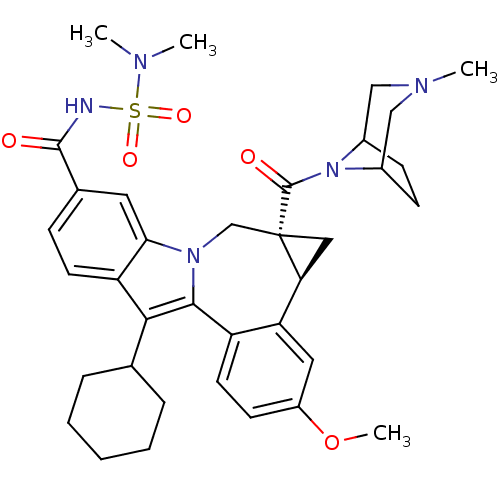
(BMS-791325 | Beclabuvir)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3C[C@]3(C[C@H]3c2c1)C(=O)N1C2CCC1CN(C)C2)C(=O)NS(=O)(=O)N(C)C |r,THB:36:35:29:31.32| Show InChI InChI=1S/C36H45N5O5S/c1-38(2)47(44,45)37-34(42)23-10-14-28-31(16-23)40-21-36(35(43)41-24-11-12-25(41)20-39(3)19-24)18-30(36)29-17-26(46-4)13-15-27(29)33(40)32(28)22-8-6-5-7-9-22/h10,13-17,22,24-25,30H,5-9,11-12,18-21H2,1-4H3,(H,37,42)/t24?,25?,30-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by FLIPR assay |
Bioorg Med Chem Lett 26: 936-40 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.058
BindingDB Entry DOI: 10.7270/Q2ZG6V33 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50142900

(CHEMBL3759768)Show SMILES [H][C@@]12C[C@@]1(Cn1c(c(C3CCCCC3)c3ccc(cc13)C(=O)NS(=O)(=O)C1CCC1)-c1ccc(OC)cc21)C(=O)N1C2CCC1CN(C)C2 |r| Show InChI InChI=1S/C38H46N4O5S/c1-40-20-25-12-13-26(21-40)42(25)37(44)38-19-32(38)31-18-27(47-2)14-16-29(31)35-34(23-7-4-3-5-8-23)30-15-11-24(17-33(30)41(35)22-38)36(43)39-48(45,46)28-9-6-10-28/h11,14-18,23,25-26,28,32H,3-10,12-13,19-22H2,1-2H3,(H,39,43)/t25?,26?,32-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by FLIPR assay |
Bioorg Med Chem Lett 26: 936-40 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.058
BindingDB Entry DOI: 10.7270/Q2ZG6V33 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50448498

(BMS-791325 | Beclabuvir)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3C[C@]3(C[C@H]3c2c1)C(=O)N1C2CCC1CN(C)C2)C(=O)NS(=O)(=O)N(C)C |r,THB:36:35:29:31.32| Show InChI InChI=1S/C36H45N5O5S/c1-38(2)47(44,45)37-34(42)23-10-14-28-31(16-23)40-21-36(35(43)41-24-11-12-25(41)20-39(3)19-24)18-30(36)29-17-26(46-4)13-15-27(29)33(40)32(28)22-8-6-5-7-9-22/h10,13-17,22,24-25,30H,5-9,11-12,18-21H2,1-4H3,(H,37,42)/t24?,25?,30-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50448498

(BMS-791325 | Beclabuvir)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3C[C@]3(C[C@H]3c2c1)C(=O)N1C2CCC1CN(C)C2)C(=O)NS(=O)(=O)N(C)C |r,THB:36:35:29:31.32| Show InChI InChI=1S/C36H45N5O5S/c1-38(2)47(44,45)37-34(42)23-10-14-28-31(16-23)40-21-36(35(43)41-24-11-12-25(41)20-39(3)19-24)18-30(36)29-17-26(46-4)13-15-27(29)33(40)32(28)22-8-6-5-7-9-22/h10,13-17,22,24-25,30H,5-9,11-12,18-21H2,1-4H3,(H,37,42)/t24?,25?,30-,36-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50448498

(BMS-791325 | Beclabuvir)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3C[C@]3(C[C@H]3c2c1)C(=O)N1C2CCC1CN(C)C2)C(=O)NS(=O)(=O)N(C)C |r,THB:36:35:29:31.32| Show InChI InChI=1S/C36H45N5O5S/c1-38(2)47(44,45)37-34(42)23-10-14-28-31(16-23)40-21-36(35(43)41-24-11-12-25(41)20-39(3)19-24)18-30(36)29-17-26(46-4)13-15-27(29)33(40)32(28)22-8-6-5-7-9-22/h10,13-17,22,24-25,30H,5-9,11-12,18-21H2,1-4H3,(H,37,42)/t24?,25?,30-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50448498

(BMS-791325 | Beclabuvir)Show SMILES COc1ccc2-c3c(C4CCCCC4)c4ccc(cc4n3C[C@]3(C[C@H]3c2c1)C(=O)N1C2CCC1CN(C)C2)C(=O)NS(=O)(=O)N(C)C |r,THB:36:35:29:31.32| Show InChI InChI=1S/C36H45N5O5S/c1-38(2)47(44,45)37-34(42)23-10-14-28-31(16-23)40-21-36(35(43)41-24-11-12-25(41)20-39(3)19-24)18-30(36)29-17-26(46-4)13-15-27(29)33(40)32(28)22-8-6-5-7-9-22/h10,13-17,22,24-25,30H,5-9,11-12,18-21H2,1-4H3,(H,37,42)/t24?,25?,30-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 1855-79 (2014)
Article DOI: 10.1021/jm4016894
BindingDB Entry DOI: 10.7270/Q2V989JD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50142901
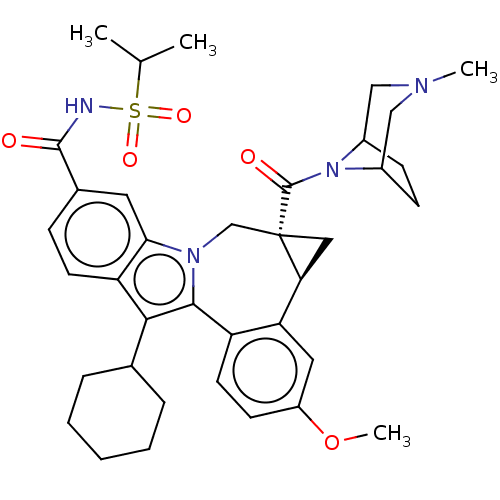
(CHEMBL3758288)Show SMILES [H][C@@]12C[C@@]1(Cn1c(c(C3CCCCC3)c3ccc(cc13)C(=O)NS(=O)(=O)C(C)C)-c1ccc(OC)cc21)C(=O)N1C2CCC1CN(C)C2 |r| Show InChI InChI=1S/C37H46N4O5S/c1-22(2)47(44,45)38-35(42)24-10-14-29-32(16-24)40-21-37(36(43)41-25-11-12-26(41)20-39(3)19-25)18-31(37)30-17-27(46-4)13-15-28(30)34(40)33(29)23-8-6-5-7-9-23/h10,13-17,22-23,25-26,31H,5-9,11-12,18-21H2,1-4H3,(H,38,42)/t25?,26?,31-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by FLIPR assay |
Bioorg Med Chem Lett 26: 936-40 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.058
BindingDB Entry DOI: 10.7270/Q2ZG6V33 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50507439

(CHEMBL4439859)Show SMILES CCN(C(=O)OC)c1cc2oc(c(C(=O)NC)c2cc1-c1ccc(OC)c(c1)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C35H32FN5O6/c1-5-41(34(44)46-4)26-19-28-24(29(32(43)37-2)30(47-28)20-7-10-22(36)11-8-20)18-23(26)21-9-12-27(45-3)25(17-21)31(42)40-35(13-14-35)33-38-15-6-16-39-33/h6-12,15-19H,5,13-14H2,1-4H3,(H,37,43)(H,40,42) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HCV genotype 2a JFH1 NS5B RNA dependent RNA polymerase infected in human HuH7 replicon cells after 3 days by luciferase reporter gene a... |
ACS Med Chem Lett 9: 1217-1222 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00379
BindingDB Entry DOI: 10.7270/Q2BZ699D |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50507440

(CHEMBL4546966)Show SMILES CCN(C(C)=O)c1cc2oc(c(C(=O)NC)c2cc1-c1ccc(OC)c(c1)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C35H32FN5O5/c1-5-41(20(2)42)27-19-29-25(30(33(44)37-3)31(46-29)21-7-10-23(36)11-8-21)18-24(27)22-9-12-28(45-4)26(17-22)32(43)40-35(13-14-35)34-38-15-6-16-39-34/h6-12,15-19H,5,13-14H2,1-4H3,(H,37,44)(H,40,43) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HCV genotype 2a JFH1 NS5B RNA dependent RNA polymerase infected in human HuH7 replicon cells after 3 days by luciferase reporter gene a... |
ACS Med Chem Lett 9: 1217-1222 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00379
BindingDB Entry DOI: 10.7270/Q2BZ699D |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50507432

(CHEMBL4534277)Show SMILES CCNc1cc2oc(c(C(=O)NC)c2cc1-c1ccc(OC)c(c1)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C33H30FN5O4/c1-4-36-25-18-27-23(28(31(41)35-2)29(43-27)19-6-9-21(34)10-7-19)17-22(25)20-8-11-26(42-3)24(16-20)30(40)39-33(12-13-33)32-37-14-5-15-38-32/h5-11,14-18,36H,4,12-13H2,1-3H3,(H,35,41)(H,39,40) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HCV genotype 2a JFH1 NS5B RNA dependent RNA polymerase infected in human HuH7 replicon cells after 3 days by luciferase reporter gene a... |
ACS Med Chem Lett 9: 1217-1222 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00379
BindingDB Entry DOI: 10.7270/Q2BZ699D |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50507441
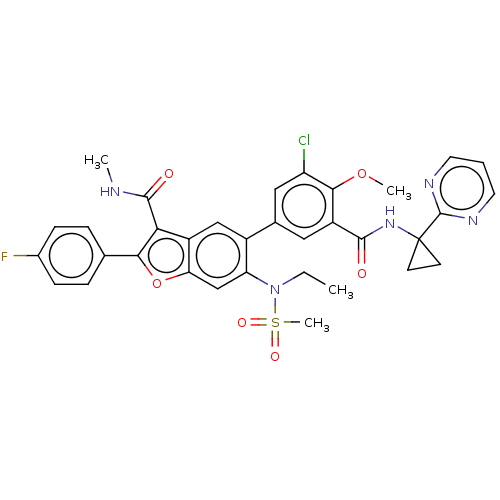
(CHEMBL4547789)Show SMILES CCN(c1cc2oc(c(C(=O)NC)c2cc1-c1cc(Cl)c(OC)c(c1)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1)S(C)(=O)=O Show InChI InChI=1S/C34H31ClFN5O6S/c1-5-41(48(4,44)45)26-18-27-23(28(32(43)37-2)29(47-27)19-7-9-21(36)10-8-19)17-22(26)20-15-24(30(46-3)25(35)16-20)31(42)40-34(11-12-34)33-38-13-6-14-39-33/h6-10,13-18H,5,11-12H2,1-4H3,(H,37,43)(H,40,42) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HCV genotype 2a JFH1 NS5B RNA dependent RNA polymerase infected in human HuH7 replicon cells after 3 days by luciferase reporter gene a... |
ACS Med Chem Lett 9: 1217-1222 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00379
BindingDB Entry DOI: 10.7270/Q2BZ699D |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50507442

(CHEMBL4474552)Show SMILES CCN(c1cc2oc(c(C(=O)NC)c2cc1-c1cc(F)c(F)c(c1)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1)S(C)(=O)=O Show InChI InChI=1S/C33H28F3N5O5S/c1-4-41(47(3,44)45)25-17-26-22(27(31(43)37-2)29(46-26)18-6-8-20(34)9-7-18)16-21(25)19-14-23(28(36)24(35)15-19)30(42)40-33(10-11-33)32-38-12-5-13-39-32/h5-9,12-17H,4,10-11H2,1-3H3,(H,37,43)(H,40,42) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HCV genotype 2a JFH1 NS5B RNA dependent RNA polymerase infected in human HuH7 replicon cells after 3 days by luciferase reporter gene a... |
ACS Med Chem Lett 9: 1217-1222 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00379
BindingDB Entry DOI: 10.7270/Q2BZ699D |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50507437

(CHEMBL4549638)Show SMILES CCN(c1cc2oc(c(C(=O)NC)c2cc1-c1cccc(c1)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1)S(C)(=O)=O Show InChI InChI=1S/C33H30FN5O5S/c1-4-39(45(3,42)43)26-19-27-25(28(31(41)35-2)29(44-27)20-9-11-23(34)12-10-20)18-24(26)21-7-5-8-22(17-21)30(40)38-33(13-14-33)32-36-15-6-16-37-32/h5-12,15-19H,4,13-14H2,1-3H3,(H,35,41)(H,38,40) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HCV genotype 1b NS5B RNA dependent RNA polymerase infected in human HuH7 replicon cells after 3 days by luciferase reporter gene assay |
ACS Med Chem Lett 9: 1217-1222 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00379
BindingDB Entry DOI: 10.7270/Q2BZ699D |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50507434
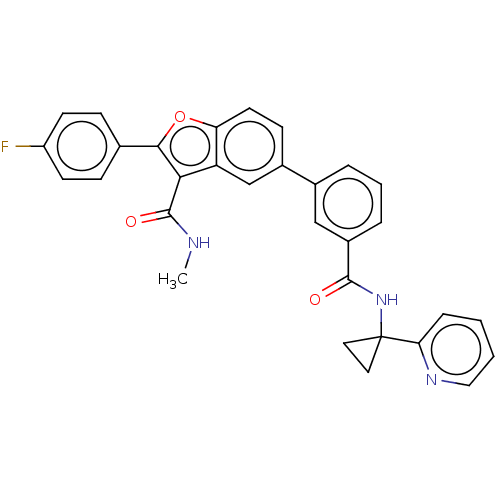
(CHEMBL4546784)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cccc(c1)C(=O)NC1(CC1)c1ccccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C31H24FN3O3/c1-33-30(37)27-24-18-21(10-13-25(24)38-28(27)19-8-11-23(32)12-9-19)20-5-4-6-22(17-20)29(36)35-31(14-15-31)26-7-2-3-16-34-26/h2-13,16-18H,14-15H2,1H3,(H,33,37)(H,35,36) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 515 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HCV genotype 2a JFH1 NS5B RNA dependent RNA polymerase infected in human HuH7 replicon cells after 3 days by luciferase reporter gene a... |
ACS Med Chem Lett 9: 1217-1222 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00379
BindingDB Entry DOI: 10.7270/Q2BZ699D |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50507443

(CHEMBL4469782)Show SMILES CCN(c1cc2oc(c(C(=O)NC)c2cc1-c1cc(C)c(OC)c(c1)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1)S(C)(=O)=O Show InChI InChI=1S/C35H34FN5O6S/c1-6-41(48(5,44)45)27-19-28-25(29(33(43)37-3)31(47-28)21-8-10-23(36)11-9-21)18-24(27)22-16-20(2)30(46-4)26(17-22)32(42)40-35(12-13-35)34-38-14-7-15-39-34/h7-11,14-19H,6,12-13H2,1-5H3,(H,37,43)(H,40,42) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HCV genotype 2a JFH1 NS5B RNA dependent RNA polymerase infected in human HuH7 replicon cells after 3 days by luciferase reporter gene a... |
ACS Med Chem Lett 9: 1217-1222 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00379
BindingDB Entry DOI: 10.7270/Q2BZ699D |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50507436
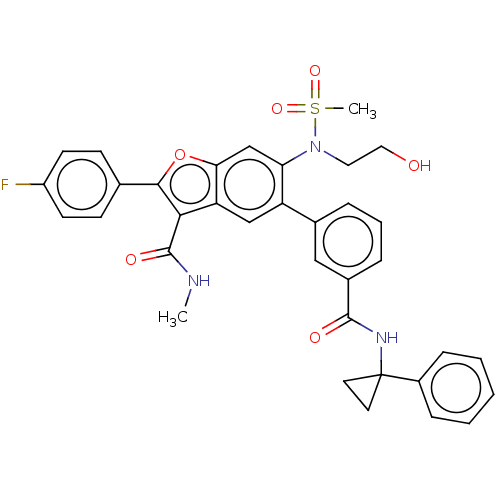
(CHEMBL4534885)Show SMILES CNC(=O)c1c(oc2cc(N(CCO)S(C)(=O)=O)c(cc12)-c1cccc(c1)C(=O)NC1(CC1)c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C35H32FN3O6S/c1-37-34(42)31-28-20-27(23-7-6-8-24(19-23)33(41)38-35(15-16-35)25-9-4-3-5-10-25)29(39(17-18-40)46(2,43)44)21-30(28)45-32(31)22-11-13-26(36)14-12-22/h3-14,19-21,40H,15-18H2,1-2H3,(H,37,42)(H,38,41) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HCV genotype 1b NS5B RNA dependent RNA polymerase infected in human HuH7 replicon cells after 3 days by luciferase reporter gene assay |
ACS Med Chem Lett 9: 1217-1222 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00379
BindingDB Entry DOI: 10.7270/Q2BZ699D |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50507437

(CHEMBL4549638)Show SMILES CCN(c1cc2oc(c(C(=O)NC)c2cc1-c1cccc(c1)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1)S(C)(=O)=O Show InChI InChI=1S/C33H30FN5O5S/c1-4-39(45(3,42)43)26-19-27-25(28(31(41)35-2)29(44-27)20-9-11-23(34)12-10-20)18-24(26)21-7-5-8-22(17-21)30(40)38-33(13-14-33)32-36-15-6-16-37-32/h5-12,15-19H,4,13-14H2,1-3H3,(H,35,41)(H,38,40) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HCV genotype 1a NS5B RNA dependent RNA polymerase infected in human HuH7 replicon cells after 3 days by luciferase reporter gene assay |
ACS Med Chem Lett 9: 1217-1222 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00379
BindingDB Entry DOI: 10.7270/Q2BZ699D |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50507435

(CHEMBL4451879)Show SMILES CNC(=O)c1c(oc2cc(N(CCO)S(C)(=O)=O)c(cc12)-c1cccc(c1)C(=O)NC1(CC1)c1ccccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C34H31FN4O6S/c1-36-33(42)30-26-19-25(22-6-5-7-23(18-22)32(41)38-34(13-14-34)29-8-3-4-15-37-29)27(39(16-17-40)46(2,43)44)20-28(26)45-31(30)21-9-11-24(35)12-10-21/h3-12,15,18-20,40H,13-14,16-17H2,1-2H3,(H,36,42)(H,38,41) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 385 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HCV genotype 1b NS5B RNA dependent RNA polymerase C316N mutant infected in human HuH7 replicon cells after 3 days by luciferase reporte... |
ACS Med Chem Lett 9: 1217-1222 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00379
BindingDB Entry DOI: 10.7270/Q2BZ699D |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50507436

(CHEMBL4534885)Show SMILES CNC(=O)c1c(oc2cc(N(CCO)S(C)(=O)=O)c(cc12)-c1cccc(c1)C(=O)NC1(CC1)c1ccccc1)-c1ccc(F)cc1 Show InChI InChI=1S/C35H32FN3O6S/c1-37-34(42)31-28-20-27(23-7-6-8-24(19-23)33(41)38-35(15-16-35)25-9-4-3-5-10-25)29(39(17-18-40)46(2,43)44)21-30(28)45-32(31)22-11-13-26(36)14-12-22/h3-14,19-21,40H,15-18H2,1-2H3,(H,37,42)(H,38,41) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HCV genotype 2a JFH1 NS5B RNA dependent RNA polymerase infected in human HuH7 replicon cells after 3 days by luciferase reporter gene a... |
ACS Med Chem Lett 9: 1217-1222 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00379
BindingDB Entry DOI: 10.7270/Q2BZ699D |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50507444

(CHEMBL4571040)Show SMILES CCN(C(=O)N(C)C)c1cc2oc(c(C(=O)NC)c2cc1-c1ccc(OC)c(c1)C(=O)NC1(CC1)c1ncccn1)-c1ccc(F)cc1 Show InChI InChI=1S/C36H35FN6O5/c1-6-43(35(46)42(3)4)27-20-29-25(30(33(45)38-2)31(48-29)21-8-11-23(37)12-9-21)19-24(27)22-10-13-28(47-5)26(18-22)32(44)41-36(14-15-36)34-39-16-7-17-40-34/h7-13,16-20H,6,14-15H2,1-5H3,(H,38,45)(H,41,44) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 165 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of HCV genotype 2a JFH1 NS5B RNA dependent RNA polymerase infected in human HuH7 replicon cells after 3 days by luciferase reporter gene a... |
ACS Med Chem Lett 9: 1217-1222 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00379
BindingDB Entry DOI: 10.7270/Q2BZ699D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data